Abstract
Geographic atrophy (GA) remains a leading cause of central vision loss with no known cure. Until recently, there were no approved treatments for GA, often resulting in poor quality of life for affected patients. GA is characterized by atrophic lesions on the retina that may eventually threaten the fovea. Emerging treatments have demonstrated the ability to reduce the rate of lesion growth, potentially preserving visual function. Avacincaptad pegol (ACP; Astellas Pharma Inc), a complement component 5 inhibitor, is an FDA-approved treatment for GA that has been evaluated in numerous clinical trials. Here we review the current clinical trial landscape of ACP, including critical post hoc analyses that suggest ACP may reduce the risk of severe loss among patients with GA.
Plain language summary
Geographic atrophy (GA) is an advanced form of eye disease age-related macular degeneration. In people with GA, light-sensitive cells at the back of the eye (the retina) start to die, forming lesions. GA lesions usually get bigger over time and can lead to blindness. New medicines are being studied that work by slowing the growth of GA lesions. Avacincaptad pegol (ACP) is one medicine that acts on the immune system and is designed to block the C5 protein, helping stop the immune system from attacking cells in the retina. Based on clinical studies, ACP was shown to slow the growth of GA over time and has been approved by the FDA. This review article summarizes research on ACP.
The complement system: a target for geographic atrophy therapeutics
Geographic atrophy (GA) remains a leading cause of vision loss.
The complement system has been implicated in GA pathophysiology and is an attractive target for GA therapeutics.
Targeting a terminal point of the complement cascade may preserve critical anti-inflammatory and host defense mechanisms upstream in the complement cascade.
Overview & mechanism of action
Avacincaptad pegol (ACP) is a pegylated ribonucleic acid aptamer that binds to C5, inhibiting its cleavage to C5a (the proinflammatory anaphylatoxin) and C5b (the initiating subunit of the membrane attack complex.
Early-phase clinical studies
To date, ACP is the only treatment for GA that has met its primary efficacy end point in two Phase 3 clinical trials.
Phase 3 Studies: GATHER1 & GATHER2
The primary efficacy end point for GATHER1 and GATHER2 was the mean rate of growth in the GA area with ACP treatment compared with sham.
GATHER1 & GATHER2 efficacy outcomes
ACP reduced the rate of GA lesion growth versus sham by as much as 32% over 18 months.
GATHER1 & GATHER2 safety outcomes through 12 Months
ACP has shown an acceptable safety profile. The most common adverse reactions were conjunctival hemorrhage, increased intraocular pressure, and choroidal neovascularization, with a low incidence of intraocular inflammation, endophthalmitis and ischemic optic neuropathy.
Post hoc analysis of vision loss risk reduction
Data from post hoc analysis suggest that ACP may reduce the risk of persistent vision loss (defined as loss of ≥15 ETDRS letters over 2 consecutive monthly visits) at 12 months.
Conclusion
Results from safety and efficacy studies evaluating ACP as a treatment option for patients with GA showed a statistically significant reduction in the rate of progression of GA and a consistent safety profile.
1. Background
Geographic atrophy (GA) secondary to age-related macular degeneration (AMD) continues to be the leading cause of severe vision loss in individuals over 55 years of age, with a global prevalence of 8.7% [Citation1,Citation2]. Although approximately 1.6 million patients are estimated to have GA in the United States, GA may be severely underdiagnosed due to a lack of knowledge surrounding the disease and its progression [Citation3].
AMD is a progressive retinal disease that can lead to the development of “wet,” or neovascular AMD (nAMD), GA, or both. The dry form of AMD is characterized by the deposition of extracellular waste products composed of lipids and proteinaceous debris, referred to as drusen, onto the retina. Drusen accumulation may lead to degeneration of photoreceptors and the retinal pigment epithelium (RPE), which may eventually result in atrophy of retinal tissue during advanced stages of the disease.
Clinicians commonly classify the severity of AMD via the size of the drusen deposits and the presence of pigmentary changes. Small drusen deposits of diameter <63 μm, or “drupelets,” are considered normal ocular characteristics of aging that do not correlate with AMD [Citation4]. Medium drusen between 63 and 125 μm in diameter with no pigmentary changes signify early AMD, while large drusen of more than 125 μm and/or any AMD pigmentary abnormalities signify intermediate AMD [Citation4]. GA, the advanced form of the disease, is characterized by loss of photoreceptors, RPE, and choriocapillaris, while nAMD is characterized by neovascularization of choroid under the retina [Citation1]. nAMD and GA both represent advanced, late-stage forms of AMD.
AMD is a complex, multifactorial disease where the interplay between modifiable and nonmodifiable environmental and genetic risk factors determines the onset and severity of the disease. Key modifiable risk factors include smoking, diet and physical activity, while the chief nonmodifiable factor is age. While the age of AMD onset varies, it generally manifests in individuals >55 years of age. The presence of GA is observed in 3.5% of individuals ≥75 years of age, with prevalence increasing exponentially with age to approximately 22% of the population over 90 years of age in the United States [Citation5].
GA may greatly affect a person's quality of life; lesion genesis and growth, even when outside the foveal region of the macula, can be detrimental to vision. When lesions encompass the fovea, there may be irreversible central vision loss, leading to extreme difficulties in important daily activities such as reading, driving and seeing in low-light environments. Although GA progression is variable based on lesion location, characteristics, size and other factors, slowing the progression of the disease from the earlier stages is paramount to preserving VA and quality of life of the patient. Standard-of-care therapeutics for other ophthalmologic diseases such as glaucoma also aim to slow disease progression but do not reverse vision loss.
Currently, there is no cure for either dry AMD or GA, and treatment options are limited. It is recommended that patients with intermediate AMD in one eye take supplements, demonstrated to potentially slow disease progression in the fellow eye, according to the Age-Related Eye Disease Study 2 (AREDS 2). These supplements include a combination of vitamins C and E, copper, zinc, lutein, and zeaxanthin [Citation6]. It is also recommended that patients cease smoking if appropriate, increase the nutritional value of their diet, and exercise regularly.
Until recently, no approved treatments have been available for GA. Pegcetacoplan (Apellis Pharmaceuticals, Waltham, MA, USA), a complement C3 inhibitor, received approval from the US Food and Drug Administration (FDA) in February 2023, becoming the first approved treatment for the disease in the United States. Despite this, treatment options for GA remain limited and there is still a large, unmet need for GA therapeutics. New and emerging treatments may further help reduce the rate of lesion growth, preserve photoreceptors and slow the loss of VA. Additionally, a deeper understanding of these emerging treatments reinforces the importance of treating the disease as early as possible to benefit a broader range of patients. One such treatment, avacincaptad pegol (APC), a C5 inhibitor for the treatment of GA, was approved by the FDA in August 2023. The following is a review of the current clinical trial landscape of ACP, including critical post hoc analyses that suggest ACP may reduce the risk of severe loss among patients with GA.
1.1. The complement system: a target for geographic atrophy therapeutics
AMD development and progression to GA have been linked to several biological pathways that have become targets for therapeutic development. Genome-wide association studies have linked genetic polymorphisms from the function of the complement system to AMD and GA [Citation7]. Immunohistochemical studies and in vitro studies also corroborate the role of the complement system in the development and progression of GA.
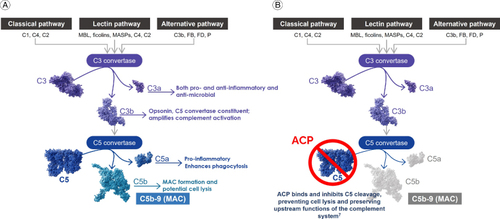
The complement system, which activates an enzymatic cascade within the innate immune response system, has been the most explored GA target to date. The innate immune response provides immediate but nonspecific defense against foreign entities within the body and is typically the first line of immunological response against infections or internal injuries. Multiple complex networks comprise the complete innate immune response, within which the complement system plays an integral role against pathogen recognition and elimination [Citation8]. The complement cascade can be activated via three separate signaling pathways: classical, lectin and alternative, but all ultimately lead to the formation of the MAC [Citation9,Citation10]. The classical pathway is activated through antigen-antibody complexes and the lectin pathway through lectin binding to polysaccharides on damaged cells [Citation10]. Distinctly, the alternative pathway is spontaneously activated by hydrolysis of thioester bonds in C3 and is constitutively active through an amplification loop of classical and lectin pathway activity [Citation10]. Despite the different sources of activation, the three pathways converge with the cleavage of complement protein C3 into C3a and C3b [Citation11]. Component C3a is a peptide anaphylatoxin that activates inflammatory cells and has highly potent antimicrobial properties [Citation10,Citation12]. C3b is an opsonin that labels targets for phagocytosis and helps amplify complement activation via the alternative pathway [Citation10,Citation13]. C3b binding to C3 convertase leads to the formation of C5 convertase and further activation of the complement system.
Complement C5 convertase functions at the terminal point of the complement cascade, cleaving the C5 protein into C5a and C5b components. Like C3a, C5a is an anaphylatoxin that attracts and activates inflammatory cells and enhances phagocytosis [Citation10,Citation12]. These inflammasomes further activate proinflammatory cytokine responses. C5b binds to complement components C6, C7, C8 and multiple C9 proteins, forming the MAC (C5b-9). The complement cascade culminates with the insertion of the MAC into the cell membrane, causing membrane lysis that may eventually lead to cell death [Citation14].
Many studies have shown a link between the various components of the complement system and the development and progression of retinal diseases, including AMD and GA, uveoretinitis, diabetic retinopathy and glaucoma [Citation15]. Increases in the accumulation of MAC, inflammatory cytokines and chemokines drive the complement system out of equilibrium in an otherwise normal retina. Aging typically increases the upregulation of genes associated with the complement system. Increases in complement factors H, B and D, along with C1, C3 and C5, have demonstrated roles in the development of GA [Citation16,Citation17]. Importantly, studies have shown that dysregulation of the complement system leads to the formation of drusen, and thus AMD with potential progression to GA [Citation18].
Despite the complexities of the complement system, it has become an attractive target for therapeutics for GA treatment. Inhibition of overactive complement components has been shown to slow GA lesion progression, with C3 and C5 showing the most promise as GA therapeutics [Citation19,Citation20].
2. Avacincaptad pegol: a C5 inhibitor for GA treatment
2.1. Overview & mechanism of action
Inhibition of C3 or C5 as core components of all three pathways has proven promising in clinical studies, suggesting an approach that targets components of all three pathways may be needed. Specifically, inhibition within a single pathway (e.g. C1q, which is the initiating molecule of the classical pathway) may not provide sufficient attenuation of the complement cascade [Citation21–23]. Inhibition of the complement cascade at C3 may lead to the loss of the anti-inflammatory and anti-infective benefits of C3, proteolytic products leading to an increased risk for infection [Citation24]. Alternatively, targeting the complement system with a terminal C5 inhibitor has also shown promise in treating GA by slowing lesion growth [Citation25]. In addition, blocking C5 specifically can preserve the host defense mechanisms upstream in the complement pathway, potentially preserving anti-inflammatory functions of C3a that may be important for phagocytosis and modulation of inflammation, while blocking the recruitment of inflammasomes and formation of MAC mediated by C5a and C5b [Citation13,Citation26].
ACP (Astellas Pharma Inc., Tokyo, Japan) is a pegylated ribonucleic acid aptamer that binds C5, inhibiting its cleavage into C5a and C5b () [Citation25]. ACP is a chemically synthesized, single-stranded oligonucleotide that demonstrates high binding affinity and specificity for C5. The pegylation of the molecule provides stability against biodegradation and delays the clearance of the drug [Citation27]. By targeting and inhibiting the terminal component of the complement cascade, ACP potentially preserves the early components of complement activation that are essential for the opsonization of microorganisms and clearance of immune complexes. Inhibition of the formation of C5a reduces phagocytosis by neutrophils while retaining anti-inflammatory functions within the pathway [Citation28]. More importantly, inhibition of the formation of C5b prevents initiation of MAC formation. As excess MAC accumulation within high-risk RPE cells may overload lysosomes and stimulate the release of pro-inflammatory factors, prevention of MAC formation has shown potential benefits in reducing these risks and preserving important anti-inflammatory functions [Citation29,Citation30]. Taken together, the inhibition of C5 potentially slows the progression of host cell degeneration within photoreceptors, RPE and choriocapillaris.
2.2. Early phase clinical studies
The safety, tolerability and pharmacokinetic profile of ACP (known as ARC1905 at the time of the study) was first evaluated in an uncontrolled, ascending dose and parallel group, open-label phase 1 study (NCT00709527) [Citation31]. In this study, ACP was administered as combination therapy with ranibizumab 0.5 mg/eye in 60 subjects with subfoveal choroidal neovascularization (CNV) secondary to AMD. Here, ACP was well tolerated with no dose-limiting toxicity and most reported adverse events were related to the injection procedure [Citation32]. ACP was further evaluated in a randomized, parallel-assigned open-label phase 2a trial to assess the safety of intravitreal administration in combination with ranibizumab in 64 treatment-naive patients with nAMD (NCT03362190) [Citation33]. Again, the treatment was well tolerated and the most commonly reported ocular treatment-emergent adverse events (TEAEs) in the study eye were related to the injection procedure. One subject was reported to have a retinal detachment, reported as not related to the study drug. In both studies, the treatment combination of ACP + ranibizumab in patients with wet AMD was well tolerated with no safety issues identified through measurement of visual acuity (VA) [Citation32,Citation33].
The safety and tolerability of ACP in patients exclusively with GA were first evaluated in an open-label, parallel-assigned phase 1/2 clinical trial (NCT00950638) [Citation34]. A total of 47 patients with GA in both eyes were randomized between two cohorts: ACP 0.3 mg and ACP 1 mg. Intravitreal injections of ACP were given at weeks 0, 4 and 8 with additional injections at weeks 24 and 36. Patients were followed up at weeks 16 and 48 to assess various safety metrics, including VA and changes in IOP. In this study, ACP was well tolerated with no associated adverse events [Citation32].
The safety and efficacy of ACP indicated for GA have been further evaluated in two phase 3, randomized, double-masked, sham-controlled studies (GATHER1: NCT02686658 and GATHER2: NCT04435366) [Citation35,Citation36]. ACP is the first investigational therapy for GA that achieved the 12-month prespecified primary objective in two phase 3 pivotal trials [Citation22,Citation25]. Overall, ACP demonstrated high statistical significance in reducing the growth of GA lesions over 12 months, with a consistent and acceptable safety profile.
2.2.1. Phase 3 studies: GATHER1 & GATHER2
In GATHER1, a total of 286 subjects were studied during 2 parts of the trial [Citation25]. In part 1, subjects were randomized 1:1:1 to receive either ACP 1 mg (n = 26), ACP 2 mg (n = 25) or sham (n = 26). During part 2, subjects were randomized 1:2:2 to receive ACP 2 mg (n = 42), ACP 4 mg (n = 83) or sham (n = 84). During both parts of GATHER1, patients were treated monthly with 100 μL injections of either ACP or sham.
In GATHER2, a total of 448 subjects were randomized 1:1 to receive either ACP 2 mg (n = 225) or sham (n = 223; 1 subject did not receive treatment after randomization) [Citation22]. In both GATHER1 and GATHER2, treatment was administered as a 100 μL intravitreal injection every month for 12 months and lesion size was measured via fundus autofluorescence (FAF) at baseline, month 6 and month 12. In GATHER1, monthly treatment continued to month 18. In GATHER2, treatment subjects were rerandomized 1:1 after month 12 to receive either monthly or every-other-month injections up to month 24.
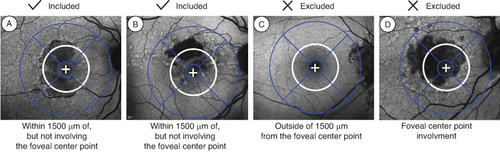
For both studies, key inclusion and exclusion criteria were the same. Subjects had to be ≥50 years of age with best-corrected visual acuity (BCVA) between 20/25 and 20/320 [Citation22,Citation25]. The GA lesion of the subject eye had to be non-center point involving and in part within 1500 μm from the foveal center, with a total area between 2.5 and 17.5 mm2 (1 and 7 disc areas [DAs]) (). In the case of multifocal lesions, at least 1 lesion had to be ≥1.25 mm2 (0.5 DA). Patients were excluded if they had evidence of CNV in either eye at baseline, GA secondary to any condition other than AMD in either eye, any prior treatment for AMD (except vitamin or mineral supplements) or any prior intravitreal treatment for any indication in either eye, any ocular condition in the study eye that could progress during the study and potentially affect central vision or otherwise act as a confounding factor, or any sign of diabetic retinopathy in either eye.
Figure 2. GATHER clinical program baseline lesion inclusion and exclusion criteria: (A) and (B) examples of lesions within 1500 microns of, but not involving, the foveal center point. (C) Outside of 1500 microns from the foveal center point. (D) Involving the foveal centerpoint.
The primary efficacy end point in GATHER1 and GATHER2 was the GA area measured by FAF at three timepoints: baseline, month 6 and month 12 [Citation22,Citation25]. The mean rate of growth in the GA area, as measured by FAF, is reflective of photoreceptor preservation, which is required for functional vision.
2.2.2. GATHER1 efficacy outcomes
In the GATHER1 trial, the primary objective was met with high statistical significance [Citation25]. Compared with the corresponding sham group, ACP 2 mg resulted in an absolute difference of 0.110 mm (95% CI: 0.030–0.190; p = 0.0072), representing a 27.4% reduction in mean change in GA area over 12 months (square root transformed), as measured by fundus autofluorescence. The analysis using observed values (non-square root transformed, representative of what is familiar in clinical practice) for this analysis shows an absolute difference in mean GA growth of 0.697 mm2, which correlates to a reduction of 30.5% when compared with its corresponding sham-control cohort (A).
Figure 3. GATHER1 efficacy outcomes for ACP 2-mg cohort compared with sham. Mean change in observed geographic atrophy (GA) area from baseline (A) through 12 months and (B) through 18 months. Least squares (LS) means based on estimates from a mixed-effects repeated measures (MMRM) model on available intention-to-treat (ITT) populations, up to Month 12 data for A and up to 18 month for B.
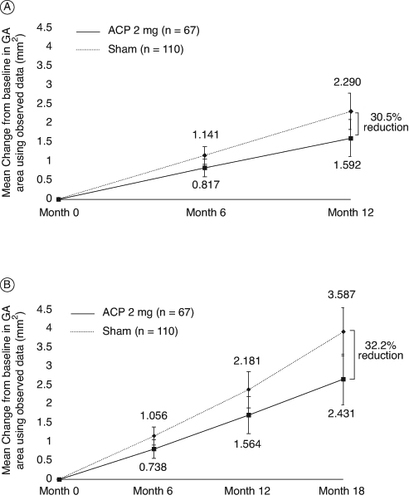
Monthly ACP treatment continued to show reductions in the progression of GA growth over 18 months in the ACP 2-mg cohort when compared with its respective sham cohort [Citation23]. Month 18 analyses are descriptive. In total, 201 subjects completed the entire treatment period of the study. Analysis using observed, non–square root transformation also showed comparable reductions in GA lesion growth rates in the ACP 2-mg treatment arm, with a 32.2% (1.156 mm2; 95% CI: 0.480–1.833) reduction when compared with its respective sham cohort (B) [Citation23]. These data are consistent with those observed during the GATHER1 12-month analysis, showing further reduction in lesion growth in ACP-treated patients compared with sham, and further supporting the potential of ACP treatment to reduce GA lesion growth.
2.2.3. GATHER2 efficacy outcomes
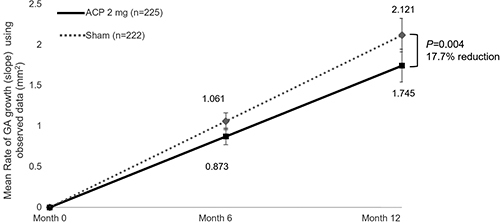
As with GATHER1, GATHER2 met its prespecified primary objection of a reduction in GA area growth at 12 months, the first time a complement inhibitor treatment for GA has met the prespecified primary end point in two phase 3 pivotal trials [Citation22]. The mean rate of square root-transformed GA area growth (slope) was 0.336 mm/year with ACP and 0.392 mm/year with sham, a statistically significant difference of 0.056 mm/year (95% CI: 0.016–0.096; p = 0.006). To demonstrate consistency in efficacy, the mean rate of GA growth via slope analysis of observed data was also performed. Similar results were seen from the analysis of observed data, with a difference of 0.376 mm2/year (95% CI: 0.122–0.631; p = 0.004), representing a 17.7% reduction when compared with sham (). In addition, subgroup slope analyses demonstrated that the mean rate of observed GA area growth was consistently lower for ACP than for sham for all patient demographics and baseline disease characteristics analyzed, including both male and female subgroups. The statistically significant GATHER2 results further demonstrate the potential for ACP 2-mg treatment to effectively slow GA lesion growth. Follow-up results for the 24-month time period will be reported upon completion of the study and subsequent analysis.
2.2.4. GATHER1 & GATHER2 safety outcomes through 12 months
In this primary safety evaluation of both pivotal phase 3 trials, we will present only the ACP 2-mg data from GATHER1 through 12 months in order to offer the most direct comparison between the two trials. Most subjects in both treatment and sham cohorts experienced a TEAE. Ocular TEAEs occurring in the study eye were higher in the ACP 2-mg treatment cohorts (GATHER1: 52.2 vs. 34.5% [sham]; GATHER2: 48.9 vs. 37.4% [sham]) [Citation22,Citation25]. A complete list of ocular TEAEs occurring in the study eye of ≥2% of patients for both GATHER1 and GATHER2 through 12 months is shown in . No serious ocular TEAEs were observed in either the treatment or sham groups in GATHER1 while two (0.9%) serious ocular TEAEs each were observed for the treatment and sham groups for GATHER2 over 12 months. No ocular TEAEs for either the treatment or sham groups led to study drug discontinuation in GATHER1 while two (0.9%) ocular TEAEs in the treatment group and none in the sham group led to study drug discontinuation in GATHER2 [Citation22,Citation25].
Table 1. TEAEs occurring ≥2% in the study eye during treatment with ACP 2 mg over 12 months in GATHER1 and GATHER2.
There were no events of intraocular inflammation, endophthalmitis, ischemic optic neuropathy, or occlusive vasculitis reported in the study eye in GATHER2 through 12 months [Citation22]. Similar results were observed for ACP 2 mg in GATHER1 with the exception of one event of intraocular inflammation at month 7 which was mild, transient, self-limiting, and deemed not related to the injection procedure or study drug by the investigator [Citation25]. The most common adverse reactions across both arms of the 2 trials were conjunctival hemorrhage, increased intraocular pressure (IOP), and CNV. Increased incidence of elevated IOP seen in GATHER trials were related to injection procedure and were anticipated with the 100 μL injection volume of ACP 2 mg [Citation22,Citation25]. Most events were transient, and mean IOP returned to near baseline levels at the next follow-up visit [Citation22,Citation25].
In both GATHER1 and GATHER2, the incidence of macular neovascularization (MNV) (inclusive of all types) was higher in the ACP 2-mg treatment arm than sham. For this analysis, the 2 MedDRA Preferred Terms (CNV and nAMD) were summed to present a complete representation of MNV conversion during the trials. Through 12 months in GATHER1, 6 (9.0%) cases of MNV conversion from the treatment cohort were reported, compared with 3 (2.7%) from the respective sham cohort [Citation25]. Through 12 months in GATHER2, 15 (6.7%) subjects from the treatment cohort developed MNV compared with 9 (4.1%) subjects from the corresponding sham cohort [Citation22].
2.2.5. Post hoc analysis of vision loss risk reduction
While both GATHER1 and GATHER2 examined supportive end points of mean change in BCVA and low-luminance BCVA (LL-BCVA) from baseline to month 12 (ETDRS letters), the trials were not designed to showcase a meaningful or significant difference in change of VA, as mean change in BCVA is not a sensitive end point in trials for GA treatments [Citation25]. Neither trial demonstrated significant mean changes from baseline in VA in the treatment arms when compared with their corresponding sham-control groups, which is expected within the clinical trial timeframe as patients may compensate through the use of remaining, viable retinal tissue. Moreover, unlike treatments for nAMD, patients may eventually have a substantial and irreversible decrease in VA once GA lesions involve the fovea. Post hoc analyses of vision loss were conducted for these trials to further quantify observed treatment effects [Citation37].
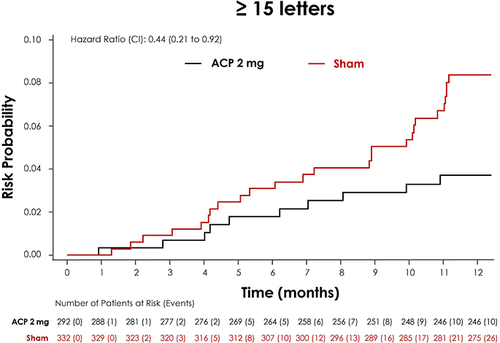
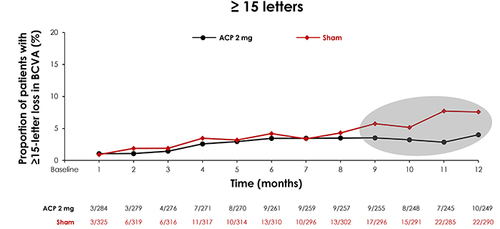
For the purposes of these analyses, GATHER1 and GATHER2 study populations were pooled, with baseline characteristics balanced between the groups: 292 subjects were in the ACP 2-mg treatment cohort and 332 subjects were in the sham cohort. Analyzing categorical change in BCVA to 12 months, a lower proportion of subjects treated with ACP 2 mg experienced BCVA letter losses of ≥15 letters from baseline when compared with sham. Overall, ≥15 letter losses were experienced by 4.0% of subjects receiving ACP 2 mg compared with 7.6% of subjects in the sham cohort at Month 12. Separation between the treatment and sham groups was evident at month 9 for ≥15 letter losses () [Citation37].
Figure 5. Post hoc analysis of the GATHER clinical program pooled data comparing proportion of patients with BCVA loss between ACP 2-mg and sham for ≥15 letter loss.
A time-to-event analysis of persistent vision loss of ≥15-BCVA letters over 12 months was also performed. Persistent vision loss was defined as occurring from baseline at 2 or more consecutive monthly visits. A similar analysis has previously been used to quantify treatment effects in early wet AMD clinical trials, demonstrating additional potential benefits of treatment [Citation38]. Results showed a 56% reduction in the relative risk of persistent vision loss of ≥15-BCVA ETDRS letters (hazard ratio: 0.44; 95% CI: 0.21–0.92) in ACP 2 mg-treated study eyes vs sham through 12 months () [Citation37].
3. Discussion
Inhibiting the complement cascade in the eye for GA treatment remains a compelling therapeutic approach. Targeting the complement cascade further downstream through inhibition of C5 preserves the immune defense mechanisms of upstream complement system effectors, while still suppressing the recruitment of inflammasomes and formation of MAC, which has been shown to play a key role in GA lesion progression [Citation39]. ACP, a pegylated ribonucleic acid aptamer that inhibits C5, has demonstrated efficacy in slowing GA lesion growth over time in two phase 3 trials [Citation22,Citation25]. In a post hoc analysis of pooled data from these 2 trials, ACP has also exhibited positive effects in reducing the risk of persistent vision loss at 12 months in patients with GA [Citation37].
Clinical trial evaluations of complement inhibitors for the treatment of GA, including DERBY, OAKS, GATHER1 and GATHER2, reveal an increased incidence of MNV conversion of the study eye during the trials. Hypotheses and speculations behind this observed increase have now been extensively documented [Citation22,Citation23,Citation40]. but ongoing analysis and evaluation of the trials and associated therapeutics continue to be conducted. In efforts to build a complete understanding of the effects of ACP on potential MNV conversion, a comprehensive MNV surveillance program has been initiated for the GATHER clinical program [Citation41]. In GATHER1, if a patient developed MNV in the study eye during treatment, the patient was withdrawn from the study. Conversely, in GATHER2, if the principal investigator suspected conversion to MNV in the study eye, a full imaging workup was triggered. This workup included color fundus photography, fluorescein angiography and optical coherence tomography (OCT), and was confirmed by an independent reading center within 1 h of submission. If the reading center confirmed the diagnosis, the patient continued receiving ACP in the trial, and the study eye was also treated with ranibizumab or aflibercept according to the country label. No patients in GATHER2 received anti-vascular endothelial growth factor (VEGF) therapy without an independent reading center confirmation of MNV conversion. All month 12 imaging was evaluated by the independent reading center regardless of suspicion by the investigator [Citation41].
Though further analysis remains to be conducted, current available knowledge suggests that thorough monitoring of patients receiving intravitreal complement inhibitor therapy for GA for MNV via OCT imaging should be a priority. The increased occurrence of new-onset MNV during clinical trials evaluating intravitreal complement inhibitors for the treatment of GA is interesting; however, it also provides further insight into the pathogenesis of GA and the function of the complement cascade therein [Citation40]. Continued in-depth analysis of MNV conversion during GA treatment, including exploration of risk factors (i.e., age, family history, smoking, and environmental factors, among others), baseline characteristics and fellow eye status, may provide a more complete picture of the relationship between these unique treatments, the complement system and GA progression.
As clinical trials concerning GA evolve, a key role of the ellipsoid zone (EZ) is emerging. Visualization of the EZ via OCT is thought to represent photoreceptor function and is used as an indicator of visual outcomes for many retinal diseases [Citation42]. Recent studies indicate that characteristics observed via OCT such as EZ integrity as well as subretinal pigment epithelium compartment features may offer critical insights into a prediction of GA development [Citation43]. Efforts are currently underway to characterize baseline EZ integrity features from the GATHER1 trial to further understand GA disease progression in the clinical trial setting. Additional analyses may evaluate the relationship between baseline measures of EZ integrity and the impact of ACP treatment on GA progression for various EZ profiles.
Quantifying visual preservation with GA treatment in the context of RPE cell loss is also an area that requires more thorough analyses. Healthy levels of RPE cell density vary as a function of location from the fovea, ranging from approximately 6000 ± 1500 cells/mm2 at the fovea to 3800 ± 1300 cells/mm2 3.5 mm nasally from the fovea [Citation44]. Additionally, the density of cone cells, the photoreceptors responsible for color vision and eye color sensitivity, can reach 324,000 cells/mm2 at the fovea [Citation45]. Together, these values suggest a richness of foveal cell density, and that observed reductions in GA lesion growth from complement inhibitor treatments may have different effects on RPE preservation, depending on lesion location. However, we acknowledge that accurate measurement and proper statistical analysis of photoreceptor and RPE cell health within treated and untreated patients is a complex and nuanced process. A complete understanding of the relationship between complement inhibition, reduction in GA progression, and preservation of viable cells clearly requires more in-depth study to truly assess the potential impact of treatment on visual outcomes. Although post hoc and exploratory, initial results from the risk reduction in vision loss analyses show promise in providing visual benefits for patients and establish an argument that various approaches to evaluating visual functions in patients with GA may be necessary.
4. Conclusion
It is undeniable that vision loss severely impacts a person's quality of life. In a poll of over 2000 US adults, 47% rated losing their vision as the worst possible health outcome, over loss of other functions such as hearing, memory, speech or even limbs [Citation46]. Additionally, elevated rates of depression and anxiety are observed among individuals with severe vision loss [Citation47].
As GA continues to be the leading cause of irreversible central vision loss, accounting for approximately 20% of all legal blindness in North America, it is evident that there is a substantial unmet need in therapeutics for GA treatment [Citation4]. Until recently, as therapeutics for GA were finally made available, identification and management of patients with GA was difficult. Lack of knowledge surrounding identification resulted in the underdiagnosis of both earlier stages of AMD and GA, which may severely impact patient prognosis [Citation48]. Additionally, the lack of treatment options to slow vision loss prevented clinicians from being able to actively manage the disease, which may result in feelings of hopelessness. However, emerging therapeutics may already be rapidly shifting the paradigm of GA treatment, reinforcing the need to improve education for eye care providers on early diagnosis. Prompt treatment will provide patients with the best possible chance to preserve their vision and the highest possible quality of life.
Targeting various stages of the complement system has revealed efficacious therapeutics for the treatment of GA. ACP, a novel C5 inhibitor, brings promise to patients with GA, demonstrating the potential to slow the progression of GA with an acceptable safety profile. By reducing the growth of GA lesions, ACP treatment may reduce the risk of severe vision loss over time. Taken together, evidence suggests that targeting the terminal, or culminating step, in the complement system through inhibition of C5 has the potential to provide a clinical benefit to patients with GA.
Author contributions
CJ Danzig: Conceptualization, writing-original draft preparation, writing-reviewing and editing (equal)
AM Khanani: Writing-reviewing and editing (equal)
A Loewenstein: Writing-reviewing and editing (equal).
Financial disclosure
CJ Danzig discloses consultancy to Adverum, Abbvie, Dutch Ophthalmic Research Center, Galimedix, Genentech, Inc., Iveric Bio, Kodiak Sciences, Novartis, Regeneron; and Regenxbio, has received grant support from Adverum, Alexion, Aviceda, Bayer, Cognition, Curacle, 4DMT, Genentech, Inc.,
Gyroscope, Iveric Bio, Kodiak Sciences, Novartis, Regeneron, Regenxbio, Rezolute, Roche and Unity, and has received speaker fees from Genentech, Inc. and Iveric Bio.
AM Khanani reports consultancy to AbbVie, Adverum, Alcon, Amgen, Annexin, Annexon, Apellis Pharmaceuticals, Aviceda Therapeutics, Beacon Therapeutics Clearside Biomedical, Complement Therapeutics, 4DMT, Exegenesis, EyePoint Pharmaceuticals, Fronterra Therapeutics, Genentech, Gyroscope Therapeutics, i-Lumen Scientific, Iveric Bio, Janssen Pharmaceuticals, Kodiak Sciences, Kriya Therapeutics, Nanoscope, Novartis, Ocular Therapeutix, Oculis, Ocuphire, OcuTerra, Olive BioPharma, Opthea, Oxular, Oxurion, Perfuse, Ray Therapeutics, Recens Medical, Regeneron Pharmaceuticals, Regenxbio, Revive, RevOpsis, Roche, Sanofi, Stealth BioTherapeutics, Thea Pharma, Unity Biotechnology, Vanotech and Vial
AM Khanani receives research support from Aviceda, Adverum, Alexion, Annexon, Apellis Pharmaceuticals, Aviceda Therapeutics, 4DMT, Eyepoint, Exegenesis, Genentech, Gyroscope Therapeutics, Iveric Bio, Janssen, Kodiak, Neurotech, Ocular Therapeutix, Oculis, OcuTerra, Opthea, Oxular, Oxurion, Regenxbio, Roche, Vanotech and Unity Biotechnology
AM Khanani has stock options in Aviceda Therapeutics, Oculis, Opthea, PolyPhotonix, Recens Medical, Perfuse, RevOpsis and Vial.
A Loewenstein reports consultancy to Allergan, Annexon, Bayer, Beyeonics, IQVIA, Iveric Bio, Johnson and Johnson, NotalVision, Novartis, Occuphire Pharma, Ocuterra, Ripple Therapeutics, Roche and Syneos.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editing assistance were provided by i2Vision, Inc. (San Diego, CA). The medical writing support was funded by Astellas Pharma Global Development, Inc. (Northbrook, IL).
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Additional information
Funding
References
- Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7:31. doi:10.1038/s41572-021-00265-2
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2:e106–e116. doi:10.1016/S2214-109X(13)70145-1
- Klein R, Chou C-F, Klein BEK, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi:10.1001/archophthalmol.2010.318
- Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi:10.1016/j.ophtha.2012.10.036
- Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25.
- Gorusupudi A, Nelson K, Bernstein PS. The age-related eye disease 2 study: micronutrients in the treatment of macular degeneration. Adv Nutr. 2017;8:40–53. doi:10.3945/an.116.013177
- Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi:10.1016/S0140-6736(18)31550-2
- Dobó J, Kocsis A, Gál P. Be on target: strategies of targeting alternative and lectin pathway components in complement-mediated diseases. Front Immunol. [ Internet]. 2018 [ cited 2022 Jan 26]; 9. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2018.01851. doi:10.3389/fimmu.2018.01851
- Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819–835. doi:10.1097/IAE.0000000000001392
- Girardi G, Lingo JJ, Fleming SD, et al. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front Immunol. 2020;11:1681. doi:10.3389/fimmu.2020.01681
- Suresh R, Chandrasekaran P, Sutterwala FS, et al. Complement-mediated ‘bystander’ damage initiates host NLRP3 inflammasome activation. J Cell Sci. 2016;129:1928–1939. doi:10.1242/jcs.179291
- Klos A, Tenner AJ, Johswich K-O, et al. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi:10.1016/j.molimm.2009.04.027
- Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi:10.1038/cr.2009.139
- Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479–492. doi:10.1016/j.semnephrol.2013.08.001
- Gehrs KM, Jackson JR, Brown EN, et al. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128:349–358. doi:10.1001/archophthalmol.2010.18
- Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016;787:94–104. doi:10.1016/j.ejphar.2016.03.001
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi:10.1056/NEJM200104053441406
- Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi:10.1073/pnas.0501536102
- Wu J, Sun X. Complement system and age-related macular degeneration: drugs and challenges. DDDT. 2019;13:2413–2425. doi:10.2147/DDDT.S206355
- Park YG, Park YS, Kim I-B. Complement system and potential therapeutics in age-related macular degeneration. Int J Mol Sci. 2021;22:6851. doi:10.3390/ijms22136851
- Yednock T, Fong DS, Lad EM. C1q and the classical complement cascade in geographic atrophy secondary to age-related macular degeneration. Int J Retina Vitreous. 2022;8:79. doi:10.1186/s40942-022-00431-y
- Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402:1449–1458. doi:10.1016/S0140-6736(23)01583-0
- Patel SS, Lally DR, Hsu J, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye. 2023;37:3551–3557. doi:10.1038/s41433-023-02497-w
- Desai D, Dugel PU. Complement cascade inhibition in geographic atrophy: a review. Eye (Lond). 2022;36:294–302. doi:10.1038/s41433-021-01765-x
- Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration. Ophthalmology. 2021;128:576–586. doi:10.1016/j.ophtha.2020.08.027
- Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194:3542–3548. doi:10.4049/jimmunol.1403068
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315–329. doi:10.2165/00063030-200822050-00004
- Huber-Lang MS, Younkin EM, Sarma JV, et al. Complement-induced impairment of innate immunity during sepsis. J Immunol. 2002;169:3223–3231. doi:10.4049/jimmunol.169.6.3223
- Lueck K, Wasmuth S, Williams J, et al. Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration. Eye (Lond). 2011;25:1074–1082. doi:10.1038/eye.2011.109
- Complement modulation reverses pathology in Y402H-retinal pigment epithelium cell model of age-related macular degeneration by restoring lysosomal function - Cerniauskas - 2020 - STEM CELLS Translational Medicine - Wiley Online Library [Internet]. [ cited 2023 Jun 1]. Available from: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.20-0211
- ARC1905 (ANTI-C5 APTAMER) given either in combination therapy with Lucentis® 0.5 mg/Eye in subjects with neovascular age-related macular degeneration. ClinicalTrials.gov identifier: NCT00709527. Updated February 2, 2024. Accessed May 2, 2024. https://www.clinicaltrials.gov/study/NCT00709527
- Ewing TM, Khan H, Wadsworth AL, et al. Update on avacincaptad pegol for geographic atrophy. TouchREVIEWS Ophthalmol. Published online. Accessed June 15, 2023. 2022;16(1):36–39. https://www.touchophthalmology.com/macular-degeneration/journal-articles/update-on-avacincaptad-pegol-for-geographic-atrophy/
- ZIMURA in combination with LUCENTIS in patients with neovascular age related macular degeneration (NVAMD). Clinical Trial.gov identifier: NCT03362190. Updated February 24, 2024. Accessed May 2, 2024. https://www.clinicaltrials.gov/study/NCT03362190
- A study of ARC1905 (anti-c5 aptamer) in subjects with dry age-related macular degeneration. ClinicalTrials.gov identifier: NCT00950638. Updated February 21, 2024. Accessed May 2, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT00950638
- Zimura in participants with geographic atrophy secondary to dry age-related macular degeneration. clinical trial.gov identifier: NCT02686658. Updated February 23, 2024. Accessed May 2, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT02686658
- A phase 3 safety and efficacy study of intravitreal administration of zimura (Complement C5 Inhibitor). ClinicalTrials.gov Identifier: NCT04435366. Updated February 23, 2024. Accessed May 2, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04435366
- Danzig CJ, Khanani AM, Kaiser PK, et al. Vision loss reduction with avacincaptad pegol for geographic atrophy: a 12-month post hoc analysis of the GATHER1 and GATHER2 trials. Ophthalmol Retina. 2024 May 6;S2468-6530(24)00224-0. doi:10.1016/j.oret.2024.04.023
- Treatment of Age-related Macular Degeneration With Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP Report 1. Arch Ophthalmol. 1999;117:1329–1345. doi:10.1001/archopht.117.10.1329
- Park DH, Connor KM, Lambris JD. The challenges and promise of complement therapeutics for ocular diseases. Front Immunol. 2019;10:1007. doi:10.3389/fimmu.2019.01007
- Kaiser PK, Jaffe GJ, Holz FG, et al. Considerations on the management of macular neovascularization in patients with geographic atrophy enrolled in clinical trials. Retina Today. March 2022. Accessed June 2, 2023. https://retinatoday.com/articles/2022-mar-supplement2/considerations-on-the-management-of-macular-neovascularization-in-patients-with-geographic-atrophy-enrolled-in-clinical-trials
- Kaiser PK. Safety of Intravitreal Avacincaptad Pegol in Geographic Atrophy: GATHER1 and GATHER2 Results. Pasadena, CA: Paper presented at: The Retina Society 55th Annual Scientific Meeting; November 3, 2022.
- Tao LW, Wu Z, Guymer RH, et al. Ellipsoid zone on optical coherence tomography: a review. Clin Exp Ophthalmol. 2016;44:422–430. doi:10.1111/ceo.12685
- Sarici K, Abraham JR, Sevgi DD, et al. Risk classification for progression to subfoveal geographic atrophy in dry age-related macular degeneration using machine learning-enabled outer retinal feature extraction. Ophthalmic Surg Lasers Imaging Retina. 2022;53:31–39. doi:10.3928/23258160-20211210-01
- Granger CE, Yang Q, Song H, et al. Human retinal pigment epithelium: in vivo cell morphometry, multispectral autofluorescence, and relationship to cone mosaic. Invest Ophthalmol Vis Sci. 2018;59:5705–5716. doi:10.1167/iovs.18-24677
- Elsner AE, Chui TYP, Feng L, et al. Distribution differences of macular cones measured by AOSLO: variation in slope from fovea to periphery more pronounced than differences in total cones. Vision Res. 2017;132:62–68. doi:10.1016/j.visres.2016.06.015
- Scott AW, Bressler NM, Ffolkes S, et al. Public attitudes about eye and vision health. JAMA Ophthalmol. 2016;134:1111–1118. doi:10.1001/jamaophthalmol.2016.2627
- Demmin DL, Silverstein SM. Visual impairment and mental health: unmet needs and treatment options. Clin Ophthalmol. 2020;14:4229–4251. doi:10.2147/OPTH.S258783
- Sunness JS. The underreporting of age-related geographic atrophy of the macula see editorial on page 287. Oph Retina. 2023;7:367–368. doi:10.1016/j.oret.2022.11.012