Abstract
Eicosanoids are lipid mediators consisting of prostaglandins, leukotrienes, lipoxins and related compounds derived primarily from arachidonic acid and to a lesser extent from eicosapentaenoic and di-homo-gamma-linolenic acids. This large class of bioactive lipids is derived from the initial release of polyunsaturated fatty acid from the sn2 position of glycerophospholipids predominantly from cytosolic phospholipase A2 and subsequent conversion by either prostaglandin H synthases-1 and -2 (PGHS-1, PGHS-2; also known as COX-1, COX-2), lipoxygenases or various members of the cytochrome P450 family. Eicosanoids control a vast array of physiological functions, including female reproductive function and parturition, platelet aggregation and vascular homeostasis, as well as renal function and roles in inflammation initiation and resolution. The authors have been studying the functions and signaling of eicosanoids, in particular the prostaglandin class of molecules, using a series of induced mutant mouse strains created in the laboratory by manipulation of the PGHS-1 and PGHS-2 genes by gene targeting in embryonic stem cells. The two strains of mice most fully characterized to date are referred to as “low-dose aspirin genetic mimic” or PGHS-1 knockdown and “selective COX-2 inhibitor genetic mimic” or PGHS-2 Y385F. This brief review describes the utility of these mouse models for unraveling new insights into eicosanoid signaling.
Introduction
There are three major enzymic pathways of eicosanoid biosynthesis via dietary essential fatty acid-catalyzed conversion by prostaglandin H synthases (PGHS), lipoxygenases (LOX) and cytochromes P450. The PGHS pathways produce prostaglandins (PGs), prostacyclin (PGI2) and thromboxane A2 (TxA2), lipoxygenase pathways give rise to leukotrienes, lipoxins, and 5-, 12- and 15-hydroxy-eicosatetraenoic acids, while the cytochrome P450 pathway metabolites are hydroxylated or epoxy-metabolites of arachidonic acid (AA). These eicosanoids possess a vast array of biological functions in pain, inflammation and many disease settings. The focus of this short review is to illuminate new understanding of the oldest class of eicosanoids, e.g. prostaglandins, by using gene-manipulated mice to alter the expression of PGHS.
Non-steroidal anti-inflammatory drugs (NSAIDs) target PGHS enzymes, which exist in two isoforms, PGHS-1 and PGHS-2. Aspirin and other traditional NSAIDs in the class are non-selective inhibitors of both PGHS-1 and PGHS-2, while newer agents termed “coxibs”, including rofecoxib, valdecoxib and celecoxib, are selective inhibitors of PGHS-2. Both PGHS isozymes are bifunctional with a cyclooxygenase (COX) activity and a functional linked peroxidase (POX) activity. The NSAIDs, including aspirin and coxibs, act via inhibition of the COX activity, while leaving POX activity intact. Aspirin is the most widely used drug in the world, and low-dose aspirin use is effective in the secondary prevention of both heart attack and stroke 1–3Citation1Citation2Citation3 and in the primary prevention of coronary heart disease in at-risk individuals 4–6Citation4Citation5Citation6. The development of coxibs was based on the notion that PGHS-2 was the major source of PGE2 and PGI2 (prostacyclin), which mediates inflammation, and that PGHS-1 was the dominant PG source for gastric cytoprotection Citation7. The authors recently created two novel mouse strains, referred to as “low-dose aspirin genetic mimic” or PGHS-1 knockdown (KD) Citation8 and “selective COX-2 inhibitor genetic mimic” or PGHS-2 Y385F Citation9Citation10 for the study of prostaglandin functions.
Prostaglandin H synthase-1 knockdown mouse model mimics low-dose aspirin treatment
Insertion of a neomycin resistance cassette within intronic sequences has been reported previously to generate a hypomorphic allele or “knockdown” of gene expression to very low levels 11–13Citation11Citation12Citation13. The PGHS-1 KD mice were generated by insertion of this cassette into intron 10 of the PGHS-1 gene through homologous recombination in murine embryonic stem cells. Follow-up characterization of the resultant mice proved that PGE2 production was diminished by 75% compared with wild-type (WT) in unstimulated peritoneal macrophages and PGHS-1 expression was suppressed similarly (70–73%) in the macrophages and other PGHS-1 KD mouse tissues tested as well, which indicates that this model is globally hypomorphic for PGHS-1 expression.
The PGHS-1 isoform, but not PGHS-2, is expressed in mature human platelets, which are the dominant source of TxA2 biosynthesis in humans Citation14. Pharmacological studies suggest that inhibition of the capacity of human platelets to generate TxA2 by more than 95% is necessary to accomplish inhibition of TxA2-dependent platelet aggregation ex vivo Citation15. The plasma thromboxane metabolite, TxB2, was depressed by 97% in PGHS-1 KD mice compared with WT controls, which is remarkably similar to the inhibition achieved by low-dose aspirin treatment in mice (96%) and agrees with the level of inhibition achieved in humans Citation15. Moreover, the platelets from both PGHS-1 KD and low-dose aspirin-treated WT mice (ASA/WT) failed to aggregate in response to various concentrations of AA that were effective in WT platelets (A). The PGHS-1 KD mouse model mimics low-dose aspirin clinical treatment regarding platelet function.
Fig. 1. Prostaglandin H synthase-1 (PGHS-1) knockdown (KD) mice represent a genetic mimic of low-dose aspirin (ASA) treatment in platelet aggregation and thrombosis studies. (A) Representative platelet aggregation tracings for PGHS-1 KD, wild-type (WT) and low-dose aspirin-treated WT mice (ASA/WT) at 3 mM arachidonic acid (AA) concentration. (B) Both PGHS-1 KD and knockout (KO) mice are more resistant to thrombosis elicited by intravenous AA injection. Percentage survival of each group is indicated (*p<0.001, as determined by Fisher's exact test) and number of mice studied is in parentheses. Data are from Yu et al. Citation8.

Protection against thrombosis and impaired inflammatory response in prostaglandin H synthase-1 knockdown mice
Since platelets from PGHS-1 KD mice have defective generation of thromboxane and impaired aggregation responses ex vivo, the finding that PGHS-1 KD mice were resistant to AA-induced thrombosis in vivo (B), while nearly all the normal WT controls died at the tested AA doses Citation8, could perhaps have been anticipated. However, since the time to arterial occlusion by clot formation was markedly prolonged in PGHS-1 KD mice compared with WT mice in a model of oxidative stress-induced arterial photochemical injury Citation8, the implications for thromboxane involvement in thrombosis are far reaching.
The role of PGHS-1-derived prostaglandins and low-dose aspirin in the setting of inflammation remains controversial. Several inflammatory models, including chemically evoked ear swelling assays by AA, capsaicin, phorbol esters and croton oil, and carrageenan-induced paw edema, were tested in PGHS-1 KD mice Citation8. PGHS-1 KD mice displayed a notably reduced inflammatory response to AA, capsaicin and carrageenan, but failed to demonstrate significant inflammatory protection in response to tetradecanoyl phorbol acetate (TPA) and croton oil, suggesting that PGHS-1-derived eicosanoids may be involved in some aspects of the inflammatory response in mice in addition to the well-known actions of PGHS-2-derived eicosanoids.
Normal parturition in prostaglandin H synthase-1 knockdown and low-dose aspirin-treated mice, but not in prostaglandin H synthase-1 null mice
During late gestation, PGHS-1 expression is up-regulated in the uterus then decreases to undetectable levels after delivery Citation16, fitting with its requirement for normal initiation of parturition in mice. PGHS-1-derived PGF2α mediates the initiation of parturition, and exogenous administration of this eicosanoid can rescue the parturition defect observed in PGHS-1 null mice Citation16 and induce a decline in serum progesterone to initiate the birthing process Citation17. Prostaglandin F receptor-deficient mice exhibit parturition failure owing to impaired withdrawal of serum progesterone at term Citation18. Uterus PGHS-1 expression in PGHS-1 KD mice was about 12% of normal at day 17.5 of gestation, and only 8% PGF2α in these mice sufficed to prepare for normal labor onset at day 19 of gestation (Table 1). By contrast, the residual synthetic capacity in PGHS-1 null mice (1.1% PGF2α) was unable to initiate normal parturition at term. Moreover, just before delivery at day 19, the striking serum progesterone fall accompanied by oxytocin induction in myometrium was observed in PGHS-1 KD mice as in WT mice. Similarly to PGHS-1 KD mice, low-dose aspirin treatment to WT mice (ASA/WT) did not affect initiation of full-term labor (Table 1). Use of these novel PGHS-1 KD mice has allowed an efficient titration of the amounts of prostaglandin necessary to achieve an important regulatory physiological process.
Table 1. Normal parturition in PGHS-1 knockdown (KD) and low-dose aspirin-treated mice, but not in PGHS-1 knockout (KO) mice
Prostaglandin H synthase-2 Y385F mice represent a novel genetic mimic of coxib administration
Tyrosine 385 of PGHS is a critical residue for initiation of COX catalysis, and the phenylalanine-385 (Y385F) mutant of PGHS lacks COX activity but retains POX activity Citation7Citation19. [Note that in this review, the tyrosine residue of PGHS-2 is numbered in parallel with ovine PGHS-1, the recognized standard in numbering nomenclature of PGHS isoforms to facilitate comparison of important residues in catalytic function. Accordingly, the actual position in mouse PGHS-2 is Tyr-371 based on numbering the amino-terminal methionine of the signal peptide as residue number 1.] To generate a mouse model that might mimic coxib administration, a Y385F point mutation was inserted into the PGHS-2 gene (known as Ptgs2) through a gene-targeting strategy Citation10. Biochemical studies in vitro confirmed that peritoneal macrophages of PGHS-2 Y385F mice lack COX activity from inducible PGHS-2 protein without disruption of POX activity, which apparently replicates the scenario with selective PGHS-2 inhibitor (coxib) treatment Citation7.
TxA2 is the major PGHS-1 product from platelets, while PGI2 is synthesized dominantly from PGHS-2, which is expressed in sheer-stimulated endothelium Citation20. Coxibs can selectively suppress circulating prostacyclin in humans Citation21. Urinary thromboxane metabolite excretion is not affected by Y385F mutation in PGHS-2 Y385F mice. In contrast, prostacyclin metabolite excretion is dramatically diminished, which is similar to celecoxib-treated mice Citation9. PGHS-2 Y385F mouse appear to represent a genuine genetic model of coxib administration in mice.
Elevated blood pressure and thrombosis susceptibility in prostaglandin H synthase-2 Y385F mice
NSAIDs, including PGHS-2 selective inhibitors, have been associated with hypertension reports or deterioration of blood pressure (BP) control in patients receiving antihypertensive drugs Citation22Citation23, and this hypertension shows a relationship to both selectivity of PGHS-2 inhibition and duration of drug exposure Citation24. Short-term studies of PGHS-2 inhibitors reveal that they evoke transient sodium retention Citation25. As expected, elevated BP, including both diastolic and systolic measures, was observed in PGHS-2 Y385F mutant and PGHS-2 selective inhibitor celecoxib-treated mice on a regular chow diet compared with WT controls (A).
Fig. 2. Both genetic disruption and pharmacological inhibition of COX activity of prostaglandin H synthase-2 (PGHS-2) increase blood pressure and susceptibility to thrombosis. (A) Blood pressure (BP) measurement by tail cuff method. Both diastolic and systolic BP were elevated significantly in PGHS-2 Y385F and celecoxib-treated mice compared with wild-type (WT) mice (*p<0.01, n=10–12). (B) Thrombotic challenge in WT and PGHS-2 Y385F mice. Data represent sudden death percentage after injection of 0.2 mg kg-1 thromboxane analog U46619; the mortality of PGHS-2 Y385F mice is significantly increased compared with WT mice (*p<0.01, n=10–14). TxA2: thromboxane A2.

Prostacyclin is the predominant PG product in endothelium, causing vasodilatation and inhibition of platelet aggregation, and preventing the proliferation of vascular smooth-muscle cells in vitro. In contrast, thromboxane has opposing actions, evoking platelet aggregation, vasoconstriction and vascular proliferation Citation20. Since the balance of these two eicosanoids was disrupted as measured by urinary metabolites, as mentioned above, the cardiovascular effects of thromboxane would be expected to be exaggerated. Consistent with these observations, genetically induced PGHS-2 inhibition in PGHS-2 Y385F mice augments the thrombotic response to the thromboxane agonist U46619 (B), and a highly selective PGHS-2 inhibitor, DFU, increases thrombosis susceptibility in a photochemical vascular injury model Citation9.
Normal neonatal ductus arteriosus remodeling in prostaglandin H synthase-2 Y385F, but not in prostaglandin H synthase-2 null mice
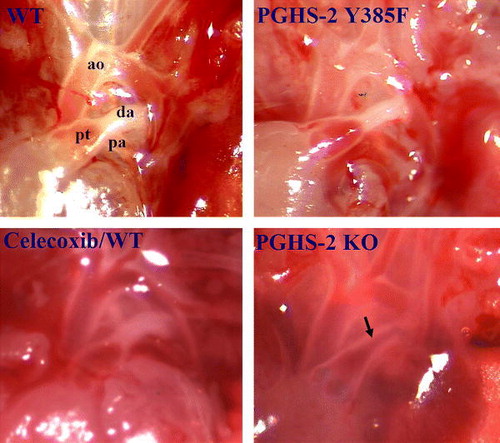
The fetal ductus arteriosus (DA) is an essential conduit for deoxygenated blood to bypass the collapsed lungs to enter via the descending aorta to the placenta. PGs are essential mediators of DA patency during fetal life and can modulate its constriction in the neonatal period Citation26. Mice deficient in PGHS-2 Citation10Citation27 or lacking the PG receptor EP4 Citation28 predispose to failure of DA remodeling at birth. Surprisingly, patent DA and resulting neonatal mortality were not observed in PGHS-2 Y385F mice and celecoxib-treated mice (). Electron microscopic analysis of the DA 4 h after delivery revealed multiple endothelial cell layers oriented toward the lumen, fragmentation of the internal elastic lamina (IEL) and smooth-muscle cell infiltration to the subendothelial region across the fragmented IEL in PGHS-2 Y385F and WT mice, indicating successful remodeling. In contrast, the patent DA in PGHS-2 null mice display a single layer of endothelial cells with an intact IEL. This normal DA remodeling in PGHS-2 Y385F mutant mice at birth might be explained by a novel means of PGHS-1/PGHS-2 heterodimer signaling in the DA at birth Citation10. Thus, molecular modeling and a variety of biochemical tests provided evidence that PGs can be synthesized from an active PGHS-1/mutant PGHS2 complex and that the two PGHS isoforms can associate Citation10.
Fig. 3. Representative morphological examination (15× magnification) of ductus arteriosus in neonatal wild-type (WT), prostaglandin H synthase-2 (PGHS-2) Y385F, PGHS-2 knockout (KO) and celecoxib-treated mice. Celecoxib/WT: WT pregnant females fed a diet containing 800 ppm celecoxib at gestation day 7. ao: aorta; pt: pulmonary track; pa: pulmonary artery; da: ductus arteriosus; arrow: patent ductus arteriosus. Data are from Yu et al. Citation10.
Summary
Both PGHS-1 KD and PGHS-2 Y385F mutant mice have provided new insights into eicosanoid signaling and will serve as important reagents to elucidate additional distinct roles of the PGHS enzymes in normal physiology and in various pathological states. Platelet activation is a hallmark of severe preeclampsia, and therapeutic intervention with low-dose aspirin in the prevention and treatment of this disease remains controversial. One concern has been that a potential benefit might be confounded by an impact of PGHS-1 inhibition on parturition. This research has shown that inhibition of platelet activation in vivo can be attained while retaining reproductive integrity in PGHS-1 KD mice, indicating the feasibility of using low-dose aspirin or selective inhibitors of PGHS-1 to prevent platelet activation in pregnant females without affecting their capacity for a normal delivery process.
The novel genetic model of selective inhibition of PGHS-2 should allow assignment of roles for COX activity of PGHS-2 in the pathogenesis of diseases in a way that does not rely on pharmacological dosing with coxibs. Comparison of PGHS-2 Y385F mutant mice with PGHS-2 null mice suggests that PG formation by PGHS-1/PGHS-2 heterodimers could explain closure of the ductus arteriosus at birth Citation10. Formation of heterodimers may explain differential tissue sensitivity to NSAIDs and perhaps can offer a novel approach to therapeutic manipulation of prostaglandin formation. Manipulation of essential fatty acid supply to the PGHS-1 KD and PGHS-2 Y385F mouse models should help to reveal new insights into eicosanoid function and signaling.
| Acronyms | ||
| AA | = | arachidonic acid |
| ASA | = | aspirin |
| BP | = | blood pressure |
| COX | = | cyclooxygenase |
| DA | = | ductus arteriosus |
| IEL | = | internal elastic lamina |
| KD | = | knockdown |
| KO | = | knockout |
| LOX | = | lipoxygenase |
| NSAID | = | non-steroidal anti-inflammatory drug |
| PG | = | prostaglandin |
| PGHS | = | prostaglandin H synthase |
| PGI2 | = | prostacyclin |
| POX | = | peroxidase |
| TPA | = | tetradecanoyl phorbol acetate |
| TxA2 | = | thromboxane A2 |
| WT | = | wild-type. |
These studies were supported by grants from the NIH (GM63130), Canadian Institutes of Health Research (MOP-79459) and Heart and Stroke Foundation of Ontario (NA-5828). CD Funk is the holder of a Tier I Canada Research Chair in Molecular, Cellular and Physiological Medicine and a Career Investigator of the Heart and Stroke Foundation of Canada.
References
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17, 187 cases of suspected acute myocardial infarction ISIS-2. Lancet 1988; ii: 349–60.
- DienerHCCunhaLForbesCSiveniusJSmetsPLowenthalA. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of strokeJ Neurol Sci1996143113
- CAST (Chinese Acute Stroke Trial). Collaborative Group, CAST randomised placebo-controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke. Lancet 1997; 349: 1641–9.
- SanmuganathanPSGhahramaniPJacksonPRWallisEJRamsayLE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trialsHeart20018526571
- PatronoCCollerBDalenJEFitzGeraldGAFusterVGentM Platelet-active drugs: the relationships among dose, effectiveness, and side effectsChest20011193963S
- US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med 2002;136:157–60.
- SmithWLDeWittDLGaravitoRM. Cyclooxygenases: structural, cellular, and molecular biologyAnnu Rev Biochem20006914582
- YuYChengYFanJChenXSKlein-SzantoAFitzGeraldGA Differential impact of prostaglandin H synthase 1 knockdown on platelets and parturitionJ Clin Invest200511598695
- ChengYWangMYuYLawsonJFunkCDFitzGeraldGA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular functionJ Clin Invest200611613919
- Yu Y, Fan J, Chen XS, Wang D, Klein-Szanto AJ, Campbell RL, . Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat Med 2006;12699–704.
- CarmelietPFerreiraVBreierGPollefeytSKieckensLGertsensteinM Abnormal blood vessel development and lethality in embryos lacking a single VEGF alleleNature19963804359
- MeyersENLewandoskiMMartinGR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombinationNat Genet19981813641
- NagyAMoensCIvanyiEPawlingJGertsensteinMHadjantonakisAK Dissecting the role of N-myc in development using a single targeting vector to generate a series of allelesCurr Biol199886614
- CatellaFFitzGeraldGA. Paired analysis of urinary thromboxane B2 metabolites in humansThromb Res19874764756
- ReillyIAFitzGeraldGA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugsBlood1987691806
- GrossGAImamuraTLuedkeCVogtSKOlsonLMNelsonDM Opposing actions of prostaglandins and oxytocin determine the onset of murine laborProc Natl Acad Sci U S A199895118759
- HortonEWPoyserNL. Uterine luteolytic hormone: a physiological role for prostaglandin F2αPhysiol Rev197656595651
- SugimotoYYamasakiASegiETsuboiKAzeYNishimuraT Failure of parturition in mice lacking the prostaglandin F receptorScience19972776813
- ShimokawaTKulmaczRJDeWittDLSmithWL. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysisJ Biol Chem1990265200736
- GrosserTFriesSFitzGeraldGA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunitiesJ Clin Invest2006116415
- McAdamBFCatella-LawsonFMardiniIAKapoorSLawsonJAFitzGeraldGA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2Proc Natl Acad Sci U S A1999962727
- ArmstrongEPMaloneDC. The impact of nonsteroidal anti-inflammatory drugs on blood pressure, with an emphasis on newer agentsClin Ther200325118
- CurhanGCWillettWCRosnerBStampferMJ. Frequency of analgesic use and risk of hypertension in younger womenArch Intern Med200216222048
- FrishmanWH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edemaAm J Cardiol2002891825D
- Catella-LawsonFMcAdamBMorrisonBWKapoorSKujubuDAntesLLasseterKC Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoidsJ Pharmacol Exp Ther199928973541
- SmithGC. The pharmacology of the ductus arteriosusPharmacol Rev1998503558
- LoftinCDTrivediDBTianoHFClarkJALeeCAEpsteinJA Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2Proc Natl Acad Sci U S A200198105964
- NguyenMCamenischTSnouwaertJNHicksECoffmanTMAndersonPA The prostaglandin receptor EP4 triggers remodeling of the cardiovascular system at birthNature19973907881