Abstract
Aim: To assess the effectiveness and safety of a new protocol for adjusting doses during interrupted subcutaneous immunotherapy maintenance, exceeding an 8-week interval, with mite allergen injections in children with allergic rhinitis. Patients & methods: 194 children with allergic rhinitis who underwent subcutaneous immunotherapy and experienced interruptions lasting more than 8 weeks during maintenance were enrolled. Following the adoption of a novel dose-adjustment protocol, a real-world study was conducted. Results: After 3 years of subcutaneous immunotherapy, the novel group exhibited a significant reduction in allergy symptoms compared with baseline. Systemic reactions related to the novel protocol did not significantly increase. Conclusion: The novel protocol was deemed safe and effective, offering advantages of time savings and reduced burdens.
Plain language summary
There is a main treatment for allergic rhinitis. it is with regular shots of a special medicine made from dust mite allergen. Patients need to take these shots in their arm for 3 years. The shot is given once a week for 14 weeks at first; then the frequency can be reduced to every 5 weeks. However, if a patient misses their scheduled shot, they may have to start getting weekly shots again. This can lead to a lot of medical waste and can be expensive for patients. Therefore, we developed a new way to give these shots. In our study, patients who needed to start weekly shots again were administered this new treatment plan. The new plan significantly reduced the number of doctor's visits and shots. This new treatment method is safe, cost-effective and patient-friendly.
Allergic rhinitis (AR) is a prevalent global allergic disease.
Subcutaneous immunotherapy is the only causal treatment capable of modifying the natural course of allergic diseases by gradually increasing subcutaneous allergen doses to induce immune tolerance.
Many subcutaneous immunotherapy patients drop out due to the added time and cost of repeating the build-up phase after treatment delays.
A retrospective, single-center, real-world study with 194 AR children assessed the efficacy and safety of a novel dose-adjustment protocol.
In the novel protocol, maintenance treatment recommenced at a fixed dose of 5000 TU after an interruption of more than 8 weeks in the maintenance phase, deviating from the package insert's recommendation of returning to 5% of the last injection dose. Conversely, the conventional protocol strictly adhered to the drug instructions.
The novel protocol demonstrated efficacy comparable to the conventional one, saving costs and time associated with repurchasing the build-up phase reagent kit and restarting injections.
Systemic reactions related to the novel schedule did not increase.
The novel protocol is deemed safe and effective, offering advantages of time savings and reduced medical and financial burdens.
1. Background
Allergic diseases are type I allergic diseases mediated by IgE, mainly including allergic rhinitis (AR), asthma, atopic dermatitis food allergy, etc [Citation1]. The WHO has listed it as one of the most serious public health problems in the 21st century [Citation2]. AR is a noninfectious chronic inflammatory disease of the nasal mucosa mediated mainly by IgE after atopic individuals are exposed to allergens [Citation3]. The main manifestations are continuous sneezing, runny nose, nasal congestion and nasal itchiness [Citation4]. Long-term AR can also lead to asthma and cardiovascular disease and its severity is closely linked to psychiatric symptoms such as anxiety [Citation5]. Treatment strategies for AR include environmental control, drug therapy, immunotherapy and health education [Citation6].
The rapid increase in the prevalence of AR in children in recent years has attracted global attention [Citation7–9]. The self-reported prevalence of AR averaged 8.5% in children aged 5–8 years and 14.6% in children aged 13–14 years, with significant differences across countries and regions [Citation10,Citation11]. Epidemiological studies in some areas of China show that the self-reported prevalence rate of AR in children aged 2–18 years is 18.10–49.68% and the diagnosed prevalence rate is 10.80–21.09% and the trend is increasing year by year [Citation12,Citation13].
The immunopathological mechanism of AR in children and adults is basically the same, mainly the IgE-mediated type I allergy triggered by inhalation of allergens in the nasal mucosa [Citation14,Citation15]. Genes and environment play important roles in the occurrence and development of AR in children. Children are more likely to be influenced by genetic factors than adults [Citation16]. The immune system of children is not yet mature and the immune response to allergens is different from that of adults and its plasticity is stronger and easier to regulate [Citation17].
The treatment of AR in children includes causative treatment and symptomatic treatment. At present, the former is mainly allergen-specific immunotherapy and the latter is mainly drug therapy [Citation18]. Subcutaneous immunotherapy (SCIT) stands out as the sole curative intervention capable of modifying the progression of allergic diseases by gradually escalating subcutaneous allergen doses to induce immune tolerance [Citation19]. When the child is exposed to the corresponding allergen again, the symptoms can be significantly reduced or even no clinical symptoms. SCIT has a long-term effect for stopping the progression of allergic disease, preventing AR from developing into asthma and reducing the generation of new sensitization [Citation20,Citation21]. It is worth noting that the dosage of different varieties of allergen vaccines has not been unified and the treatment plan is not the same, so on the premise of ensuring the safety of treatment, the treatment plan can be adjusted according to the condition and actual situation of children according to the existing clinical research evidence [Citation22,Citation23]. At present, immunotherapy methods commonly used in clinical practice are divided into two phases: a build-up phase and a maintenance phase. Numerous studies have indicated that a minimum 3-year course of SCIT is necessary to attain sustained efficacy [Citation24]. Through standardized comprehensive prevention and treatment, various symptoms of children can be controlled for a long time and the quality of life can be significantly improved.
Considering the developmental maturity of the child's immune system and the safety of the treatment, SCIT is usually carried out in children older than 5 years [Citation25]. Children receiving SCIT and/or their guardians are required to sign informed consent to increase their awareness of SCIT and clarify treatment goals through adequate and good communication, thereby improving compliance and confidence in treatment [Citation26,Citation27]. SCIT must be carried out under the supervision of a physician, by trained medical personnel in a healthcare facility and with emergency plans in place [Citation28].
However, since the onset of the COVID-19 pandemic, many SCIT patients in our hospital have faced disruptions in their scheduled subcutaneous injections due to isolation or infection [Citation29].
In light of the absence of evidence-based dose adjustment protocols following delayed treatment and prolonged intervals, doses are typically repeated or reduced based on experiential grounds [Citation30]. The package insert suggests initiating treatment at 5% of the last dose for maintenance injections interrupted for over 8 weeks [Citation31]. The definition of interruption of AIT referred to the time interval since the patient's last subcutaneous injection. Numerous studies have demonstrated that a significant number of patients discontinue SCIT due to the added time and cost of repeating the build-up phase after treatment delays [Citation32]. Consequently, it is imperative to formulate an optimal strategy for dose adjustment post-SCIT treatment delays. Given that the majority of SCIT patients at our hospital's allergy center fall within the 5–18 years age range, we conducted a study on a novel dose-adjustment protocol following delayed treatment in the maintenance phase of SCIT. We compared the efficacy and safety of this novel protocol with the conventional one. The objective of this study is to establish a reliable foundation for crafting a scientifically sound dose adjustment protocol in real-world research.
2. Patients & methods
This retrospective study was conducted as a single-center, real-world research initiative. Approval for the study was obtained from the Ethics Committee of the Third People's Hospital of Changzhou (02A-A20200006) and all patients provided informed consent before participating.
2.1. Patient selection
The study retrospectively selected patients aged 5–18 with AR who underwent SCIT with mite allergen injections at the Otolaryngology Allergy Center of the Third People's Hospital of Changzhou from February 2020 to November 2023. These patients experienced interruptions of more than 8 weeks during maintenance treatment. A total of 194 cases were included and the inclusion criteria were: (1) meeting the latest diagnostic criteria for AR [Citation4]; (2) absence of asthma; (3) no occurrence of severe systemic reactions (SRs); (4) reaching the dose maintenance period with the total treatment duration not exceeding 104 weeks, but with an interruption duration exceeding 8 weeks; (5) completion of the 3-year course with the adoption of the novel dose-adjustment protocol. Additionally, a control group comprised 145 children aged 5–18 with AR, who underwent mite allergen injection SCIT and received conventional treatment from February 2016 to November 2019. The inclusion criteria for this group were the same as the novel protocol group, except for completing a 3-year course with conventional treatment.
2.2. Treatments
Both groups received mite allergen injection SCIT using Novo–Helisen–Depot (NHD). Its active ingredients were 50% dermatophagoides farinae and 50% house dust mites. The treatment course consisted of a build-up phase and a maintenance phase. In the build-up phase (weeks 1–14), the initial dose was 5 TU, with subsequent weekly increments of 10, 20, 40, 50, 100, 200, 400, 500, 1000, 2000, 3000, 4000 and 5000 TU. The dose maintenance phase (15–52 weeks of the first year, 53–104 weeks of the second year, 105–156 weeks of the third year) involved a constant dose of 5000 TU, administered once every 5 weeks from the 15th week of the first year, totaling 142 weeks [Citation33].
In the novel protocol, maintenance treatment recommenced at a fixed dose of 5000 TU after an interruption of more than 8 weeks in the maintenance phase, deviating from the package insert's recommendation of returning to 5% of the last injection dose. Conversely, the conventional protocol strictly adhered to the drug instructions. Under aseptic conditions, the nurse slowly injected subcutaneously at 1/2 of the upper arm of the child and applied pressure to hemostasis for 5 min after injection. Subjects were permitted to use loratadine (tablets) and budesonide (nasal spray) as symptom relief drugs during the treatment period [Citation34].
2.3. Observation indicators
2.3.1. Clinical response
For subsequent analysis, data encompassing baseline information before treatment, the baseline at the restart of the maintenance phase (R0)/baseline of maintenance phase (M0) and efficacy assessments after 3 years of treatment (R3/M3) were utilized. The primary index for assessing clinical efficacy was the combined symptom and medication score (CSMS), with the total nasal symptom score (TNSS) and medication score serving as secondary efficacy indicators [Citation35]. Nasal congestion, nasal itching, sneezing and a runny nose were incorporated into TNSS. Severity ratings, ranging from 0 (asymptomatic) to 3 (severe, intolerable, affecting daily life and sleep), were assigned for each of the four nasal symptoms. TNSS, the sum of these symptoms, was calculated to range from 0 to 12. MS, evaluated on a 3-point scale based on the specific drug used, comprised 1 point for antihistamines, 2 points for nasal or inhaled corticosteroids and 3 points for oral corticosteroids. CSMS was calculated as follows: CSMS = TNSS/4 + MS, where symptom and drug scores held equal weight.
2.3.2. Adverse reactions
Children were maintained in a quiet demeanor for 30 min both before and after each injection. Adverse reactions were closely monitored by medical staff, with specific manifestations, occurrence time, possible causes, severity, treatment methods and final outcomes being recorded. Adverse reactions primarily included local reactions and SRs, with SRs being considered the key safety indicator for SCIT [Citation36]. SRs were categorized into grades 1–5 per the WAO 2010 grading system, considering grade 3 or above as severe. Grade 1: itching, urticaria, skin redness, sneezing, runny nose, itchy nose, stuffy nose, itchy eyes, cough, etc. Grade 2: asthma (response to inhaled bronchodilators), vomiting, diarrhea, etc. Grade 3: Asthma (no response to inhaled bronchodilators), laryngeal edema, etc. Grade 4: Respiratory failure or hypotension; Grade 5: Death. Treatment for adverse reactions was administered in adherence to allergen-specific immunotherapy (AIT) guidelines [Citation37].
2.4. Statistical analysis
Statistical analysis was carried out using SPSS 26.0 software (IBM Corp., NY, USA). Mean ± SEM represented the results of symptom and medication scores. Mann–Whitney U and two independent-sample t-tests were used to assess the differences between the two groups, while adverse reaction incidences between the novel and conventional groups were compared using the chi-square test. p-values <0.05 were considered significant.
3. Results
3.1. Demographic characteristics
Following 3 years of treatment, we conducted a comprehensive analysis and comparison of the efficacy and safety outcomes for 194 children in the novel group and 145 children in the conventional group. The trial process details are depicted in . Demographic characteristics and baseline scores for both groups are presented in . At the initiation of SCIT, there were no notable differences in age, gender, symptom scores or other clinical indicators between the two groups. Similarly, there were no significant differences in the scores of the novel group at the restart of maintenance treatment (R0) and the conventional group at the initiation of maintenance treatment (M0).
Figure 1. The trial flow chart illustrates the novel dose-adjustment protocol and the conventional one for subcutaneous immunotherapy in allergic rhinitis due to delayed injection. Baseline: baseline before treatment; M0: the maintaining pretreatment baseline of the conventional group; R0: the maintaining pretreatment baseline of the novel dose-adjustment protocol group; M3: the end of 3-year treatment in the conventional group; R3: the end of 3-year treatment in the novel dose-adjustment protocol group.
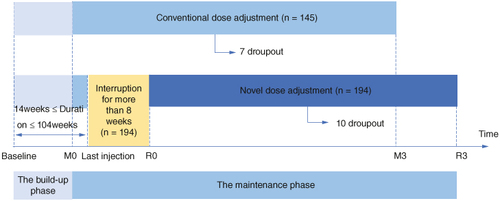
Table 1. Demographic characteristics comparison between the novel group and the conventional group.
3.2. Clinical efficacy evaluation
Individual symptom scores (A–D), TNSS (E), MS (F) and CSMS (G) demonstrated significant improvement after 3 years of SCIT in both the novel and conventional groups compared with their respective maintenance phase baselines (R0/M0) (p <0.001). This suggests a favorable therapeutic effect of SCIT in both groups.
Figure 2. Comparison of therapeutic effects between the novel dose-adjustment protocol group and the conventional protocol group in subcutaneous immunotherapy. (A) Nasal congestion score; (B) itchy nose score; (C) sneezing score; (D) runny nose score; (E) TNSS; (F) MS; (G) CSMS. Baseline: baseline before treatment; M0: the maintaining pretreatment baseline of the conventional group; R0: the maintaining pretreatment baseline of the novel dose-adjustment protocol group; M3: the end of 3-year treatment in the conventional group; R3: the end of 3-year treatment in the novel dose-adjustment protocol group.
CSMS: Combined symptom and medication score; MS: Medication score; TNSS: Total nasal symptom score.
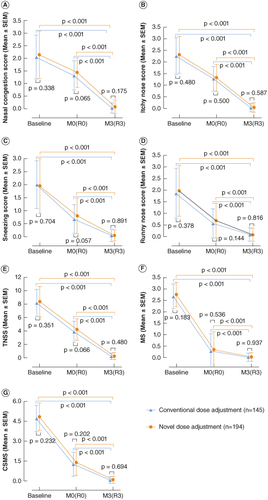
At the conclusion of the 3-year treatment period, there were no statistically significant differences between the two groups in individual symptom scores (p = 0.175, p = 0.587, p = 0.891, p = 0.816, A–D), TNSS (p = 0.480, E), MS (p = 0.937, F) and CSMS (p = 0.694, G).
In comparison with the maintenance pretreatment baseline (R0/M0), the changes in TNSS (-3.96 ± 1.42 vs. -3.68 ± 1.50, p = 0.078, A), MS (-0.32 ± 0.76 vs. -0.27 ± 0.78, p = 0.547, B) and CSMS (-1.31 ± 0.93 vs. -1.19 ± 0.92, p = 0.231, C) showed no statistical significance. Similarly, compared with the baseline before treatment, the changes in TNSS (-8.12 ± 2.32 vs. -7.94 ± 2.10, p = 0.460, A), MS (-2.71 ± 0.58 vs. -2.63 ± 0.59, p = 0.233, B) and CSMS (-4.74 ± 1.10 vs. -4.62 ± 1.04, p = 0.303, C) also showed no statistical significance.
Figure 3. Comparison of changes in TNSS, MS and CSMS before and after treatment between the novel dose-adjustment protocol group and the conventional group. (A) Change in TNSS; (B) change in MS; (C) change in CSMS. R3-R0: The change at the end of 3-year treatment compared with the pretreatment baseline of the re-maintenance in the novel dose-adjustment protocol group; M3-M0: the change at the end of 3-year treatment compared with the baseline of maintenance treatment in the conventional group; R3-Baseline: the change from baseline to the end of 3-year treatment in the novel dose-adjustment protocol group; M3-Baseline: the change from baseline to the end of 3-year treatment in the conventional group.
CSMS: Combined symptom and medication score; MS: Medication score; TNSS: Total nasal symptom score.
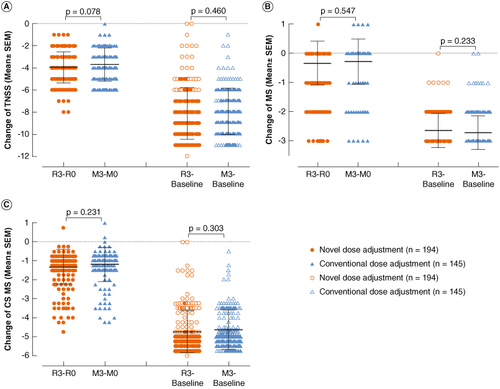
These findings suggest that the efficacy of SCIT in children with the new regimen was similar to that of children with continuous injection for 3 years.
3.3. Safety evaluation
The occurrence of SRs during the maintenance phase in children from the novel and conventional groups is detailed in . SRs manifested 43-times in 30 children in the novel group and 31-times in 21 children in the conventional group. There were no significant differences between the two groups in the occurrence rate of patients with SRs (15.46 vs 14.48%; p = 0.803) and the occurrence rate of injections with SRs (0.82 vs 0.79%; p = 0.870). Therefore, the novel dose-adjustment protocol did not lead to an increased incidence of SRs during the maintenance phase. All SRs appeared within 30 min after injection, primarily presenting as rash, pruritus, nasal congestion, sneezing, etc. and alleviated after the use of loratadine (tablet) or budesonide (nasal spray).
Table 2. The occurrence of SRs in the maintenance phase of children with allergic rhinitis in the novel dose-adjustment protocol group and the conventional group.
3.4. Dropped out of the study
In the novel dose-adjustment protocol group, where treatment interruption exceeded 8 weeks, 10 children (10/194, 5.15%) discontinued participation during the maintenance phase. One child was unable to continue injections due to a change in residence and nine children withdrew from the study due to concerns about infection. In the conventional group, seven children (7/145, 4.83%) dropped out during the maintenance phase. Two children were lost to follow-up due to a change in the injection site and five children discontinued participation due to a lack of confidence in the efficacy of the treatment.
4. Discussion
Challenges in patient compliance with SCIT arise from factors such as the prolonged treatment duration, elevated costs, potential adverse reactions and uncertainties regarding the prevention of new sensitization [Citation38]. Particularly noteworthy is the impact of the COVID-19 epidemic, where patients faced hindrances in adhering to the subcutaneous injection regimen due to infections or isolation, leading to substantial interruptions in treatment [Citation39]. Delayed injection is the most common condition in which SCIT requires dose adjustment and other conditions include adverse reactions. According to the American Academy of Allergy, Asthma and Immunology survey, 84.2% of allergy physicians make dose adjustments based on the interval after a patient delays treatment [Citation40]. After delayed treatment, it is necessary to actively communicate with the patient to understand the reasons for the delay, which can effectively avoid the subsequent treatment of the patient.
Although many scholars in this field have been studying AIT dose adjustment, there is still a lack of evidence-based dose adjustment protocols in the world. When the interval between treatments is longer, physicians often repeat or lower the dose based on their own experience. The European Institute of Allergy and Clinical Immunology has proposed that patients who have discontinued maintenance injections for more than 16 weeks should restart treatment [Citation41]. The American Academy of Allergy, Asthma and Immunology proposed that in the dose accumulation phase, the injection delay time exceeds 28 days, the original injection dose needs to be reduced by 50% and the adjustment of the maintenance phase dose needs to refer to the accumulation phase dose adjustment scheme [Citation40]. A survey involving 327 SCIT patients revealed that 31.8% dropped out during the pandemic due to delayed injections [Citation42]. Another study identified the additional time and costs associated with the conventional dose-adjustment protocol after delayed treatment as a significant contributor to patient dropout from SCIT [Citation43]. These findings underscore the importance of developing a scientifically sound and easily acceptable dose-adjustment protocol for SCIT in real-world clinical settings.
A report assessing safety and adherence to venom immunotherapy during the COVID-19 pandemic indicated that satisfactory therapeutic outcomes could be achieved without SRs even when injections were delayed for an extended period [Citation44]. Moreover, the maintenance phase could be directly resumed without restarting the build-up phase. Consequently, for children experiencing an interruption of more than 8 weeks in their maintenance phase in this study, the dose-adjustment protocol facilitated the direct resumption of the maintenance phase without reinitiating the build-up phase. Limited literature exists on the use of mite allergen injections for treating SCIT interruptions lasting over 8 weeks, especially concerning the efficacy and safety of dose-adjustment regimens in real-world scenarios for children aged 5–18 years.
The novel dose-adjustment protocol implemented in this study represents a pioneering effort. Nearly 200 children receiving mite allergen injections for SCIT, who had experienced an interruption of more than 8 weeks during the maintenance phase, underwent adjustments and successfully completed a 3-year follow-up during treatment. The outcomes of this study offer valuable insights into the efficacy and safety of dose-adjustment in children undergoing SCIT.
As the allergic reaction progresses, the prevalence of various concomitant diseases changes accordingly, so the direct cost of AR medical treatment is not only the direct cost, but also the further economic burden of conditions such as asthma and sinusitis. This study demonstrates that the efficacy of the novel dose-adjustment protocol is on par with that of the conventional approach. Additionally, the novel protocol presents cost savings by obviating the need to repurchase the build-up phase reagent kit and reducing the time required to recommence the injection of the build-up phase. Aytekin et al. reported a 28.7% dropout rate for SCIT in children during the COVID-19 pandemic, primarily attributed to fear of infection [Citation45]. In contrast, the dropout rate for the novel group in our study was 5.15%, slightly higher than pre-pandemic levels but significantly lower than the reported rate. Possible reasons for this discrepancy include the fact that our study focused on children, whose health is highly prioritized by Chinese parents. Additionally, effective epidemic prevention strategies in Changzhou played a crucial role in containing the outbreak [Citation46]. During the COVID-19 containment period, our team actively organized telephone follow-ups and online questionnaires, maintaining close attention to the physical and psychological well-being of children and their parents [Citation47]. Furthermore, it was demonstrated that a well-designed dose adjustment was beneficial in reducing the dropout rate of SCIT.
No significant differences were observed in TNSS, MS and CSMS between children who completed 3 years of treatment in the novel group and those in the conventional group. This suggests that re-maintenance treatment after a prolonged interruption does not adversely affect the overall efficacy of SCIT. Therefore, when the treatment interval is extended, active encouragement of patient visits and prompt adjustments and counseling are recommended to prevent unnecessary wastage of time, costs and potential dropout.
Considering the unfamiliarity of the novel protocol, it was imperative to assess and ensure the safety of children. Four measures were implemented to enhance the security of the novel protocol [Citation48]. First, to prevent severe SRs, the novel regimen excluded patients with asthma and those with a history of severe SRs [Citation49]. Second, all children were required to sit for 30 min before each injection and remain for at least 30 min after injection to monitor adverse reactions and ensure prompt treatment [Citation50]. Third, for children with prior SRs, subsequent injections were recommended to administer half a dose separately in the left and right upper arms. Fourth, histamine is the core inflammatory mediator in the pathogenesis of AR. Antihistamines can relieve sneezing, runny nose and itchy nose symptoms of AR by competitively binding histamine H1 receptors. Therefore, loratadine (tablet) and budesonide (nasal spray) were designated as rescue drugs for adverse reactions post-injection [Citation51].
While our study confirmed the efficacy and safety of the novel dose-adjustment regimen in pediatric patients undergoing SCIT after discontinuation for more than 8 weeks, it is essential to acknowledge the retrospective and nonrandomized nature of the study, potentially introducing bias. Furthermore, future investigations should delve into dose adjustment programs tailored for different populations, especially those with asthma, a history of serious SRs and other underlying diseases. Caution should be exercised when restarting treatment in such cases. Additionally, children undergoing continuous treatment for over 104 weeks, discontinued for more than 8 weeks, were not included in this study, warranting attention to their long-term efficacy in future research. Hence, prospective and randomized studies with larger sample sizes and diverse research populations are recommended to validate these findings. We have initiated follow-up records of TNSS, MS and CSMS at 6 months, 1 year, 2 years and 3 years after completion of SCIT in children to further evaluate the long-term benefits of the two different protocols.
5. Conclusion
This study demonstrates that a novel dose-adjustment protocol in children with AR undergoing SCIT after discontinuation of the maintenance phase for more than 8 weeks is equally effective as the conventional approach. The novel protocol emerges as a safe alternative, offering advantages such as time and cost savings and a reduced dropout rate.
Author contributions
Both authors participated in the writing and revision of the manuscript and approved the final draft. JRX was responsible for the analysis and interpretation of data.
Financial disclosure
This work is funded by The Joint Project of “Changzhou Medical Center” of Nanjing Medical University, No. CMCC202208 & “Medical Star” Youth Science and Technology Talent Promotion Project of Changzhou, CZSLCYX-202203. The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study received approval from the Ethics Committee of the Third People's Hospital of Changzhou (02A-A20200006). All experiments were conducted in adherence to relevant guidelines and regulations of the Ethics Committee of the Third People's Hospital of Changzhou. Written informed consent was obtained from subjects or their legal representatives.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Additional information
Funding
References
- Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi:10.1038/s41572-020-00227-0
- Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020;13(2):100080. doi:10.1016/j.waojou.2019.100080
- Aït-Khaled N, Pearce N, Anderson HR, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64(1):123–148. doi:10.1111/j.1398-9995.2008.01884.x
- Wheatley LM, Togias A. Clinical practice. Allergic Rhinitis. N Engl J Med. 2015;372(5):456–463. doi:10.1056/NEJMcp1412282
- Xi L, Cao F, Zhang Y, et al. Severity of nasal obstruction can predict the anxiety status of patients with allergic rhinitis but not patients with vasomotor rhinitis. Int Forum Allergy Rhinol. 2016;6(11):1196–1203. doi:10.1002/alr.21802
- Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(Suppl. 1):S1–S43. doi:10.1177/0194599814561600
- Bakhshaee M, Ashtiani SJ, Hossainzadeh M, et al. Allergic rhinitis and dental caries in preschool children. Dent Res J (Isfahan). 2017;14(6):376–381. doi:10.4103/1735-3327.218560
- Chen H, Meng X, Yu Y, et al. Greenness and its composition and configuration in association with allergic rhinitis in preschool children. Environ Res. 2024;251(Pt 2):118627. doi:10.1016/j.envres.2024.118627
- Rajapakse S, Amarasiri L, Yasaratne D, et al. Temporal variation and factors associated with allergic rhinitis in a cohort of rural preschool children from Sri Lanka. J Trop Pediatr. 2022;68(2):fmac017. doi:10.1093/tropej/fmac017
- Montefort S, Ellul P, Montefort M, et al. A decrease in the prevalence and improved control of allergic conditions in 13- to 15-yr-old Maltese children (ISAAC). Pediatr Allergy Immunol. 2011;22(1 Pt 2):e107–111. doi:10.1111/j.1399-3038.2010.01058.x
- Montefort S, Muscat HA, Caruana S, et al. Allergic conditions in 5-8-year-old Maltese schoolchildren: prevalence, severity and associated risk factors [ISAAC]. Pediatr Allergy Immunol. 2002;13(2):98–104. doi:10.1034/j.1399-3038.2002.00063.x
- Zhang YM, Zhang J, Liu SL, et al. Prevalence and associated risk factors of allergic rhinitis in preschool children in Beijing. Laryngoscope. 2013;123(1):28–35. doi:10.1002/lary.23573
- Chen Y, Zhu J, Lyu J, et al. Association of maternal prepregnancy weight and gestational weight gain with children's allergic diseases. JAMA Netw Open. 2020;3(9):e2015643. doi:10.1001/jamanetworkopen.2020.15643
- Gowthaman U, Chen JS, Eisenbarth SC. Regulation of IgE by T follicular helper cells. J Leukoc Biol. 2020;107(3):409–418. doi:10.1002/JLB.3RI1219-425R
- Han X, Krempski JW, Nadeau K. Advances and novel developments in mechanisms of allergic inflammation. Allergy. 2020;75(12):3100–3111. doi:10.1111/all.14632
- Devillier P, Bousquet PJ, Grassin-Delyle S, et al. Comparison of outcome measures in allergic rhinitis in children, adolescents and adults. Pediatr Allergy Immunol. 2016;27(4):375–381. doi:10.1111/pai.12561
- Schuler Iv CF, Montejo JM. Allergic rhinitis in children and adolescents. Immunol Allergy Clin North Am. 2021;41(4):613–625. doi:10.1016/j.iac.2021.07.010
- Brożek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
- Huang Y, Wang C, Wang X, et al. Efficacy and safety of subcutaneous immunotherapy with house dust mite for allergic rhinitis: a meta-analysis of randomized controlled trials. Allergy. 2019;74(1):189–192. doi:10.1111/all.13583
- Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi:10.1111/all.13317
- Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. 2019;74(5):855–873. doi:10.1111/all.13749
- Halken S, Larenas-Linnemann D, Roberts G, et al. EAACI guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28(8):728–745. doi:10.1111/pai.12807
- Vogelberg C, Klimek L, Kruppert S, et al. Long-term effects of pollen allergoid tyrosine-adsorbed subcutaneous immunotherapy on allergic rhinitis and asthma. Clin Exp Allergy. 2024;54(4):253–264. doi:10.1111/cea.14444
- Jutel M, Bruggenjurgen B, Richter H, et al. Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthma. Allergy. 2020;75(8):2050–2058. doi:10.1111/all.14240
- Wang C, Bao Y, Chen J, et al. Chinese guideline on allergen immunotherapy for allergic rhinitis: the 2022 update. Allergy Asthma Immunol Res. 2022;14(6):604–652. doi:10.4168/aair.2022.14.6.604
- Feng M, Zeng X, Li J. House dust mite subcutaneous immunotherapy in Chinese patients with allergic asthma and rhinitis. J Thorac Dis. 2019;11(8):3616–3625. doi:10.21037/jtd.2019.06.35
- Eguiluz-Gracia I, Parkin RV, Layhadi JA, et al. Nasal allergen-neutralizing antibodies correlate closely with tolerated intranasal allergen challenge dose following grass pollen subcutaneous immunotherapy in patients with local allergic rhinitis. Allergy. 2024. doi:10.1111/all.16083
- Huang HH, Xu C, Liu L, et al. [Efficacy comparison and safety analysis of subcutaneous specific immunotherapy with standardized house dust mite allergen in patients with single and multiple allergic rhinitis]. Zhonghua Yu Fang Yi Xue Za Zhi. 2022;56(6):774–783. doi:10.3760/cma.j.cn112150-20220120-00071
- Zhang Y, Zhang L. Management practice of allergic rhinitis in China during the COVID-19 pandemic. Allergy Asthma Immunol Res. 2020;12(4):738–742. doi:10.4168/aair.2020.12.4.738
- Wang C, Bao Y, Chen J, et al. Chinese guideline on allergen immunotherapy for allergic rhinitis: the 2022 update. Allergy Asthma Immunol Res. 2022;14(6):604–652. doi:10.4168/aair.2022.14.6.604
- Alvarez-Cuesta E, Bousquet J, Canonica GW, et al. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl. 82):1–20. doi:10.1111/j.1398-9995.2006.01219_1.x
- Villalobos Violan V, Gandolfo Cano MDM, Vicente EM, et al. Influence of the COVID-19 pandemic on the prescription and adherence to allergen-specific immunotherapy. Clin Exp Allergy. 2022;52(7):916–917. doi:10.1111/cea.14115
- Kim JY, Jang MJ, Kim DY, et al. Efficacy of subcutaneous and sublingual immunotherapy for house dust mite allergy: a network meta-analysis-based comparison. J Allergy Clin Immunol Pract. 2021;9(12):4450–4458 e6. doi:10.1016/j.jaip.2021.08.018
- Liu W, Zeng Q, He C, et al. Compliance, efficacy and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr Allergy Immunol. 2021;32(1):86–91. doi:10.1111/pai.13332
- Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy. 2014;69(7):854–867. doi:10.1111/all.12383
- Zhu W, Gao P, Zhang Q, et al. Efficacy and safety of subcutaneous immunotherapy for local allergic rhinitis: a meta-analysis of randomized controlled trials. Am J Rhinol Allergy. 2022;36(2):245–252. doi:10.1177/19458924211050547
- Cox L, Larenas-Linnemann D, Lockey RF, et al. Speaking the same language: the World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125(3):569–574; 574.e1–574.e7. doi:10.1016/j.jaci.2009.10.060
- Lao-Araya M, Sompornrattanaphan M, Kanjanawasee D, et al. Allergen immunotherapy for respiratory allergies in clinical practice: a comprehensive review. Asian Pac J Allergy Immunol. 2022;40(4):283–294. doi:10.12932/AP-260722-1418
- Aarestrup FM, Taketomi EA, Santos Galvao CE, et al. Good clinical practice recommendations in allergen immunotherapy: position paper of the Brazilian Association of Allergy and Immunology - ASBAI. World Allergy Organ J. 2022;15(10):100697. doi:10.1016/j.waojou.2022.100697
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(Suppl. 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Alvarez-Cuesta E, Bousquet J, Canonica GW, et al. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl. 82):1–20. doi:10.1111/j.1398-9995.2006.01219_1.x
- Yegit OO, Demir S, Unal D, et al. Adherence to subcutaneous immunotherapy with aeroallergens in real-life practice during the COVID-19 pandemic. Allergy. 2022;77(1):197–206. doi:10.1111/all.14876
- Koca Kalkan I, Ates H, Aksu K, et al. Real-life adherence to subcutaneous immunotherapy: what has changed in the era of the COVID-19 pandemic. World Allergy Organ J. 2021;14(7):100558. doi:10.1016/j.waojou.2021.100558
- Bilo MB, Braschi MC, Piga MA, et al. Safety and adherence to venom immunotherapy during COVID-19 pandemic. J Allergy Clin Immunol Pract. 2021;9(2):702–708. doi:10.1016/j.jaip.2020.11.030
- Aytekin ES, Soyer O, Sekerel BE, et al. Subcutaneous allergen immunotherapy in children: real life compliance and effect of COVID-19 pandemic on compliance. Int Arch Allergy Immunol. 2021;182(7):631–636. doi:10.1159/000514587
- Cheng X, Hu J, Luo L, et al. Impact of interventions on the incidence of natural focal diseases during the outbreak of COVID-19 in Jiangsu Province, China. Parasit Vectors. 2021;14(1):483. doi:10.1186/s13071-021-04986-x
- Zhou S, Liu Y, Xue J, et al. Sustained impact of subcutaneous immunotherapy among patients with allergic rhinitis who experienced treatment delay due to the COVID-19 pandemic: a multicenter, two-arm, real-world study. Clin Transl Allergy. 2022;12(3):e12122. doi:10.1002/clt2.12122
- Zhang Y, Lan F, Zhang L. Advances and highlights in allergic rhinitis. Allergy. 2021;76(11):3383–3389. doi:10.1111/all.15044
- Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61(7):855–859. doi:10.1111/j.1398-9995.2006.01068.x
- Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41(9):1177–1200. doi:10.1111/j.1365-2222.2011.03794.x
- Cheng L, Chen J, Fu Q, et al. Chinese society of allergy guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2018;10(4):300–353. doi:10.4168/aair.2018.10.4.300
