?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Determination of the chemical composition of biomaterial is important for their valued utilization in biorefinery. In this study, the chemical composition of seven wood species, i.e. lambu (Khaya anthotheca), raj-koroi (Albizia richardiana), jhau (Casuarina equisetifolia), sil-koroi (Albizia procera), katbadam (Terminalia catappa), jolpai (Elaeocarpus robustus), and arjun (Terminalia arjuna) were examined. The chemical characterization of these wood species can expedite a further study on the extraction of cellulose, lignin, and extractive. α-cellulose content was in the range of 37.0% to 42.1% and lignin content was 20.4% to 34.1%. The solubility in 1% caustic soda was 16.1% to 24.3%. The α-cellulose and lignin content were similar to other wood species. Therefore, these species can be a potential source of raw material for biorefinery.
1. Introduction
Wood is a hygroscopic material mainly composed of a complex matrix of tree biopolymers. It is heterogeneously aggregated of cell wall fibers composed primarily of cellulose and hemicellulose, joined by major cell wall constituent polymers of lignin. Cellulose is the major component in the walls of plant cells, helping the plant to remain stiff and upright. Holocellulose is the total polysaccharide fraction of wood that is composed of cellulose and all of the hemicelluloses. Hemicellulose is branched, and it is composed of several different kinds of hexose and pentose sugar monomers (Wiedenhoeft Citation2010). Lignin is often called the cementing agent that binds individual cells together (Boerjan et al. Citation2003, Wiedenhoeft Citation2010). Extractives include resigns, fats, oils, gum, starch, waxes, tannins, and coloring materials. These components contribute to various wood properties, including odor, color, taste, hygroscopicity, density, and decay resistance (Wiedenhoeft Citation2010).
Wood is one of the prime sources of feedstock for the production of pulp and paper, boards, and furniture, and increasingly popular bio-refinery products (biochemicals, biomaterials, biofuels, and others). The currently growing market demand for naturally derived compounds and the need to replace synthetic ones have led to the increasingly efficient utilization of wood (reuse, recycling, recovery), as well as increasing interest in studying the individual chemical components of wood and identifying novel application pathways and value-added solutions for particular bioactive molecules (Royer et al. Citation2012, Routa et al. Citation2017). The amount of chemical constituents, i.e. cellulose and lignin, is an important factor for considering its suitability as a raw material for bio-refinery and selecting the optimum conversion method.
Therefore, knowledge of the chemical composition of wood is inevitable for its better utilization. It is important to expand knowledge of the chemical constituents of this wood so that the region’s timber resources will have greater potential and utility in the technological and industrial process such as the production of pulp and paper, tannins, and as natural wood preservatives (Hillis Citation1971, Argyropoulos Citation2001, Das et al. Citation2015, Citation2016). On the other hand, the alternative wood species need to be examined for reducing the raw material shortage issue for wood-based industries. There are seven wood species, i.e. Khaya anthotheca, Albizia richardiana, Casuarina equisetifolia, Albizia procera, Terminalia catappa, Elaeocarpus robustus, and Terminalia arjuna, which chemical composition haven’t been studied. K. anthotheca can grow in deep fertile moist soils and it can be 60 m in height (Alam et al. Citation2012). Similarly, A. procera (Troup, Citation1921), E. robustus (Zmarzty, Citation2001), and T. arjuna (Kumar and Maulik, Citation2013) prefer moist soils but they can grow at a different height. The maximum height of A. procera (Troup, Citation1921), E. robustus (Zmarzty, Citation2001), and T. arjuna (Kumar and Maulik, Citation2013) is 25, 20, and 20–30 m, respectively. On the other hand, A. richardiana can grow in dry to moderately moist soil and it can be 30–40 m tall (Rahman et al. Citation2013). However, T. catappa can grow in dry to moist soils and saline soils. It can attain a maximum height of 35 m (Göltenboth et al. Citation2006). The habitat of C. equisetifolia is different from the other six species. It prefers a hot sub-humid area to grow and can be 10–40 m in height (Göltenboth et al. Citation2006). The study on the chemical profiles of these can provide a better understanding of their utilizations.
That’s why this study has been conducted to analyse the chemical components of seven wood species. Cellulose, lignin, and extractive content were determined. Cold water, hot water, and caustic soda (NaOH) solubility of each species were also studied.
2. Material and methods
2.1. Raw materials
A 20–30 years old and defect-free seven timber species, i.e. Khaya anthotheca, Albizia richardiana, Casuarina equisetifolia, Albizia procera, Terminalia catappa, Elaeocarpus robustus, and Terminalia arjuna, were selected in this study. There were three trees of each species were collected for the analysis of chemical composition.
Reagent grade (≥95% purity) sodium hydroxide (NaOH), acetic acid (CH3COOH), Sodium chlorite (NaClO2), and sulfuric acid (H2SO4) were received from Carolina Biological Supply Company, New York City, USA. Analytical grade (≥95% purity) benzene and ethanol were sourced from Merck KGA, Darmstadt, Germany.
2.2. Preparation of raw material
The trees were cut at 15–25 cm above ground level and then subdivided equally into the top, middle and basal portions according to their total length. Each part was debarked and converted into boards. The boards were chipped using an electric planning machine. The chips were dried in the sun and the dried chips were then milled by a Wiley mill to get wood powder. The powder of three portions was mixed thoroughly to obtain a representative sample of a whole tree. The powders were then sieved to obtain a 40–60 mesh. This procedure was applied for all seven species used in this study. The powders were stored in an airtight container for further analysis.
2.2. Chemical analysis
Chemical analysis of seven timber species was conducted based on TAPPI standards. To determine the cellulose content of seven wood species, holocellulose and α-cellulose were analysed by following the standard of T 249-75 and T 203 cm-99, respectively. Lignin content was analysed based on the T-222 cm-02 standard. Extractive content was determined following the T-204 cm-97 standard. The polymer degradation was investigated by the analysis of solubility. T-207 cm-99 standard was used to anlayse cold and hot water solubility and alkaline solubility was determined by T-212 om-02 standard. The carried out analyses are described in this section.
2.2.1. Holocellulose
A 2 g extractive-free sample, 160 ml of distilled water, 0.2 ml of cold CH3COOH, and one gram of NaClO2 were placed in a 250 ml flask. It was then kept in a water bath at 70–80°C for 5 h. One gram of NaClO2 and 0.2 ml of cold CH3COOH were added to the mixture with continuous stirring in a 1 h interval. At the end of five hours, the flask was placed in an ice-water bath and filtered into a known weight of coarse porosity fritted-glass crucible. The residue was washed with acetone followed by ethanol. The crucible containing residue was then oven-dried at 105°C and weighed until a constant weight was reached. The following equation was used to calculate the holocellulose content.
where W1 is alcohol-toluene extractive content (percent), W2, W3, and W4 were weight (dry mass) of samples, residue and crucible, and crucible, respectively.
2.2.2. α-cellulose
A 3 g of oven-dried holocellulose sample was placed in a 250 mL Erlenmeyer flask and it was then put in a water bath maintaining a temperature of 20°C. Then, 50 ml of 17.5% NaOH solution was poured into the flask and mixed thoroughly for one minute. After that, the reaction between the sample and solution was continued for 29 min followed by adding 50 ml of distilled water and mixing for another minute. The mixture was kept for another 5 min to complete the reaction and it was filtered using a known weight of fritted-glass crucible by the aid of vacuum suction. The residue was washed with 50 ml of 8.3% NaOH followed by 40 ml of 10% CH3COOH, and 1000 ml of hot distilled water. The crucible was oven-dried in an oven at 103 ± 2°C until obtaining a constant weight. α-cellulose was calculated using the following formula.
where W1 is holocellulose content (percent), W2, W3, and W4 were weight (dry mass) of holocellulose sample, residue and crucible, and crucible, respectively.
2.2.3. Lignin
A 2 g of extractive free sample and 40.0 ml of 72% H2SO4 were kept in a beaker followed by continuous stirring with a glass rod until the complete dispersion of the sample. It was then transferred to the flask and 1540 ml of hot water was added to the mixture. The mixture was boiled for 4 h, maintaining constant volume by the addition of hot water frequently. After that, it was kept overnight. Then, it was filtered using a known weight of fritted-glass crucible by the aid of vacuum suction and washed with hot water to remove the residual acid from the lignin. The crucible containing the residue was oven-dried at 103 ± 2°C until obtaining a constant weight. Lignin content was calculated using the following equation.
where W1, W2, and W3 were weight (dry mass) of sample, residue and crucible, and crucible, respectively.
2.2.4. Extractive
A 5 g sample was used for extractive analysis. The solvent was a mixture of benzene and ethanol in the ratio of 2:1 and kept in a flask. Soxhlet apparatus was used to extract extractive for a period of 4–5 h. The solvent was evaporated and the flask was dried at 105 ± 3°C for 1 h followed by cooling in a desiccator and weighing. The extractive content was measured using the following equation.
where We, Wp, and Wb are weight (dry mass) of flask with extractive, wood powder and flask, respectively.
2.2.5. Cold water solubility
A 2 g sample was placed in a 500 ml beaker, and 300 ml of distilled water was added to the beaker. After wetting the sample, extraction was carried out with constant stirring at room temperature (23°C) for 48 h. The mixture was then transferred to a tared filtering crucible and washed with 200 ml of cold distilled water. After that, it was dried at 105°C to a constant weight. The following equation was used to calculate the cold water solubility.
where A and B are before and after extraction weight (dry mass) of the test specimen, respectively.
2.2.6. Hot water solubility
A 2 g sample and 100 ml of hot distilled water were put in a 250 ml conical flask. The mixture was placed in a boiling water bath and digested by the reflux condenser for 3 h. Then, the mixture was transferred to a tared filtering crucible and washed with 200 ml of hot distilled water. It was dried at 105°C until reaching constant weight. The calculation of hot water solubility was done using the following equation.
where A and B are before and after extraction weight (dry mass) of the sample, respectively.
2.2.7. Alkaline solubility
A 2 g sample and 100 ml of 1% NaOH solution were kept in a 500 ml conical flask. The mixture was stirred with a glass rod, and it was then placed in a water bath at 97–100°C for 60 min. After that, it was transferred to a tared filtering crucible and washed with 100 ml of hot water. It was then soaked in 25 ml of 10% CH3COOH for 1 min followed by soaking in 15 ml of 10% CH3COOH for 1 min. Finally, it was washed with hot water until the removal of the remaining acid. Alkaline solubility was calculated using the following equation.
where A and B are before and after extraction weight (dry mass) of the sample, respectively.
3. Results and discussion
3.1. Cellulose
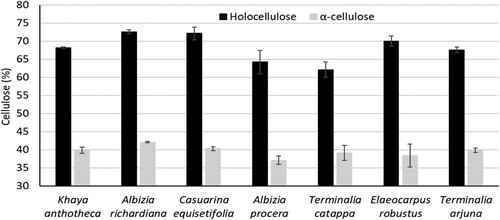
Holocellulose and α-cellulose content of seven wood species are presented in . The holocellulose and α-cellulose content of seven species were in the range of 62.1% to 72.6% and 37.0% to 42.1%, respectively. A. richardiana showed the highest amount of holocellulose (72.6%) and α-cellulose (42.1%) than K. anthocera, C. equisetifolia, A. procera, T. catappa, E. Robustus, and T. arjuna. The α-cellulose content of Eucalyptus spp. (Pereira et al. Citation2013), Acacia mangium (Pinto et al. Citation2005), and Populus spp (Kacik et al. Citation2012) were 46.1% to 48.8%, 46.5%, and 43.1% to 45.9%, respectively in previous studies. The holocellulose content of Acacia mangium (Pinto et al. Citation2005) and Populus spp (Kacik et al. Citation2012) were 70.9% and 81.7% to 82.9%, respectively. In comparison to other wood species, seven wood species used in this study showed more or less similar content of α-cellulose and holocellulose contents.
3.2. Lignin
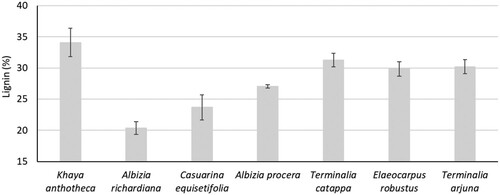
The measured lignin content of seven wood species has been shown in . The observed lignin content of seven wood species was 20.4% to 34.1% and it was significantly different from each other. The lowest lignin content (20.4%) was observed for A. richardiana and it was the highest (34.1%) for K. anthocera. In previous studies, the lignin content of Eucalyptus spp. (Pereira et al. Citation2013), Acacia mangium (Pinto et al. Citation2005), and Populus spp (Kacik et al. Citation2012) were 28.8% to 31.4%, 27.1%, and 17.7% to 23.7%, respectively. The lignin content of seven wood species was comparatively similar to other wood species observed in previous investigations.
The variability of the lignin content within these species could indicate better possible end-uses, with K. anthotheca, T. catappa, E. robustus, and T. arjuna showing high contents that could be exploited for energy purposes (biocoal production) or aiming at this rich polyphenolic pool, while A. richardiana, with low lignin content, appears to be more suited for pulp or polysaccharides production (maybe even ethanol).
3.3. Solubility
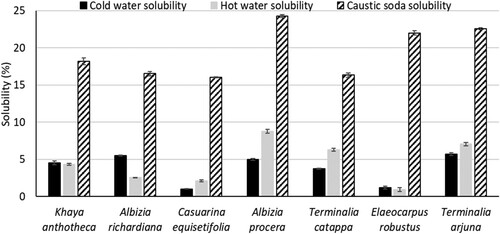
represents the solubility of seven wood species. The range of cold water, hot water, and 1% caustic soda (NaOH) solubility were 1.0% to 5.7%, 0.9% to 8.8%, and 16.1% to 24.3%, respectively. The highest hot water (8.8%) and NaOH solubility (24.3%) was found for A. procera while T. arjuna showed the highest solubility (5.7%) in cold water. Again, the lowest cold water (1.0%) and NaOH (16.1%) solubility were observed for C. equisetifolia. Meanwhile, E. Robustus showed the lowest (0.9%) hot water solubility. On the other hand, all species were more soluble in NaOH solution comparison to in cold and hot water. Cellulose is more resistant but hemicellulose is prone to degrade by the presence of NaOH (Horvath Citation2006) which may cause the higher solubility in 1% NaOH solution. The cold water solubility of Cedrus libani and Cedrus penhallowii was 0.8% and 3.3%, respectively (Horvath Citation2006). Again, the water solubility of Pinusbrutia, Pinusnigra, Fagus orientalis, and Fagussy lvatica was 1.5%, 4.2%, 2.3%, and 3.4%, respectively (Horvath Citation2006). The findings of water solubility for seven wood species were in the range of previous studies. According to the results, the polysaccharides of A. procera and T. arjuna are easier to degrade, which indicates that these can consume less chemical during bioconversion, i.e. pulping. This may provide the idea for selecting the parameters, i.e. chemical charge, time, and temperature, during the conversion process.
3.4. Extractive
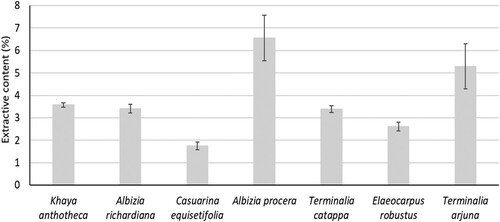
The benzene-ethanol extractive content of seven wood species is shown in and it was in the range of 1.7% to 6.6%. The highest extractive content (6.6%) was found for A. procera, and it was the lowest (1.7%) for C. equisetifolia. The extractive content of Eucalyptus spp. (Pereira et al. Citation2013) and Populus spp (Kacik et al. Citation2012) was 3.1% to 5.0% and 1.7% to 3.8%, respectively. On the other hand ethanol-toluene, dichloromethane, and methanol-water extractive of A. mangium were 4.5%, 1.3%, and 4.1%, respectively (Pinto et al. Citation2005). The extractive content of seven wood species of this study was in the range of other wood species except for A. procera and T. arjuna.
4. Potential applications
Cellulose and lignin are the most valuable resources for developing value-added products. Extraction of extractive can contribute to employ in advanced applications, i.e. biomedical and biochemical. The significant content of cellulose and lignin of seven species compared to other wood species can appeal to consider them as an alternative source of cellulose and lignin. These have the potential for application in bioenergy generation, biochemical production, and biomedical applications. However, based on the chemical analysis, A. richardiana and C. equisetifolia can be targeted for their polysaccharides content while K. anthotheca, T. catappa, E. serratus, and T. arjuna can be focused on for their lignin content. In addition, the extractive content of A. Procera and T. arjuna can be considered for possible applications in biochemical extraction and biomedical.
5. Conclusions and recommendations
The chemical composition of seven wood species was investigated in this study. Cellulose and lignin content was more or less close to other wood species used for commercial purposes. K. anthotheca, T. catappa, E. robustus, and T. arjuna had high lignin contents while A. richardiana and C. equisetifolia showed high cellulose content. The high extractive content was observed in A. Procera and T. arjuna. The solubility analysis showed that A. procera and T. arjuna can be easier to apply in bioconversion. Therefore, these seven wood species indicate the potential source of raw material for developing value-added products. Further studies are needed to develop a suitable extraction method of cellulose, lignin, and extractive for these new raw materials.
CRediT author statement
Mohammad Jakir Hossain: Conceptualization, Data acquisition, Visualization, Literature review, Writing – Original draft, Writing – Review & Editing, Rupak Kumar Ghosh: Writing – Review & Editing, Atanu Kumar Das: Conceptualization, Literature review, Writing – Original draft, Writing – Review & Editing, Visualization Shambhu Chandra Nath: Visualization, Writing – Review & Editing, Md. Rakibul Islam: Writing – Review & Editing, Shaheen Akhter: Writing – Review & Editing, Md. Saidur Rahman: Writing – Review & Editing.
Ethical approval
Not required.
Acknowledgements
The authors wish to express their gratitude to the Ministry of Environment, Forest and Climate change for their financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Not applicable.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Alam, M. K., Basak, S. R. and Alam, S. (2012) Khaya anthotheca (Welw.) C. DC. (Meliaceae) – An exotic species in Bangladesh. Bangladesh Journal of Plant Taxonomy, 19(1), 95–97.
- Argyropoulos, D. S. (2001) Wood and Cellulosic Chemistry. Second Edition, Revised and Expanded Edited by David N.-S. Hon (Clemson University) and Nubuo Shiraishi (Kyoto University). Marcel Dekker: New York and Basel. 2001. vii + 914 pp. $250.00. ISBN 0-8247-0024-4. Journal of the American Chemical Society, 123(36), 8880–8881. doi:10.1021/ja015237p
- Boerjan, W., Ralph, J. and Baucher, M. (2003) Lignin biosynthesis. Annual Review of Plant Biology, 54, 519–546. doi:10.1146/annurev.arplant.54.031902.134938
- Das, A. K., Nakagawa-izumi, A. and Ohi, H. (2015) Evaluation of pulp quality of three non-wood species as alternative raw materials for paper production. Japan Tappi Journal, 69, 548–554.
- Das, A. K., Nakagawa-izumi, A. and Ohi, H. (2016) Quality evaluation of dissolving pulp fabricated from banana plant stem and its potential for biorefinery. Carbohydrate Polymers, 147, 133–138.
- Göltenboth, F., Langenberger, G. and Widmann, P. (2006) Chapter 15 – beach forests. In F. Göltenboth, K. H. Timotius, P. P. Milan, and J. Margraf (eds.), Ecology of Insular Southeast Asia, 281–295, Amsterdam, Elsevier B.V.
- Hillis, W. E. (1971) Distribution, properties and formation of some wood extractives. Wood Science and Technology, 5(4), 272–289. doi:10.1007/BF00365060
- Horvath, A. L. (2006) Solubility of structurally complicated materials: I. Wood. Journal of Physical and Chemical Reference Data, 35(1), 77–92. doi:10.1063/1.2035708
- Kacik, F., Durkovic, J. and Kacikova, D. (2012) Chemical profiles of wood components of poplar clones for their energy utilization. Energies, 5(12), 5243–5256. doi:10.3390/en5125243
- Kumar, S. and Maulik, S. K. (2013) Chapter 39 – effect of Terminalia arjuna on cardiac hypertrophy. In R. R. Watson and V. R. Preedy (eds.), Bioactive Food as Dietary Interventions for Cardiovascular Disease (San Diego: Bioactive Food as Dietary Interventions for Cardiovascular Disease), pp. 673–680.
- Pereira, B. L. C., Carneiro, A. D. O., Carvalho, A., Colodette, J. L., Oliveira, A. C. and Fontes, M. P. F. (2013) Influence of chemical composition of eucalyptus wood on gravimetric yield and charcoal properties. Bioresources, 8(3), 4574–4592.
- Pinto, P. C., Evtuguin, D. V. and Neto, C. P. (2005) Chemical composition and structural features of the macromolecular components of plantation Acacia mangium wood. Journal of Agricultural and Food Chemistry, 53(20), 7856–7862. doi:10.1021/jf058081b
- Rahman, M. M., Das, A. K., Asaduzzaman, M., Biswas, S. K. and Hannan, M. O. (2013) Physical and mechanical properties of Raj Koroi (Albizia richardiana) plywood. African Journal of Wood Science and Forestry, 2(1), 098–103.
- Routa, J., Brännström, H., Anttila, P., Mäkinen, M. A., Jänis, J. and Asikainen, A. J. (2017) Wood extractives of Finnish pine, spruce and birch@ availability and optimal sources of compounds.
- Royer, M., Houde, R., Viano, Y. and Stevanovic, T. (2012) Non-wood forest products based on extractives – a new opportunity for the Canadian forest industry part 1: Hardwood forest species. Journal of Food Research, 1(3), 8–45.
- Troup, R. S. (1921) The Silviculture of Indian Trees (Oxford, UK: Clarendon Press), 1195.
- Wiedenhoeft, A. (2010) Chapter 03: Structure and function of wood. In Wood Handbook. General Technical Report FPL-GTR-190. Madison, WI: U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: 3-1 – 3-18.
- Zmarzty, S. (2001) Revision of Elaeocarpus (Elaeocarpaceae) Section Elaeocarpus in Southern India and Sri Lanka. Kew Bulletin, 56(2), 405–447.