Abstract
A well-integrated inflammatory response and its natural resolution are essential to homeostasis. Hence, it is important to achieve a complete understanding of the molecular events that govern termination of acute inflammation. Recent studies uncovered endogenous pathways in inflammatory exudates taken from the resolution phase that actively generate new families of locally acting mediators from the essential fatty acids eicosapentaenoic acid and docosahexaenoic acid. These new chemical families were coined resolvins and protectins because in animals specific members of these families control the duration and magnitude of inflammation. Hence, mapping of these resolution circuits, mediators and their target signaling pathways of these potent agonists of resolution has provided new avenues for appreciating the molecular basis of many inflammatory diseases. This overview covers recent advances on the biosynthesis and actions of these novel anti-inflammatory lipid mediators, with a focus on the stereochemical basis of the potent actions of resolvin E1 and protectin D1. These previously unappreciated families of lipid-derived mediators were originally isolated from experimental murine models of acute inflammation captured during natural self-limited resolution. Since they have proven anti-inflammatory, proresolving, and protective properties in experimental models of disease, it follows that potential defective resolution mechanism(s) may underlie the current appreciation of inflammatory disease phenotype(s).
Introduction
The essential roles of omega-3 (n-3) polyunsaturated acids (PUFAs) in health were evident as early as 1929 Citation1. Although studied until the present, the mechanism of action of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the major n-3 PUFAs in human systems, remains a topic of debate 2–5Citation2Citation3Citation4Citation5. Inflammation has emerged in recent years as playing a key role in many prevalent diseases not previously known to involve inflammation. These include Alzheimer's disease and cardiovascular disease Citation6, as well as cancer Citation7, which now join the well-known inflammatory disorders such as arthritis and periodontal disease 8–10Citation8Citation9Citation10. The molecular mechanism(s) underlying the many reports of the beneficial actions of n-3 PUFAs remains an important challenge for molecular medicine. To this end, novel oxygenated products were identified, generated by enzymic pathways initiated from the precursors EPA and DHA. These new compounds are biosynthesized and possess potent actions in the resolution of inflammatory exudates 11–13Citation11Citation12Citation13 and are also neuroprotective Citation14Citation15. The term resolvins (resolution phase interaction products) was first introduced to signify that the new structures were endogenous mediators possessing very potent anti-inflammatory and immunoregulatory actions Citation12, which include reducing neutrophil traffic, cytokine and reactive oxygen species regulation, as well as lowering the magnitude of the inflammatory response in vivo Citation11Citation12. The terms protectin and neuroprotectin, when generated in the neural tissues Citation16, were introduced given the anti-inflammatory Citation13 as well as the protective actions of the 10,17-docosatriene in neural systems Citation15, stroke Citation14 and animal models of Alzheimer's disease Citation10.
In contrast to the earlier oxygenated products characterized as being formed from n-3 PUFAs that are similar in structure to known arachidonic acid (AA)-derived eicosanoids, but less potent or devoid of bioactions Citation17Citation18, specific members of the resolvin and protectin families evoke potent biological actions demonstrable in the nanomolar and picomolar ranges in vitro and in vivo 10–15Citation10Citation11Citation12Citation13Citation14Citation15 (Tables 1 and 2).
Table 1. Actions of E and D series resolvins
Table 2. Protectins: formation and actions of protectin D1/neuroprotectin D1
Chemical mediators in inflammation and resolution
Early studies by Bergström, Samuelsson and colleagues established that AA is transformed into many potent bioactive compounds such as prostaglandins, leukotrienes and lipoxins Citation27Citation28. The departure of fatty acids from simply playing structural roles in cell membranes or as energy stores came with the recognition that AA is transformed by both cyclooxygenase and lipoxygenase mechanisms to potent eicosanoid mediators, which led to a Nobel prize in 1982 Citation27Citation29Citation30. Most of the classic prostaglandins and leukotriene mediators are proinflammatory and also play specific roles in the reproductive system. It was assumed that these same mediators were formed and served in the initiation and termination of acute inflammation () as well as in the turn from acute to chronic inflammation Citation33. However, in sharp contrast, it has become clear that counter-regulatory substances, such as lipoxins, are generated during the resolution of acute inflammation to serve in healthy termination of an acute response Citation34Citation35. It is important to note that these chemical mediators serve as agonists for endogenous anti-inflammatory and proresolving mechanisms. Hence, the first evidence that the resolution of inflammation, which was once thought to be a passive process Citation36, is actually an active biochemical process that turns on specific proresolution biochemical signaling circuits came with the identification of specialized chemical mediators biosynthesized during the resolution phase such as the resolvins that actively promote resolution Citation34Citation37Citation38 and the return to homeostasis Citation37 (see below).
Fig. 1. Progression and outcomes of acute inflammation: roles of chemical mediators. A temporal illustration of the fate of acute inflammation Citation31. The chemical mediators involved in the initiation of acute inflammation such as prostaglandins and leukotrienes are assumed to be the same as those involved in the termination or resolution of inflammation. Specialized chemical mediators appear to be programmed in a tissue-level response to participate actively in leukocyte responses required for resolution. (See text and ref. 32.)
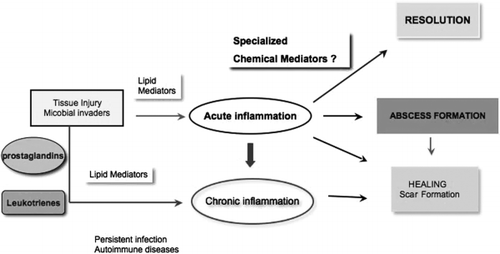
In general, this is a departure from the well-appreciated proinflammatory roles of lipid mediators Citation28. The recognition that resolution is an active process and that activation of the lipoxin biosynthetic circuit and lipoxins themselves, as well as aspirin-triggered lipoxins and their stable analogs, are potent agonists of anti-inflammation in vivo and in many disease models Citation37, has now focused our attention on understanding the biochemical events activated during healthy resolution of acute inflammatory responses and ischemia reperfusion injury.
A compelling connection for n-3 polyunsaturated fatty acids
The GISSI results showed, from a randomized study of over 11 000 patients with cardiovascular disease, a reduction in sudden death of around 45% in those taking almost 1 g of n-3 per day Citation39Citation40. In view of these findings, the present author questioned the mechanism of this n-3 action in humans with vascular disease. Inspection of the GISSI protocol indicated that patients in each arm of the study groups were taking aspirin daily. However, the contributions of ongoing aspirin therapy to the beneficial outcomes with the group taking n-3 were not taken into account Citation39. In addition, an abundant literature with n-3 PUFAs given at doses of milligrams to grams per day pointed to beneficial actions in many diseases including inflammatory diseases as well as cancer Citation2Citation41. Each of the three major human lipoxygenases (5-LO, 12-LO, 15-LO) can convert n-3 PUFAs to various monohydroxy-containing products. The biological importance of these products, if any, was not known Citation18Citation42Citation43. DHA can also be non-enzymically oxygenated to isoprostane-like compounds termed neuroprostanes that reflect oxidative stress in the brain Citation44 or can undergo autooxidization to products that are monohydroxy racemates Citation45. Thus, despite the many decades of research with n-3 PUFAs, the cellular and molecular basis underlying their regulatory and immunoprotective actions remained for the most part unknown.
Aspirin and specialized chemical mediators in resolution
Aspirin is in many over-the-counter remedies, making it difficult to control for in many human studies. Moreover, although it is clear that aspirin inhibits prostaglandin formation and hence a key mechanism in anti-inflammatory therapy Citation29, and that aspirin has well-appreciated clinical uses and an ability to limit leukocyte traffic into sites of inflammation, the key player in propagating inflammation remained unknown. To address this, studies by this group first used murine dorsal skin pouches Citation11Citation12 known to resolve spontaneously in rats Citation38Citation46. This system was adapted for study in mice, in order to assess genetic contributions to resolution and develop mediator informatics and lipidomics using liquid chromatography (LC)–ultraviolet (UV)–tandem mass spectrometry (MS-MS)-based analyses of inflammatory exudates. The author's group also constructed lipid mediator libraries with physical properties, such as MS and MS/MS spectra, elution times and UV spectra for matching studies, as well as to assess whether known and/or potential novel lipid mediators were present within the exudates Citation47Citation48. These databases and informatics were geared to evaluate whether novel lipid mediators are indeed generated during the resolution phase of inflammation Citation11Citation12.
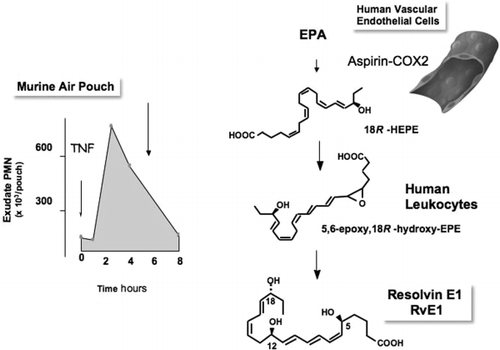
In this experimental model of a contained inflammation (), after 4 h, polymorphonuclear neutrophil (PMN) numbers drop within the exudates Citation11Citation12, namely, the cellular definition of resolution Citation31. Exudates were taken in these intervals, focusing on the period of “spontaneous resolution” Citation46; namely, when neutrophils are lost from the exudate and the tissue sites appear to resolve (, left). In this phase of the response in vivo, lipid mediator profiles were recorded using tandem LC-UV-MS-MS to identify lipid mediators present within the exudates. When novel bioactive mediators were encountered, their structures were elucidated. This was accomplished by carrying out retrograde analysis for both biogenic (i.e. using recombinant enzymes) and total organic synthesis. This approach also permitted assessment of structure–activity relationships as well as the scale-up required to confirm bioactions characterized for the novel compounds identified Citation19Citation47.
Fig. 2. Biosynthesis of resolvin E1. Left: murine air pouch model of contained inflammation and self-limited resolution. The arrow denotes decreasing neutrophil numbers at the dorsal inflammatory site and the points when resolving pathways and products were obtained for mass spectral and bioassay analysis. Right: human endothelial cells expressing cyclooxygenase-2 (COX-2) treated with aspirin transform eicosapentaenoic acid (EPA) by abstracting hydrogen at carbon 16 to give R insertion of molecular oxygen to yield 18R-hydro(peroxy)eicosapentaenoic acid [18R-H(p)EPE]. They are further converted via sequential actions of leukocyte 5-lipoxygenase and lead to the formation of resolvin E1. TNF: tumor necrosis factor; PMN: polymorphonuclear neutrophil. (See text and refs 11, 12 and 19.)
Resolvins: 18R E series and 17R D series
Resolving exudates contained 18R-hydroeicosapentaenoic acid (18R-HEPE) (, right) as well as several other related bioactive compounds Citation11. These novel compounds were produced from EPA. The first bioactive compound was isolated from exudates and found to reduce inflammation in vivo as well as block human neutrophil transendothelial migration. Structural elucidation was carried out using both gas chromatography-MS and LC-MS-MS analysis of bioactive fractions obtained following extraction and reverse-phase high-performance liquid chromatography. The basic structure of the potent bioactive product generated in exudates from EPA proved to be 5,12,18R-trihydroxyeicosapentaenoic acid Citation11. Isolated cyclooxygenase-2 (COX-2) treated with aspirin generates 18R-HEPE as well as 15R-HEPE, which are blocked by selective COX-2 inhibitors. This 15R-HEPE is a precursor to the 15-epi-lipoxin A5 series from EPA that shares the potent anti-inflammatory actions of lipoxins of the 4 series. The 5 series EPA-derived lipoxins were identified earlier as major endogenous mediators in fish Citation49. Surprisingly, acetaminophen and indomethacin, at clinically used doses, permitted oxygenation of EPA to both 18R-HEPE and 15R-HEPE with isolated COX-2, although the levels of both were significantly reduced. These results indicate that the oxygenation of n-3 PUFAs to generate novel bioactive mediators can also involve certain widely used anti-inflammatory drugs, but not selective COX-2 inhibitors Citation11.
Next, the most likely human pathway that was a source of these bioactive mediators within the inflammatory exudates was reconstructed in vitro and found to involve cell–cell interactions that could potentially contribute to the generation of these bioactive mediators in vivo within the exudates. As determined with isolated human cells, vascular endothelial cells treated with aspirin convert EPA to 18R-HEPE, which is released and then rapidly converted by activated human PMN to a 5Citation6 epoxide-containing intermediate that is converted to the bioactive 5,12,18R-trihydroxy-EPE. This bioactive compound was termed resolvin E1 (RvE1), because it proved to be a potent regulator of PMN transmigration and inflammation in vivo. RvE1 possesses an interesting and novel distinct structure consisting of a conjugated triene plus conjugated diene chromophore present within the same molecule. Both biogenic Citation11 and total organic synthesis was achieved and its complete stereochemical assignment was recently established Citation19. RvE1 proved to be 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid. This compound displayed potent stereoselective actions in vivo and with isolated cells (Table 1 and references therein). Therefore, evidence was sought for the ligand-specific receptors for RvE1 ligand that were identified using a signal screening approach and a synthetic radiolabeled RvE1. Other geometric isomers related to RvE1 were prepared and proved to be less potent than RvE1 and did not share the physical properties of endogenous RvE1 (see ref. 19, along with the supplemental material).
The resolving exudates from mice given aspirin plus DHA also contained novel 17R-hydroxydocosahexaenoic acid (17R-HDHA) and two separate novel families of bioactive compounds (). Here, too, the biosynthetic pathways were reconstructed in vitro to establish a potential origin for these novel compounds found in vivo. Along these lines, hypoxic human microvascular endothelial cells treated with aspirin released 17R-HDHA and DHA, which are converted by isolated human recombinant COX-2 to 13-hydroxy-DHA. With aspirin, this switches to 17R-oxygenation with molecular oxygen to give an epimeric aspirin-triggered form at sites of exudate formation () and also in the brain Citation12Citation13 of both families, resolvins and protectins, which carry a 17R alcohol group configuration instead of 17S when biosynthesized via lipoxygenase mechanisms (see and below).
Fig. 3. Overview of resolvins and protectins: biosynthesis from docosahexaenoic acid (DHA). DHA is the precursor to and is enzymically transformed to two families of chemically distinct mediators that demonstrate potent actions in vitro and in vivo. These include resolvins of the D series and the protectins. The distinguishing features of these two families of chemical structures are as follows. The protectins carry three of their six double bonds in a conjugated triene system and thus display a distinct conjugated triene chromophore. The resolvins of the D series are subject to two sequential lipoxygenation steps and carry four of their six double bonds in a conjugated tetraene structure, and exhibit a characteristic tetraene chromophore. Resolvins and protectins each carry a 17-position alcohol group that is inserted via lipoxygenase in the S configuration. Both families are also generated in an aspirin-dependent fashion that inserts oxygen at the 17-position in the R configuration, demonstrated with recombinant acetylated cyclooxygenase-2. Hence, the aspirin-triggered epimeric forms of the protectins and resolvins are denoted AT-resolvins and AT-protectins. When protectin D1, which is a demonstrated anti-inflammatory and has potent actions in neural systems, is generated within neural systems, it is prefixed with neuro to give its organ location, since the same biochemical pathway and utilization of DHA exist in both immune and neurological systems. LOX: lipoxygenase. (See text and for details.)
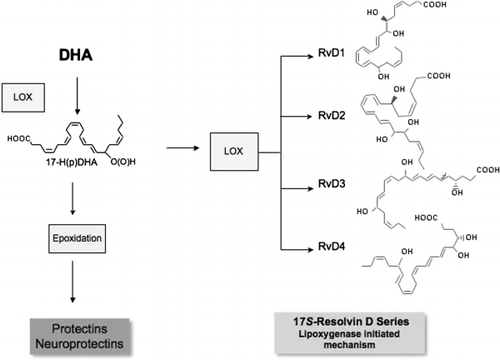
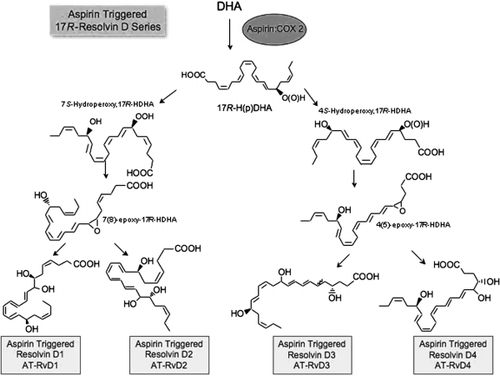
Fig. 4. Conversion of docosahexaenoic acid (DHA) to aspirin-triggered D series resolvins. The 17R series resolvins are produced from DHA in the presence of aspirin. Human endothelial cells expressing cyclooxygenase-2 (COX-2) treated with aspirin transform DHA to 17R-HDHA. Human polymorphonuclear neutrophils can then convert 17R-HDHA to two separate intermediates via 5-lipoxygenation Citation11 that are each rapidly transformed into two epoxide intermediates, 7Citation8-epoxide (left) and another 4Citation5-epoxide-containing intermediate (right). These two novel epoxide intermediates open via enzymic hydration to give the stereochemistry required for the bioactive products denoted 17S series RvD1 to RvD4. RvD1 to RvD4 appear to be functionally redundant positional isomers generated via the actions of specific enzymes. Their complete stereochemical assignments are in progress and are depicted as tentative geometries of their respective double bonds. Aspirin triggers the same compound, but chirality at carbon 17 is in the R configuration from the involvement of COX-2 when acetylated by aspirin.
Resolvins of the 17S D series and protectins
Using this lipid mediator informatics approach, it was found that neither aspirin nor exogenous DHA was required to produce these compounds in vivo. The endogenous DHA was converted in vivo to a 17S alcohol-containing series of resolvins, depicted in and , as well as docosatriene-containing structures via lipoxygenase-initiated mechanisms Citation13Citation14. The complete stereochemistry of protectin D1 (PD1), which carries a 10,17-dihydroxydocosatriene structure Citation12Citation13, was recently established. Total organic synthesis of related isomers and matching studies with biologically derived materials showed that endogenous PD1, called neuroprotectin D1 (NPD1) when produced by neural tissues, was recently established in isolated human cells in vitro and murine cells in vivo as 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid Citation16. The geometry of the double bonds in PD1 and their positions during biosynthesis from the key intermediates in its biosynthesis in situ, namely via an epoxide intermediate at the 16Citation17 position, indicated that PD1 biosynthesis requires enzymic steps to generate the potent bioactive molecule from DHA ().
Fig. 5. Protectin/neuroprotectin D1 biosynthetic pathway. The initial enzymic product 17S-hydro(peroxy)docosahexaenoic acid [17S-H(p)DHA] is converted to a 16(17)-epoxide containing intermediate by leukocyte and microglial cells and enzymically converted to the 10,17-dihydroxytriene-containing bioactive product Citation13 denoted earlier as 10,17S-docosatriene Citation14 and recently coined neuroprotectin D1 based on its potent actions in vivo Citation14Citation15. (See text and Table 2 for further details.) The complete stereochemistries of the bioactive mediators and related isomers were established and are shown as indicated Citation16. DHA: docosahexaenoic acid.
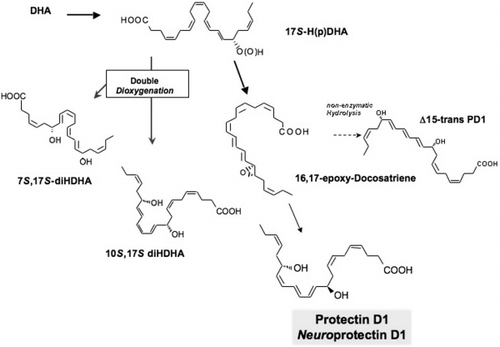
PD1 proved to be log orders of magnitude more potent than its precursor DHA, indicating that it is a chemical autacoid Citation13. The formation of potent bioactive mediators from DHA was proposed as early as 1984 by Bazan Citation50. In collaboration with Bazan and colleagues Citation10Citation14Citation15, the present author documented the formation of NPD1 and identified actions that suggest that it is an important mediator in neural systems (Table 2). Identification of the resolvins and protectins generated from DHA now opens exploration into the essential roles of these pathways and mediators in human systems. The structures and pathways of the D-series resolvins are shown in Figs. 3–5. Their basic structures and actions have been determined (see below) (Table 2). The complete stereochemical assignments of resolvins D1 to D4 are in progress and will be reported elsewhere.
Resolvins and protectins in disease models: formation and actions
Resolvins of the E series comprise several molecules. Among them, RvE1 was the first to be isolated and studied in depth. At nanomolar levels, RvE1 dramatically reduced neutrophil transendothelial migration, dermal inflammation Citation12Citation19Citation51, peritonitis, dendritic cell migration and interleukin-12 production (Table 1). Administration of synthetic RvE1 blocks PMN infiltration in periodontal disease in a rabbit model Citation22 and protects against the development of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis Citation21. Therefore, it was of interest to determine the receptors involved in its bioactions. To this end, screening a library of G-protein-coupled receptors (GPCRs) Citation19, the orphan receptor ChemR23 was found to bind specifically to 3H-labeled RvE1, thereby attenuating nuclear factor-κB signaling. Using a structure–function approach, a second high-affinity GPCR was identified, which signals in response to RvE1 Citation19. The main second messenger for RvE1 agonist actions via these GPCRs appears to be activation of intracellular phosphorylation pathways, rather than mobilization of intracellular calcium or cyclic adenosine monophosphate (cAMP) as with most proinflammatory mediators Citation19Citation21. Thus, in several animal models of inflammatory diseases, RvE1 displays potent counter-regulatory actions that protect against leukocyte-mediated tissue injury and excessive proinflammatory gene expression Citation19Citation21Citation22.
Resolvins of the D series block tumor necrosis factor-α-induced interleukin-1β transcripts in microglial cells and also act as potent regulators limiting PMN infiltration into inflamed brain, skin and peritonitis 12–14Citation12Citation13Citation14. Direct comparisons between the resolvin E versus both of the D series (17S and 17R epimer aspirin-triggered series) at equal doses demonstrated that the new 17S series generated by lipoxygenase-initiated mechanisms and those of the 17R series RvDs that are triggered by aspirin treatment administered intravenously at 100 ng [∼3 µg kg-1] in mice display essentially similar actions, reducing PMN infiltration by approximately 50% in peritonitis, compared with around 75–80% inhibition by RvE1. For the purpose of direct comparison, indomethacin, a widely used anti-inflammatory, at the same doses produced a reduction in leukocyte infiltration of about 25% Citation12Citation13. Thus, both the RvDs (17S series) and aspirin-triggered RvDs (17R series) are potent regulators of PMN infiltration in vivo, and the S to R switch with aspirin treatment in the biosynthesis of the 17 position alcohol in the omega side-chain does not diminish their activity. This implicates that the aspirin-triggered 17R epimers of protectins and resolvins may each serve as the body's own anti-inflammatory mediators in response to aspirin.
Protectins
Protectins possess a conjugated triene-containing structure as the key feature of this new family of compounds derived from DHA. The 10,17S-docosatriene formed in the NPD1 pathway ( and ) proved to be a potent regulator of PMN influx in exudates at the site where it is formed from endogenous precursors Citation12Citation13, and limits stroke brain injury Citation14 and retinal cells Citation15. Other dihydroxy-docosanoids (both positional and geometric isomers) were substantially less bioactive Citation13Citation15Citation16. PD1, also referred to as NPD1 when generated in the nervous system, possesses potent actions in vitro and in vivo (Table 2). The complete stereochemistry of PD1, namely chirality of the alcohol groups and geometry of the conjugated triene, was recently established in human cells as 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid Citation16.
Synthetic PD1 at 10 nM attenuates human neutrophil transmigration by about 50% in vitro, whereas its Δ15-trans-isomer is essentially inactive. PD1 is also a potent regulator of PMN in vivo by reducing PMN infiltration (∼40% at 1 ng per mouse) in murine peritonitis. PD1 also reduced PMN infiltration when administered after the initiation of inflammation in vivo, as well as acting in an additive fashion with RvE1 to stop PMN infiltration. These results indicate that PD1 is a potent, stereoselective anti-inflammatory molecule Citation12Citation13Citation16. Moreover, these results and those obtained together with Bazan and colleagues in neural tissues demonstrate that PD1 displays potent immunoregulatory Citation12Citation13Citation16 and neuroprotective actions Citation10Citation14Citation15 as well as promoting wound healing capacity Citation25. PD1/NPD1 limits stroke brain injury Citation14 and retinal pigmented cellular damage and promotes brain cell survival, suppressing Aβ42-induced neurotoxicity Citation15 (Table 2). The protection upon oxidative stress with NPD1 involves up-regulation of the antiapoptotic Bcl-2 and Bcl-x(L) in response to NPD1 treatment, whereas the proapoptotic Bax and Bad are down-regulated, resulting in reduced caspase-3 cleavage Citation10. Taken together, these are the first results indicating that novel lipid mediators are generated and present in resolving exudates derived from n-3 fatty acids. This work also established that these novel compounds derived from EPA and DHA carry anti-inflammatory properties and that they are log orders more potent than their precursors in vivo.
Conclusion
The new families of EPA- and DHA-derived chemical mediators reviewed here, namely the resolvins and protectins, now open new avenues to design resolution-targeted therapies to control unwanted side-effects of aberrant inflammation. These n-3-derived lipid mediators act as agonists at different steps in the progression of inflammation that appear to be temporal and spatially distinct within the resolution phase Citation32. It follows that endogenous lipid mediators play key roles in controlling and programming resolution of inflammation as an active bioprocess Citation11. Identification of anti-inflammatory bioactions of the lipoxins and of aspirin-triggered lipoxin dispel the dogma that all AA-derived mediators are solely proinflammatory Citation35. These findings lead to a deeper appreciation of how acute inflammation and its natural resolution are regulated.
Systematic studies focusing on mechanisms of resolution and specialized chemical mediators generated within resolution Citation11Citation12 have uncovered n-3 PUFAs as endogenous precursors of resolvins and protectins. The small molecule members of these families have unique structures and independent routes of biosynthesis, yet share anti-inflammatory and proresolving properties in vivo. Their aspirin-triggered epimeric forms of both families of compounds, namely the aspirin-triggered resolvins and protectin family epimers, are endogenously generated with aspirin treatment in murine models in vivo Citation12Citation13. These aspirin forms share the characteristic features dampening inflammation and PMN-mediated injury from within, key culprits in many widespread human diseases. These findings suggest that aspirin is unique among anti-inflammatory drugs because it jump-starts resolution by generating epimers of resolution mediators. In addition, the studies summarized here underscore the role(s) of PUFA-derived local mediators in resolution and tissue catabasis or the return to homeostasis Citation20Citation32. These actions provide evidence for endogenous anti-inflammatory and proresolving mechanism(s) that may account in part for some of the beneficial actions of dietary n-3 PUFAs. Given their potent bioactivity and specific actions within resolution, the lipoxins Citation35, resolvins and protectins (Tables 1 and 2) and their mimetics now qualify as agonists for resolution in this new arena of resolution-modulation and tissue protection.
| Acronyms | ||
| AA | = | arachidonic acid |
| COX | = | cyclooxygenase |
| DHA | = | docosahexaenoic acid |
| EPA | = | eicosapentaenoic acid |
| GPCR | = | G-protein-coupled receptors |
| HDHA | = | hydroxydocosahexaenoic acid |
| HEPE | = | hydroxyeicosapentaenoic acid |
| LC | = | liquid chromatography |
| LO | = | lipoxygenase |
| MS-MS | = | tandem mass spectrometry |
| NPD1 | = | neuroprotectin D1 |
| PD1 | = | protectin D1 |
| PMN | = | polymorphonuclear neutrophil |
| PUFA | = | polyunsaturated fatty acid |
| RvE1 | = | resolvin E1 |
| UV | = | ultraviolet. |
Studies in the author's laboratory were supported in part by National Institutes of Health grants GM38675, DK074448 and P-50 DE-016191. The author also thanks Stacey Lindberg and Mary Halm Small for assistance in preparing this manuscript.
References
- BurrGOBurrMM A new deficiency disease produced by the rigid exclusion of fat from the dietJ Biol Chem19298234567
- Lands WEM, ed. Proceedings of the AOCS Short Course on Polyunsaturated Fatty Acids and Eicosanoids. Champaign IL: American Oil Chemists’ Society; 1987.
- BazanNGSupply of n-3 polyunsaturated fatty acids and their significance in the central nervous systemNutrition and the brainWurtmanRJWurtmanJJRaven PressNew York1990122
- SimopoulosAPLeafASalemNJr Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acidsJ Am Coll Nutr1999184879
- SalemNJrLitmanBKimH-YGawrischK Mechanisms of action of docosahexaenoic acid in the nervous systemLipids20013694559
- HanssonGRobertsonAKLSoderberg-NauclerC Inflammation and atherosclerosisAnnu Rev Pathol Mech Dis20061297329
- ErlingerTPPlatzEARifaiNHelzlsouerKJ C-reactive protein and the risk of incident colorectal cancerJAMA200429158590
- NathanC Points of control in inflammationNature200242084652
- Van DykeTESerhanCN Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseasesJ Dent Res2003828290
- LukiwWJCuiJGMarcheselliVLBodkerMBotkjaerAGotlingerK A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer diseaseJ Clin Invest2005115277483
- SerhanCNClishCBBrannonJColganSPChiangNGronertK Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processingJ Exp Med20001921197204
- SerhanCNHongSGronertKColganSPDevchandPRMirickG Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signalsJ Exp Med2002196102537
- HongSGronertKDevchandPMoussignacR-LSerhanCN Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammationJ Biol Chem20032781467787
- MarcheselliVLHongSLukiwWJHua TianXGronertKMustoA Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expressionJ Biol Chem20032784380717
- MukherjeePKMarcheselliVLSerhanCNBazanNG Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stressProc Natl Acad Sci U S A200410184916
- SerhanCNGotlingerKHongSLuYSiegelmanJBaerT Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienesJ Immunol2006176184859
- CoreyEJShihCCashmanJR Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesisProc Natl Acad Sci U S A19838035814
- LeeTHMencia-HuertaJ-MShihCCoreyEJLewisRAAustenKF Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophilsJ Clin Invest198474192233
- AritaMBianchiniFAlibertiJSherAChiangNHongS Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1J Exp Med200520171322
- BannenbergGLChiangNArielAAritaMTjonahenEGotlingerKH Molecular circuits of resolution: formation and actions of resolvins and protectinsJ Immunol2005174434555
- AritaMYoshidaMHongSTjonahenEGlickmanJNPetasisNA Resolvin E1, a novel endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against TNBS-induced colitisProc Natl Acad Sci U S A200510276716
- HasturkHKantarciAOhiraTAritaMEbrahimiNChiangN RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitisFASEB J2006204013
- DuffieldJSHongSVaidyaVLuYFredmanGSerhanCNBonventreJV. Resolvin D series and protectin D1 mitigate acute kidney injuryJ Immunol2006117590211
- ArielALiP-LWangWTangW-XFredmanGHongS The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clusteringJ Biol Chem20052804307986
- GronertKMaheshwariNKhanNHassanIRDunnMSchwartzmanML A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defenseJ Biol Chem20052801526778
- González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, . Docosahexanenoic acid (DHA) ameliorates necroinflammatory liver injury by conversion to protective lipid mediators: protectin D1 and 17-hydroxy-DHA. FASEB J 2006;( in press DOI: 10/096/fj06-6250fje(http://www.fasebj.org)).
- Samuelsson B. From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes. In: Les Prix Nobel: Nobel Prizes, presentations, biographies and lectures. Stockholm: Almqvist & Wiksell; 1982. p. 153–74.
- SamuelssonB Leukotrienes: mediators of immediate hypersensitivity reactions and inflammationScience198322056875
- Vane JR. Adventures and excursions in bioassay: the stepping stones to prostacyclin. In: Les Prix Nobel: Nobel Prizes, presentations, biographies and lectures. Stockholm: Almqvist & Wiksell; 1982. p. 181–206.
- Bergström S. The prostaglandins: from the laboratory to the clinic. In: Les Prix Nobel: Nobel Prizes, presentations, biographies and lectures. Stockholm: Almqvist & Wiksell; 1982. p. 129–48.
- Cotran RS, Kumar V, Collins T, eds. Robbins pathologic basis of disease. 6th edn. Philadelphia PA: WB Saunders; 1999.
- SerhanCNSavillJ Resolution of inflammation: the beginning programs the endNature Immunol2005611917
- LawrenceTWilloughbyDAGilroyDW Anti-inflammatory lipid mediators and insights into the resolution of inflammationNat Rev Immunol2002278795
- LevyBDClishCBSchmidtBGronertKSerhanCN Lipid mediator class switching during acute inflammation: signals in resolutionNature Immunol200126129
- Serhan CN, ed. Special Issue on lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukot Essent Fatty Acids 2005;73(3–4).
- MajnoGJorisICells, tissues and disease: of general pathologyBlackwell ScienceCambridge MA1996
- SerhanCN Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolutionProstaglandins Other Lipid Mediat200268–6943355
- GilroyDWLawrenceTPerrettiMRossiAG Inflammatory resolution: new opportunities for drug discoveryNat Rev Drug Discov2004340116
- GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardicoLancet199935444755
- MarchioliRBarziFBombaEChieffoCDi GregorioDDi MascioR Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-PrevenzioneCirculation20021051897903
- BazanNG Supply, uptake, and utilization of docosahexaenoic acid during photoreceptor cell differentiationNestle Nutrition Workshop Series19922812133
- HambergMSamuelssonB On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidaseJ Biol Chem1967242532935
- SawazakiSSalemNJrKimH-Y. Lipoxygenation of docosahexaenoic acid by the rat pineal bodyJ Neurochem199462243747
- ReichEEZackertWEBrameCJChenYRobertsLJ IIHacheyDL Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acidBiochemistry200039237683
- VanRollinsMBakerRCSprecherHWMurphyRC Oxidation of docosahexaenoic acid by rat liver microsomesJ Biol Chem1984259577683
- Winyard PG, Willoughby DA, eds. Inflammation protocols. Totowa NJ: Humana; 2003.
- Lu Y, Hong S, Gotlinger K, Serhan CN. Lipid mediator informatics and proteomics in inflammation-resolution. ScientificWorldJournal 2006;6: 589–614.
- LuYHongSTjonahenESerhanCN Mediator-lipidomics: databases and search algorithms for PUFA-derived mediatorsJ Lipid Res200546790802
- RowleyAFLloyd-EvansPBarrowSESerhanCN Lipoxin biosynthesis by trout macrophages involves the formation of epoxide intermediatesBiochemistry19943385663
- BazanNGBirkleDLReddyTS. Docosahexaenoic acid (22:6, n-3) is metabolized to lipoxygenase reaction products in the retinaBiochem Biophys Res Commun19841257417
- Arita M, Bianchini F, Aliberti J, Sher A, Yang R, Petasis NA,. Novel omega-3 autacoid resolvin E1: receptor identification and anti-inflammatory properties. J Leukoc Biol 2003;Suppl:31 ( Abstract 72).