ABSTRACT
To improve the resistance of wood to biological decay the Maillard reaction between introduced amines and wood cell-wall polymers can be utilised. However, initial studies in wood modification showed almost complete leaching of bicine and tricine from treated wood and the loss of beneficial effects. The objective of this study was to assess whether possible reactions of bicine or tricine with wood could be further enhanced and reaction products stabilised through the addition of glucose and/or citric acid. Thus, Scots pine sapwood specimens were impregnated with tricine or bicine, with or without glucose and citric acid, and then heated to a temperature of 160°C. The dimensional stability, degree of chemical leaching and mechanical properties were assessed. Overall, it was concluded that neither the presence of glucose nor citric acid did appear to enhance the reactivity of tricine or bicine. Anti-swelling efficiency (ASE) of 50% was observed for combined treatments of bicine/tricine and citric acid but the leaching resistance originated mainly from citric acid and glucose, with no indication for the retention of bicine or tricine. The presence of citric acid led to a strongly reduced modulus of rupture.
Introduction
Wood continues to increase in popularity in use, but the majority of commercially available species require additional protection against moisture and biological decay. Two general methods, chemical and thermal modification, can enhance water resistance and biological durability (Sandberg et al. Citation2021).
The Maillard reaction has been considered as a possible combined method for wood modification because it is an aqueous process that requires high temperature, making it relatively straightforward to apply to wood with chemical and thermal modification via commercially available processing techniques. This potential was explored by Peeters et al. (Citation2018), using a combination of glycine, sugar, and maleic acid, at a maximum heating temperature of 120°C, though exhibited excessive chemical leaching after prolonged water exposure. Hauptmann et al. (Citation2015) treated wood with tricine at 103°C, inducing the Maillard reaction and improving tensile strength and hardness despite leaching and low weight percent gain (WPG). Jones et al. (Citation2022) assessed the effects of tricine and bicine, respectively, in combination with thermal modification at 160°C. These treatments resulted in a WPG of about 6–7%, decreasing to 5% with further thermal treatment. Biological durability was improved by the treatment, but to retain the improved properties, the leaching resistance needs to be significantly improved. One method might be the promotion of the Maillard reaction by the addition of a reducing sugar such as glucose or the cross-linkage of reaction products through esterification with citric acid.
The objective of this study was to explore a combined chemical treatment and thermal modification using bicine and tricine with added glucose and citric acid to improve leaching resistance and dimensional stability.
Material and methods
For this study, defect-free and straight-grained Scots pine (Pinus sylvestris L.) sapwood with growth rings parallel to the tangential face were prepared, with specimens of dimension 21 × 20 × 10 mm (radial x tangential x longitudinal) used for determining anti-swelling efficiency (ASE) and specimens 10 × 10 × 200 mm for determining bending strength parallel to the grain, respectively. Analytical-grade (>99%) bicine (C6H13NO4), analytical-grade (>99%) tricine (C6H13NO5) (both from Apollo Scientific Ltd, Cheshire, UK), D-(+)-glucose (C6H12O6) (Merck KGaA, Darmstadt, Germany), and analytical-grade (99.9%) citric acid (C6H8O7) (VWR International AB, Stockholm, Sweden) were used in this study.
Specimens were placed in a container and fully submerged with an aqueous solution of chemicals. The impregnation solutions used contained either a single compound of 3 wt% amino acid (bicine or tricine), 2 wt% glucose, or 15 wt% citric acid or combinations of these compounds. The specimens were pressure-impregnated using the full-cell process which applies 30 min of vacuum, followed by 1 h pressure at 15 bar. Afterwards the specimens were oven-dried to 0% moisture content for 24 h at 70°C and 16 h at 103°C. The specimens were then tightly wrapped in aluminium foil and heat-treated for 4 h at 160°C in an open oven system. Treatments where further explored by varying the chemical loading and increasing the heat treatment temperature to 200°C. An untreated and solely heat-treated groups were added as control groups. WPG after impregnation and after heat treatment were determined.
ASE was determined for all treatment groups in 3 wet-dry cycles according to the method developed by Stamm (Citation1964). Mass and dimensions were measured in each step and mass change and volumetric changes were calculated as in Scharf et al. (Citation2023). 5 replicates were used for each treatment group. Modulus of elasticity (MOE) and modulus of rupture (MOR) were determined in conjunction with a four-point bending test according to the EN 408 standard (CEN Citation2010) on certain treatment groups, with 12 replicates each. The specimens were loaded in a universal testing machine (MTS System Corporation, Eden Prairie, MN, USA) equipped with a 10 kN load cell.
Results and discussion
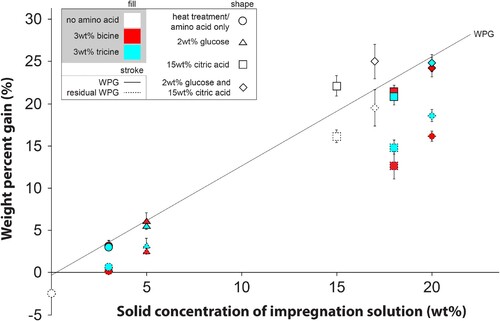
shows the WPG before and after heat treatment. As expected, treatments containing citric acid exhibited higher values, due to added chemical content. The difference of WPG before and after heat treatment, i.e. heat treatment-induced mass loss, was similar between heat treatment only and all treatments without citric acid indicating no additional thermal degradation of wood or chemicals due to the presence of these compounds. The mass loss was larger in treatments with citric acid, which was assumed to be due to the formation of a cyclic anhydride from citric acid accompanied by the release of water. Additionally, slightly greater mass loss was observed when citric acid was used in combination with bicine than with tricine.
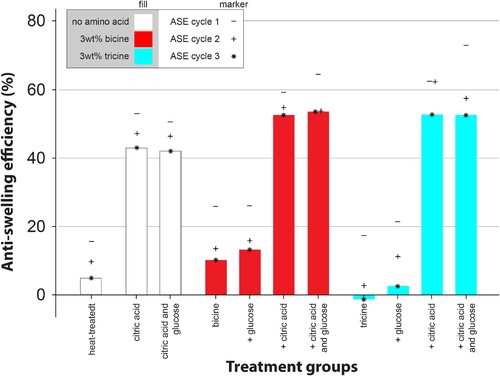
shows the ASE measured over 3 wet-dry cycles. Modification with tricine or bicine with and without glucose did not have any significant positive influence on ASE after one cycle. The addition of citric acid strongly improved the ASE, with the best results obtained at the highest chemical content. While a combination of bicine/tricine with citric acid led to an increase ASE compared to citric acid only, the addition of glucose had no influence.
Figure 2. Anti-swelling efficiency (ASE) over 3 wet-dry cycles. The used chemical concentrations in the solutions are as in .
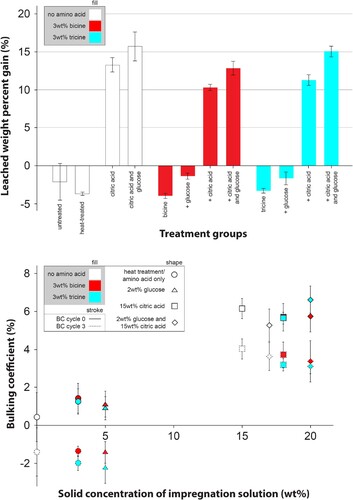
shows the mass and volumetric changes due to leaching. These measurements correlate to the result of the ASE test, where only groups containing citric acid exhibited a positive WPG after leaching due to the formation of cross links. After leaching, the WPG was similar in groups treated with only bicine or tricine as well as in the heat treatment control group, suggesting that the chemicals appear to have been completely removed, as observed in previous works (Hauptmann et al. Citation2015, Jones et al. Citation2022). The addition of glucose increased the WPG indicating stronger leaching resistance, but no positive influence on ASE could be seen. The reduction in bulking coefficient over the treatment ((b)) of bicine and tricine groups was relatively similar indicating that these components remained water-soluble and were leached out. Thus, it is likely that any bulking effects stem from the esterification reaction between citric acid and cell-wall components.
Figure 3. (a) Weight percent gain after leaching over the three wet-dry cycles, and (b) bulking coefficient before (BC cycle 0) and after the ASE procedure (BC cycle 3).
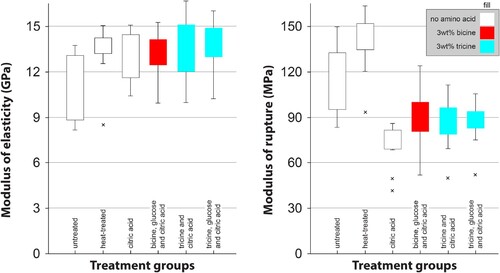
shows the bending properties of chosen groups. The addition of citric acid had a strongly negative effect on the bending strength with slight increases on the MOE. Combining citric acid with bicine, tricine and glucose did not change the MOR, all being lower than untreated samples or those only heat treated.
The combination of 3 wt% tricine, 2 wt% glucose and citric acid was further explored, by varying the citric acid content of the impregnation solution to 10 or 20 wt%. Additionally, the original combination was tested at a treatment temperature of 200°C, a temperature where bicine and tricine may thermally degrade, but conventionally used in thermal modification of wood. Changes in the amount of citric acid had the expected influence on WPGs and bulking coefficients, but the influence on ASE and MOR was less significant compared to an increase of the treatment temperature from 160°C to 200°C. A higher treatment temperature led to a larger heat treatment-induced mass loss indicating stronger degradation of the chemicals and cell wall components during the heat treatment. None of the results pointed to a promotion of the Maillard reaction at these changed treatment conditions.
Conclusion
From this study, it was possible to conclude that the addition of glucose or citric acid to bicine or tricine did not promote a Maillard reaction between wood cell-wall polymers and the amino acids. Anti-swelling efficiency was improved to above 50% by the addition of citric acid to bicine or tricine. However, results showed that leaching resistance originated mainly from citric acid and glucose, with no indication for the retention of bicine or tricine. Modulus of rupture (MOR) decreased with combined thermal and chemical modification compared to thermal modification alone, probably due to citric acid-catalysed degradation of wood components. Changes in the amount of citric acid had a smaller influence on dimensional stability and MOR, than an increase of the treatment temperature from 160°C to 200°C. Studies will continue to assess if there are alternative ways to improve possible reactions of wood with bicine and tricine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- CEN, 2010. EN 408: 2010 + A1: 2012; Timber Structures. Structural timber and glued laminated timber. determination of some physical and mechanical properties. Brussels: European Committee for Standardization.
- Hauptmann, M., et al., 2015. Wood modification with tricine. Holzforschung, 69 (8), 985–991. doi:10.1515/hf-2014-0122
- Jones, D., et al., 2022. Evaluation of the effect of a combined chemical and thermal modification of wood though the use of bicine and tricine. Forests, 13, 834. doi:10.3390/f13060834
- Peeters, K., et al., 2018. An examination of the potential for the use of the Maillard reaction to modify wood. International Wood Products Journal, 9 (3), 108–114. doi:10.1080/20426445.2018.1471840
- Sandberg, D., et al., 2021. Wood modification technologies. Principles, sustainability and the need for innovation. Boca Raton, FL: CRC Press.
- Scharf, A., et al., 2023. Wood modification using imidazole and succinimide: effects on dimensional stability and bending properties. Forests, 14, 1976. doi:10.3390/f14101976
- Stamm, A.J., 1964. Wood and cellulose science. New York, NY: Ronald Press.