Abstract
Phospholipids are an important constituent of the cell plasma membrane and are also present in most common dietary products, being particularly abundant in milk, egg, meat and beans. Phospholipids are hydrolysed by different phospholipases to generate multiple breakdown products that affect the fate of the cells. Most phospholipids such as phosphatidylcholine, lysophosphatidylcholine, phosphatidylinositol and platelet activating factor are important for cell survival and thus may promote tumorigenesis and inflammation. Sphingomyelin is unique in the sense that its hydrolysis by sphingomyelinase and ceramidase generates several lipid messengers such as ceramide and sphingosine that inhibit cell proliferation and induce apoptosis. In the intestinal tract there is a specific type of sphingomyelinase called alkaline sphingomyelinase, which can hydrolyse sphingomyelin in both the cell membrane and the diet. The enzyme may play important roles in preventing colon cancer development and inflammation by hydrolysing sphingomyelin to generate anticancer molecules, and by counteracting the cancer-promoting effects of other phospholipids such as lysophosphatidylcholine and platelet activating factor. This mini-review highlights the signal transduction pathways activated by different phospholipids, with special attention being paid to potential implications in the development of colon cancer.
Introduction
Intestinal epithelial cells originate from one stem cell at the bottom of the crypt. The cell keeps dividing and migrating upwards along the crypt–villus axis. At the villus region, the crypt cells differentiate into mature absorptive epithelial cells, and at the tip of the villus, the epithelial cells undergo apoptosis and are shed into the intestinal lumen. The proliferation and apoptosis occur all the time and the two processes must be tightly regulated and well balanced. Overproliferation and failure of apoptosis are risk factors leading to tumorigenesis.
Phospholipids are important constituents of cells. Based on their backbone structure, phospholipids can be divided into glycerophospholipids and sphingophospholipids. In cell plasma membrane, phospholipids are distributed in a non-random manner, with phosphatidylinositol (PI) and phosphatidylserine (PS) predominantly located in the inner leaflet, and phosphatidylcholine (PC) and sphingomyelin (SM) in the outer leaflet. In the intestinal mucosa, phospholipids account for about 50% of the total lipids, of which about 41% is PC, 25% is phosphatidylethanolamine (PE), 12.5% is SM, 10% is PS and 5.6% is PI Citation1. Besides serving as structural compounds, phospholipids in the cells play important roles in regulating the fate of the cells by generating different lipid messengers affecting various signal transduction pathways. In the intestinal tract, the link between phospholipid metabolism and colon cancer development has been intensively studied. According to the major outcomes on the colonic cells, phospholipids can be roughly divided into two groups: proliferative and antiproliferative. The main members of the former group are PI, PC, lyso-PC and platelet activating factor (PAF), and the main one in the latter group is SM. This review outlines these signal transduction pathways affected by phospholipids and their potential implications in the development of colon cancer.
Signalling pathways affected by proliferative phospholipids and relation to colon cancer
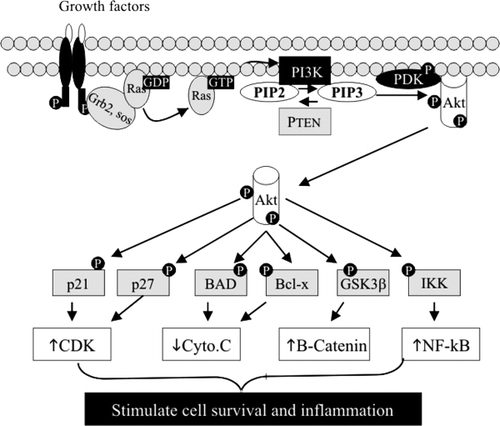
PI was the first phospholipid found to have signalling effects. It is well known that phosphatidylinositol 4,5-bisphosphate (PIP2), the position 4 and 5 phosphorylated PI, can be hydrolysed by a PI-specific phospholipase C (PLC) to generate diacylglycerol and inositol triphosphate, leading to activation of protein kinase C (PKC). Different types of PKC catalyse the phosphorylation of many proliferative molecules located in the cell membrane, cytoplasm and nucleus, such as vascular endothelial growth factor (VEGF) receptor, mitogen activated protein kinase (MAPK), nuclear factor-κB (NF-κB) and ribosomal S6 kinase. Recent studies found that PI can also be phosphorylated at the Δ3 position by a lipid kinase called PI3 kinase (PI3K) to form phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 can activate PI3K-dependent kinase, which in turn phosphorylates and activates Akt (also called protein kinase B). Akt is a key molecule that phosphorylates many target proteins that are important for cell-cycle regulation, such as p21 and p27, for cytochrome C release and apoptosis, such as Bcl-x and Bcl-XL/Bcl-2-associated death promoter (BAD), and for cell proliferation and inflammation, such as glycogen synthase kinase-3β (GSK-3β) and inhibitor of κB kinase (IKK), resulting in cell survival and cancer promotion (). The activation of the PI3K pathway is triggered by Ras, which is activated by many growth factors through tyrosine kinase receptors. As shown in , the effect of PI3K is counteracted by PTEN (phosphatase and tensin homologue deleted on chromosome 10), which is a phosphatase that dephosphorylates PIP3 to PIP2. The PI3K pathway plays important roles in tumorigenesis in the colon. Activating mutation of Ras is an early event in colon cancer progression, which could lead to a sustained activation of PI3K, as demonstrated in several colon cancer cell lines. Up-regulation of PI3K and Akt and inactivation of PTEN are frequently identified in colon cancer tissues Citation2Citation3. Inhibition of Ras, PI3K or Akt suppresses cell proliferation, enhances the efficacy of anticancer drugs and sensitizes the responsiveness of the cancer cells to radiation treatment.
Fig. 1. Phosphatidylinositol 3-phosphate kinase (PI3K)–protein kinase B (Akt) pathway. The pathway is triggered by many growth factors, which induce activation of PI3K and Akt. By phosphorylation of targeting proteins, Akt stimulates cell proliferation and inflammation and inhibits apoptosis. BAD: Bcl-associated death promoter; CDK: ceramide dependent kinase; cyto.C: cytochrome C; GDP: guanosine diphosphate; Grb: growth factor receptor bound protein; GSK: glycogen synthase kinase; GTP: guanosine triphosphate; IKK: inhibitor of κB kinase; PDK: PIP3 dependent kinase; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol 3,4,5-triphosphate; NF-κB: nuclear factor-κB; PTEN: phosphatase and tensin homologue deleted on chromosome 10; SOS: son of sevenless.
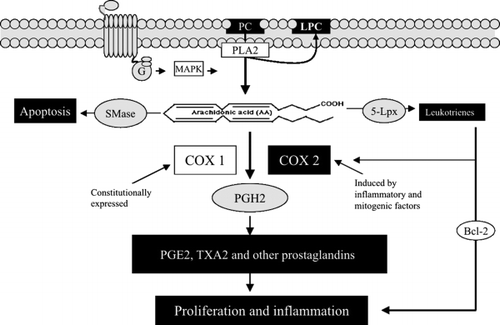
PC is the most abundant phospholipid in the intestinal mucosa and may have crucial implications in colonic tumorigenesis. The fatty acid in the sn-2 position of PC is rich in arachidonic acid, and is cleaved by phospholipase A2 (PLA2), which can be activated by MAPK and up-regulated in colon cancer. As shown in , arachidonic acid may stimulate apoptosis by a pathway presumably related to sphingomyelinase (SMase). However, the major metabolic pathway for arachidonic acid is to be converted to prostanoids by cyclooxygenase (COX). There are two isoforms of COX. COX1 is constitutionally expressed, and is important for physiological functions of prostaglandins (PGs), such as regulating renal flow, gastric mucosa and platelet aggregation, whereas COX2 expression is induced by many proinflammatory and mitogenic factors in the diseased tissues. High levels of COX2 have been found in ulcerative colitis, colon cancer tissues and other cancerous tissues. It increases the production of PG and many of them, particularly PGE2, stimulates cell proliferation and inflammation. COX2 may counteract the effect of adenomatous polyposis coli (APC) protein; in APC knockout mice, further mutation of COX2 significantly reduces cancer development Citation4. Non-steroidal anti-inflammatory drugs (NSAIDs) targeting COX2 have been shown to be chemopreventive against colon cancer Citation5. Arachidonic acid, apart from forming prostanoids, can be converted to leukotrienes by lipoxygenase (Lpx), which is up-regulated in colon cancer tissue Citation6. Leukotrienes are potent proinflammatory molecules, and LD4 has been found to stimulate cell proliferation and inhibit apoptosis by up-regulation of COX2 and Bcl-2 in colonic cancer cells Citation7.
Fig. 2. Phosphatidylcholine (PC)–phospholipase A2 (PLA2) pathway. PC is hydrolysed by PLA2 to generate arachidonic acid, which can be converted to prostaglandins and leukotrienes by cyclooxygenase-2 (Cox2) and 5-lipoxygenase (5-Lpx), respectively, leading to cell proliferation and inflammation. G: G protein; LPC: lysophosphatidylcholine; MAPK: mitogen-activated protein kinase; PG: prostaglandin; SMase: sphingomyelinase; TX: thromboxane.
Hydrolysis of PC by PLA2 generates not only arachidonic acid, but also lysophosphatidylcholine (lysoPC). LysoPC is a risk factor, as it can be further hydrolysed by lysophospholipase D to lysophosphatidic acid (LPA). The LPA formed in the membrane will be released from the cells and binds the membrane receptors of its host cell or neighbouring cells. At least three types of LPA receptor coupled with different G proteins have been identified. The main receptor expressed in colonic cancer cells is LPA2, and in some cases, LPA1. Recent studies show that LPA plays important roles in cell migration and cancer metastasis Citation8Citation9. When cultured with colon cancer cells, LPA stimulates cell migration and increases secretion of interleukin (IL)-8 and VEGF, two key molecules in cancer metastasis. The effects can be inhibited by inhibitors of Akt and PLC?, indicating upstream involvement of Akt, PLC? and MAPK. Other studies showed that, through activation of PLC and PKC, LPA affects Wingless type signals and increases the translocation of ?-catenin into the nucleus.
Sphingomyelin pathway and colon cancer
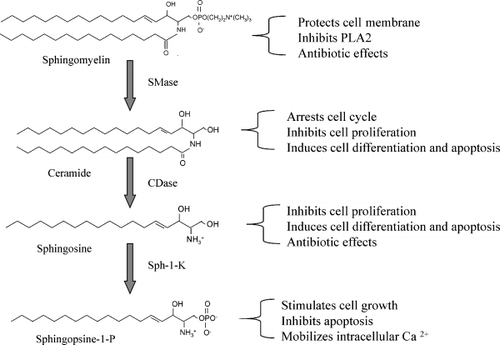
SM is a type of phospholipid, which is composed of a sphingosine backbone, a fatty acid and a phosphocholine head group. SM is an important membrane constituent that maintains membrane integrity. In the intestine, it may protect the membrane against toxic compounds such as bile salts. As shown in , SM is hydrolysed by sphingomyelinase (SMase), which cleaves the phosphocholine headgroup and turns SM to ceramide. Ceramide in turn is hydrolysed by ceramidase (CDase) to sphingosine and fatty acid. Since the end of 1980s, sphingosine and ceramide have been identified as potent antiproliferative and apoptotic molecules, which inhibit cell proliferation, arrest cell cycle, induce cell differentiation and stimulate apoptosis Citation10. In contrast with PI and PC, metabolism of SM generates lipid messengers with antiproliferative properties, and thus may have important physiological implications in cancer prevention.
Fig. 3. Hydrolysis of sphingomyelin (SM) and the biological effects of the metabolites. The hydrolytic process of SM and the enzymes involved are shown on the left and the biological effects of the metabolites on the right. CDase: ceramidase; PLA2: phospholipase A2; SMase: sphingomyelinase; Sph: sphingopsine.
The mechanism underlying the effects of ceramide is mainly related to its ability to activate several types of phosphatase Citation11 (), which induce dephosphorylation and inactivation of several antiapoptotic and proliferative molecules, such as (i) Akt and PKC, the important kinases activated by PI and PC metabolites, (ii) Bcl-2, the major inhibitor of cytochrome c release from mitochondria and a key molecule that suppresses apoptosis, and (iii) pRb, the regulator of the cell cycle in the nucleus, which inhibits the cell cycle in dephosphorylated form. Ceramide is also able to bind cathepsin D, a lysosomal protease, and induce autoproteolysis and activation of the enzyme Citation12. The activated cathepsin D in turn hydrolyses and activates caspase 3, a key enzyme for the execution of apoptosis.
Fig. 4. Signal pathway affected by ceramide. Ceramide induces dephosphorylation of many proliferative and antiapoptotic molecules, activation of cathepsin D, and activation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK). The main outcome by ceramide is the inhibition of cell proliferation and stimulation of apoptosis. Akt: protein kinase B; KSR: kinase suppressor of Ras; NF-κB: nuclear factor-κB; PKC: protein kinase C.
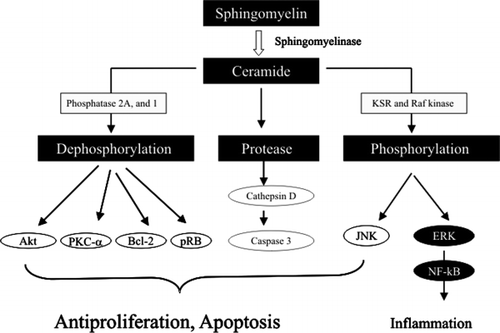
Besides phosphatase, several types of kinase have been found to be activated by ceramide, such as kinase suppressor of Ras (KSR) and Raf kinase. Activation of these kinases affects the members of MAPK family, such as c-jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK). Activation of JNK may stimulate apoptosis, whereas activation of ERK may stimulate NF-κB and enhance inflammation.
Sphingosine, derived from the hydrolysis of ceramide by ceramidase, was originally found to be an endogenous inhibitor of PKC. Apart from inhibiting PKC, sphingosine has diverse effects on different types of MAPK; for example, it inhibits ERK and activates JNK and p38MAPK. As a consequence, sphingosine inhibits cell proliferation and facilitates apoptosis. As shown in , sphingosine is phosphorylated by sphingosine kinase to sphingosine-1-phosphate (S1P). An important feature is that when sphingosine is converted to S1P, the antiproliferative property of the SM pathway turns to proliferative, as S1P is a strong mitogenic factor that stimulates cell proliferation, migration and angiogenesis. Many factors, such as platelet-derived growth factor (PDGF), nerve growth factor (NGF), tumour necrosis factor (TNF)-α and IL-1β, through binding their receptors, activate and translocate sphingosine kinase to the plasma membrane. The formed S1P, like LPA, is released from the cells and binds to S1P receptors, serving as an extracellular signal. There are several types of S1P receptor coupled with G proteins. S1P has been found to increase the activity of PLC and activate the MAPK cascade, and to stimulate cell migration in a Rho-dependent manner Citation13. High levels of sphingosine kinase have been recently found in colon cancer tissues and the increase is parallel with that of COX2 Citation14. Besides functioning as an extracellular signal, S1P may also serve as a second messenger in the cytosol to mobilize intracellular calcium.
It is clear that the SM pathway generates both antiproliferative and proproliferative molecules, and the final outcome is determined by the dynamic balance of ceramide and sphingosine versus S1P. The balance is affected by several enzymes, including SMase, CDase, sphingosine kinase and S1P lyase, and also the enzymes responsible for de novo synthesis of ceramide and its conversion to other glucosylceramides.
Although SM metabolism has implications in cancer development in general, a specific link between SM metabolism and colon cancer has been indicated in the past decade. SM hydrolysis in colonic mucosa in mice treated with the chemical carcinogen dimethylhydrazine was found to be inhibited before the onset of the tumours Citation15, whereas in human colonic carcinoma tissues, both SM and ceramide levels were significantly decreased Citation1. Supplementation of SM in the diet significantly inhibited the formation of aberrant crypt foci, the early marker of dysplasia, and the formation of carcinomas in the colon of mice treated with chemical carcinogen Citation16Citation17. The chemopreventive effects of SM can be reproduced by feeding ceramide and other sphingolipids that generate ceramide, indicating that the hydrolysis of SM is necessary for such preventive effects of SM.
Alkaline sphingomyelinase, neutral ceramidase and colon cancer
Intestinal mucosa expresses a specific type of SMase called alkaline SMase (alk-SMase) Citation18, whose activity is 100–1000 times higher than other types of SMase in the gut. Recent cloning studies found that the enzyme shares no structural similarities to other types of SMase and belongs to the nucleotide pyrophosphatase/phosphodiesterase (NPP) family Citation19. As a novel member of the NPP family, alk-SMase is also named NPP7. Alkaline SMase is an ectoenzyme anchoring on the surface of the microvilli via its C-terminal transmembrane domain; its active site is located in the intestinal lumen. The enzyme can be dissociated from the mucosa by either pancreatic trypsin or bile salts. Both the free form and the membrane-bound form of alk-SMase are able to hydrolyse both dietary SM in the lumen and the SM in the plasma membrane in a bile salt-dependent manner. The properties of the enzyme have been summarized recently in an review article Citation20. In the intestinal tract, the ceramide generated by alk-SMase is further hydrolysed by neutral ceramidase (N-CDase) to sphingosine. N-CDase is also a brush-border enzyme and is present in both intestinal lumen and mucosa Citation18. Along the intestinal tract, N-CDase distributes in parallel with alk-SMase and the two enzymes co-operate in the hydrolysis of SM and regulation of ceramide levels Citation21.
Studies by the author's group indicate that alk-SMase may be a novel gene product in the intestine that prevents colonic tumorigenesis. The activity of alk-SMase is decreased by 20% in longstanding ulcerative colitis, by 50% in sporadic colonic adenomas and by 75% in colonic carcinomas Citation20Citation22, indicating that the reduction in enzyme activity occurs early and progresses with the process of colonic carcinogenesis. Studies from this group also showed that purified alk-SMase added in the medium, or the cDNA transfected into colon cancer cells, inhibited cell proliferation and DNA synthesis by about 50%. The inhibited cell proliferation was associated with a reduced SM level and a sharp increase in ceramide in the cells Citation23. Mutations of alk-SMase caused by alternative splice have been found in HT29 colon cancer cells and in human colon cancer biopsy samples, and these mutations abolish the enzyme activity Citation24. Whether alk-SMase affects apoptosis is not fully clear. Studies with normal animals show that alk-SMase activity correlates positively with caspase 3 activity Citation25. However, in studies with colon cancer cells, alk-SMase was found to affect only cell proliferation, not apoptosis Citation23.
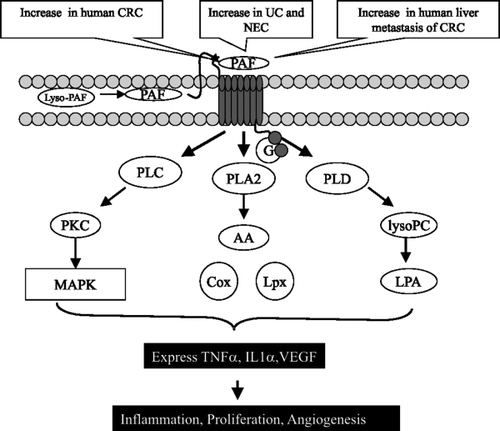
As well as hydrolysing SM, alk-SMase can hydrolyse PAF, another type of phospholipid, by a PLC activity Citation26. PAF can be synthesized in colonic cells and PAF receptors have been found in colonic mucosa. PAF is a proinflammatory factor involved in several intestinal diseases such as diarrhoea, ulcerative colitis, necrotizing enterocolitis and cancer. A striking feature of PAF is its ability to increase several types of phospholipases, including PLA2, PLC and PLD (), leading to cytokine release, MAPK activation and cell proliferation. The levels of PAF are significantly increased in ulcerative colitis and colon cancer, and also in liver metastasis of colon cancer. By cleaving the phosphocholine moiety, alk-SMase completely abolishes PAF-induced MAP kinase activation and cytosine release in colon cancer cells, and chemotaxis in macrophages Citation26. In addition to PAF, alk-SMase can hydrolyse lyso-PC to monoglyceride Citation19. The effect may also be important, because in this manner alk-SMase counteracts lysophospholipase D, and may reduce the formation of LPA. Taken together, alkaline SMase is an enzyme with three arms to protect the colonic mucosa from carcinogenesis: by forming ceramide, inactivating PAF and reducing LPA production.
Fig. 5. Signal transduction pathways affected by platelet activating factor (PAF). PAF binds G-protein-coupled receptors and activates several types of phospholipase (PL), resulting in inflammation, proliferation and angiogenesis. Cox: cyclooxygenase; CRC: ??; IL: interleukin; LPA: lysophosphatidic acid; Lpx: lipoxygenases; lysopC: ??; MAPK: mitogen-activated protein kinase; NEC: ??; PKC: protein kinase C; TNF: tumour necrosis factor; UC: ??; VEGF: vascular endothelial growth factor.
The expression of alk-SMase and N-CDase is susceptible to change by dietary factors and anticancer and anti-inflammatory agents. A high-fat diet significantly inhibits the levels of alk-SMase in the colonic mucosa, whereas the water-soluble fibre psyllium increases alk-SMase expression and decreases N-CDase activity Citation25. Beef, cellulose, fish oil and arachidonic acid in the diet have no effect on alk-SMase. Several compounds that have anti-inflammatory or anticancer properties, such as 5-aminosalicylic acid and ursodeoxycholic acid, may enhance the expression of alk-SMase; the effects are mainly exerted on differentiated epithelial cells Citation27Citation28. Ursolic acid, a triterpenoid widely present in fruit and vegetables, increases alk-SMase activity Citation29, whereas most phospholipids inhibit alk-SMase activity in the test-tube Citation30.
The link between SM and colon cancer raises several questions. Can a supplement of dietary SM, which is abundant in milk and eggs, prevent the development of colon cancer in those at high risk? Can measuring alk-SMase activity in a colonic biopsy or the faeces be valuable in diagnosis or population screening? Is alk-SMase effective in preventing or treating patients with ulcerative colitis and colonic adenocarcinomas? Collaborations among the scientists, drug companies and the food industry may solve these questions and generate novel means to fight colon cancer.
Biological effects of phosphatidylserine
Compared with other phospholipids, much less is known about the signalling effects of PS. PS has been shown to enhance the binding of some types of PKC in the inner side of the membrane and thus to contribute to PKC activation. However, when cells undergo apoptosis, PS was found to be translocated to the outer leaflet where SM had been hydrolysed Citation31. Translocation of PS to the outer leaflet labels the dying cells, which may be important for them to be identified and cleared up by neighbouring cells or professional phagocytes by phagocytosis, an important process for avoiding inflammation.
| Acronyms | ||
| Akt | = | protein kinase B |
| alk-SMase | = | alkaline sphingomyelinase |
| APC | = | adenomatous polyposis coli |
| CDase | = | ceramidase |
| COX | = | cyclooxygenase |
| ERK | = | extracellular signal regulated kinase |
| GSK | = | glycogen synthase kinase |
| IKK | = | inhibitor of κB kinase |
| IL | = | interleukin |
| JNK | = | c-Jun N-terminal kinase |
| KSR | = | kinase suppressor of Ras |
| LPA | = | lysophosphatidic acid |
| LPX | = | lipoxygenase |
| MAPK | = | mitogen-activated protein kinase |
| N-CDase | = | neutral ceramidase |
| NF-κB | = | nuclear factor-κB |
| NGF | = | nerve growth factor |
| NPP | = | nucleotide pyrophosphatase/phosphodiesterase |
| NSAID | = | non-steroidal anti-inflammatory drug |
| PAF | = | platelet activating factor |
| PC | = | phosphatidylcholine |
| PDGF | = | platelet-derived growth factor |
| PE | = | phosphatidylethanolamine |
| PG | = | prostaglandin |
| PI | = | phosphatidylinositol |
| PI3K | = | phosphatidylinositol 3-phosphate kinase |
| PIP2 | = | phosphatidylinositol 4,5-bisphosphate |
| PIP3 | = | phosphatidylinositol 3,4,5-triphosphate |
| PKC | = | protein kinase C |
| PLA2 | = | phospholipase A2 |
| PLC | = | phospholipase C |
| PLD | = | phospholipase D |
| PS | = | phosphatidylserine |
| PTEN | = | phosphatase and tensin homologue deleted on chromosome 10 |
| S1P | = | sphingosine-1-phosphate |
| SM | = | sphingomyelin |
| SMase | = | sphingomyelinase |
| TNF | = | tumour necrosis factor |
| VEGF | = | vascular endothelial growth factor. |
The works cited from the author's laboratory were supported by grants from Swedish Cancer Society, Albert Påhlsson's Foundation, The Swedish Research Council and the Research Foundation of Lund University Hospital.
References
- MerchantTEKasimosJNde GraafPWMinskyBDGierkeLWGlonekT. Phospholipid profiles of human colon cancer using 31P magnetic resonance spectroscopyInt J Colorectal Dis199161216
- IkenoueTKanaiFHikibaYObataTTanakaYImamuraJ Functional analysis of PIK3CA gene mutations in human colorectal cancerCancer Res20056545627
- RoyHKOlusolaBFClemensDLKarolskiWJRatashakALynchHT AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesisCarcinogenesis2002232015
- OshimaMDinchukJEKargmanSLOshimaHHancockBKwongE Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2)Cell1996878039
- HulsGKoornstraJJKleibeukerJH. Non-steroidal anti-inflammatory drugs and molecular carcinogenesis of colorectal carcinomasLancet20033622302
- NielsenCKOhdJFWikstromKMassoumiRParuchuriSJuhasM The leukotriene receptor CysLT1 and 5-lipoxygenase are upregulated in colon cancerAdv Exp Med Biol20035252014
- OhdJFNielsenCKCampbellJLandbergGLofbergHSjolanderA. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomasGastroenterology20031245770
- YangMZhongWWSrivastavaNSlavinAYangJHoeyT G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathwayProc Natl Acad Sci USA2005102602732
- YunCCSunHWangDRusoviciRCastleberryAHallRA LPA2 receptor mediates mitogenic signals in human colon cancer cellsAm J Physiol Cell Physiol2005289C211
- HannunYALinardicCM. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipidsBiochim Biophys Acta1993115422336
- PettusBJChalfantCEHannunYA. Ceramide in apoptosis: an overview and current perspectivesBiochim Biophys Acta2002158511425
- HeinrichMWickelMWinoto-MorbachSSchneider-BrachertWWeberTBrunnerJ Ceramide as an activator lipid of cathepsin DAdv Exp Med Biol200047730515
- SpiegelSMilstienS. Sphingosine-1-phosphate: an enigmatic signalling lipidNat Rev Mol Cell Biol20034397407
- KawamoriTOstaWJohnsonKRPettusBJBielawskiJTanakaT Sphingosine kinase 1 is up-regulated in colon carcinogenesisFASEB J2006203868
- BrasitusTADudejaPKDahiyaR. Premalignant alterations in the lipid composition and fluidity of colonic brush border membranes of rats administered 1,2 dimethylhydrazineJ Clin Invest19867783140
- DillehayDLWebbSKSchmelzE-MMerrillAH. Dietary sphingomyelin inhibits 1,2-dimethylhydrazine-induced colon cancer in CF1 miceJ Nutr199412461520
- LemonnierLADillehayDLVespremiMJAbramsJBrodyESchmelzEM. Sphingomyelin in the suppression of colon tumors: prevention versus interventionArch Biochem Biophys200341912938
- Nilssonå. The presence of sphingomyelin- and ceramide-cleaving enzymes in the small intestinal tractBiochim Biophys Acta196917633947
- DuanRDBergmanTXuNWuJChengYDuanJ Identification of human intestinal alkaline sphingomyelinase as a novel ecto-enzyme related to the nucleotide phosphodiesterase familyJ Biol Chem20032783852836
- DuanRD. Alkaline sphingomyelinase: an old enzyme with novel implicationsBiochim Biophys Acta2006176128191
- OlssonMDuanRDOhlssonLNilssonA. Rat intestinal ceramidase: purification, properties, and physiological relevanceAm J Physiol Gastrointest Liver Physiol2004287G92937
- HertervigENilssonANybergLDuanRD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinomaCancer19977944853
- HertervigENilssonAChengYDuanRD. Purified intestinal alkaline sphingomyelinase inhibits proliferation without inducing apoptosis in HT-29 colon carcinoma cellsJ Cancer Res Clin Oncol200312957782
- WuJChengYNilssonADuanRD. Identification of one exon deletion of intestinal alkaline sphingomyelinase in colon cancer HT-29 cells and a differentiation-related expression of the wild-type enzyme in Caco-2 cellsCarcinogenesis200425132733
- ChengYOhlssonLDuanRD. Psyllium and fat in diets differentially affect the activities and expressions of colonic sphingomyelinases and caspase in miceBr J Nutr20049171523
- WuJNilssonAJonssonBAStenstadHAgaceWChengY Intestinal alkaline sphingomyelinase hydrolyses and inactivates platelet-activating factor by a phospholipase C activityBiochem J2006394299308
- ChengYTauschelHDNilssonåDuanRD. Administration of ursodeoxycholic acid increases the activities of alkaline sphingomyelinase and caspase-3 in rat colonScand J Gastroenterol19993491520
- LiuFChengYWuJTauschelHDDuanRD. Ursodeoxycholic acid differentially affects three types of sphingomyelinase in human colon cancer Caco 2 cellsCancer Lett20062351416
- AnderssonDNilssonADuanR. Ursolic acid and other pentacyclic triterpenoids stimulate intestinal alkaline sphingomyelinase in vitroEur J Lipid Sci Technol20061081038
- LiuJJNilssonåDuanRD. Effects of phospholipids on sphingomyelin hydrolysis induced by intestinal alkaline sphingomyelinase: an in vitro studyJ Nutr Biochem2000111927
- TepperADRuursPWiedmerTSimsPJBorstJvan BlitterswijkWJ. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphologyJ Cell Biol200015015564