Abstract
Cytokine release syndrome (CRS) is immune dysregulation phenomenon that is associated with immune checkpoint inhibitors. It is still difficult to distinguish CRS from other dangerous, acute and life-threatening medical disorders.
We present a case of delayed grade 4 CRS following treatment of lung adenocarcinoma with ipilimumab plus nivolumab that warranted intensive care level treatment with abundant fluid resuscitation, two-tire vasopressor support, high-flow nasal oxygenation, corticosteroids in high dosages, as well as sustained low-efficiency daily diafiltration with CytoSorb hemadsorption and tocilizumab. Initial treatment of presumed septic shock of unknown origin did not yield results.
After initiation of corticosteroids and particularly CytoSorb hemadsorption and tocilizumab, prompt clinical and laboratory improvement was observed.
Plain language summary
This case report describes a 62-year-old woman who experienced a life-threatening immune system reaction, 2 weeks after receiving immunotherapy for lung cancer. This reaction, called cytokine release syndrome (CRS), caused her organs to malfunction. The patient was treated with high-dose steroids, a blood purification technique (SLEDD with CytoSorb), and the medication tocilizumab. Her condition stabilized after initiation of SLEDD with CytoSorb and dramatically improved after receiving tocilizumab. This case highlights the importance of considering CRS in patients who experience severe illness after receiving immunotherapy.
Advances in immunotherapy, particularly checkpoint inhibitors (CPI), have improved outcomes in various cancers, but also increased the incidence of immune-related adverse events such as cytokine release syndrome (CRS).
CRS is a hyperactivation of the immune system commonly seen in CAR-T cell therapy but can also occur with PD-1 and CTLA-4 inhibitors. Severe forms are characterized by fulminant systemic inflammation, often requiring intensive care and multifaceted treatment.
A 62-year-old female with lung adenocarcinoma, post-treatment with ipilimumab and nivolumab, developed life-threatening grade 4 CRS two weeks after her last immunotherapy session. She presented with symptoms mimicking septic shock (malaise, fever and hypotension), leading to an initial misdiagnosis of sepsis. She was treated with fluids, antibiotics and corticosteroids.
The clinical deterioration, high IL-6 levels and lack of infectious source led to the diagnosis of grade 4 CRS. Elevated IL-6 (10,8878 pg/ml) was a critical diagnostic marker.
The patient received high-dose methylprednisolone, tocilizumab and rescue therapy with SLEDD and CytoSorb hemadsorption. Hemodynamic stabilization was observed after initiating SLEDD, with significant improvement following tocilizumab infusion (decreased need for vasopressors, improved mental status and recovery of kidney function). She was discharged from the ICU after 4 days and from the hospital after 23 days with tapering dose of methylprednisolone.
The case highlights the importance of considering CRS in patients treated with immune checkpoint inhibitors, especially when presenting with severe symptoms. Elevated IL-6 is a key biomarker for CRS, and distinguishing CRS from sepsis is critical for appropriate treatment.
For patients on immune checkpoint inhibitors presenting with acute severe illness, comprehensive testing including IL-6 is recommended. Hemadsorption therapy may be a viable rescue option in severe cases of CRS.
1. Background
In recent years, advances in immunotherapy treatment have dramatically improved the prognoses of certain malignancies. One of the more prominent therapeutic options is checkpoint inhibitors, most effective in skin, lung and renal cell carcinomas, but also used in other malignancies [Citation1–5]. With novel treatment options targeting specific immune pathways, there is a concomitant rise in previously unrecognized immune related adverse events. One of the recently and increasingly recognized immune-related adverse event is cytokine release syndrome (CRS), a form of immune system hyperactivation [Citation6–9]. CRS is widely recognized in CAR-T trials for B-cell acute lymphoblastic leukemias and large cell lymphoma with an incidence of 57–100% and fewer than 5% treatment-related deaths [Citation10]. It is a somewhat less frequent complication of PD-1 or CTLA-4 inhibitors, especially CRS of higher grades and life-threatening courses [Citation7,Citation11–14]. Glucocorticoids are standard first-line treatment with tocilizumab in severe cases or in cases where glucocorticoids might deplete or eradicate the infused CAR-T cells. Some experts suggest that tocilizumab should be the initial and, if possible, only treatment of mild and severe CRS with addition of glucocorticoids only exceptionally [Citation7,Citation15]. The incidence of high-grade CRS differs with treatment modality, greatest being after CAR-T cell treatment [Citation16]. Literature on most immunotherapeutics is still case-report based [Citation13,Citation17].
In our case, we are presenting a patient with life-threatening case of grade 4 CRS [Citation18] 2 weeks after the last ipilimumab plus nivolumab immunotherapy for lung adenocarcinoma, requiring intensive care setting treatment, with two-tier vasopressor therapy, high dosages of methylprednisolone, one-time sustained low-efficiency daily diafiltration (SLEDD) with cytokine adsorption using a CytoSorb membrane, high-flow nasal cannula (HFNC) oxygenation and infusion of the IL-6 blocker tocilizumab. An initial raise in mean blood pressure (BP) was observed after SLEDD with CytoSorb initialization, but marked improvement was observed only after tocilizumab infusion.
2. Case presentation
A 62-year-old female patient with history of acute biliary pancreatitis in December 2021, but with no regular therapy, was routinely followed by gastroenterology because of an intraductal papillary mucinous neoplasm. She had a routine magnetic resonance cholangiopancreatography in May 2023 that showed no changes regarding intraductal papillary mucinous neoplasm, but suspicious consolidations in the bilateral basal parts of the lungs. In the next 3 months, a complete evaluation was performed with computed tomography (CT) scans of the thorax, abdomen and cranium and a CT-guided biopsy of the left lower lung lobe consolidation. The incidentally found consolidation was confirmed as metastatic mucinous adenocarcinoma, KRAS-positive, MetFusion PDL-1 0%, grade T2N0M1a. On 22 August 2023, she was started on chemo/immunotherapy with nivolumab, ipilimumab, PEMEtrexed and CARBOplatin [Citation19]. On 12 September 2023, the second cycle with CARBOplatin, PEMEtrexed and nivolumab was administered, with the third cycle containing ipilimumab plus nivolumab (CTLA-4 and PD-1 blocker respectively) administered on 5 October 2023. There were no side effects reported with any of the three applications.
On 19 October 2023 at 12:50 (14 days after the last ipilimumab plus nivolumab application) she presented in our infectious disease emergency department with symptoms of malaise, fatigue, nausea, vomiting and fever. She denied pain, dyspnea, urinary and gastrointestinal abnormalities. She informed us that she had symptoms of an upper respiratory tract infection with nasal congestion and a sore throat one week ago, which had completely resolved. Clinical examinations showed hypotension with a systolic BP of 95 mmHg, sinus tachycardia of 125/min, decreased saturation of 92%, an inguinal maculopapular rash, and signs of dehydration. Laboratory results showed high values of CRP and PCT, mild acute liver and kidney injury, mildly prolonged international normalized ratio, hypoxemia, negative base excess with compensated metabolic acidosis, lactate was negative and urinalysis was not significant. Abdominal ultrasound was normal, and chest x-ray showed no new infiltrates, just basal tumorous consolidations and mild interstitial edema. Blood and urine samples were taken for microbiological analysis. During the first few hours in the emergency department, she received a total of 3000 ml of balanced crystalloids and 2 l/min oxygen through a binasal cannula. She was diagnosed with sepsis of unknown origin and treated with a combination of ceftriaxone 2 g daily and flucloxacillin 2 g every 6 h. She was then admitted to the Department of pulmonary diseases at 16:50. Up until the next morning she received an additional 3000 ml of balanced crystalloids, paracetamol 1000 mg every 8 h because of fever, and antibiotics as planned. Despite therapy, her BP kept decreasing, and so did her mental state. A cardiology consultant performed an echocardiography that showed normal heart function with marked intravascular hypovolemia.
On 20 October at 14:45, she received the first stress dose of 100 mg of hydrocortisone for the treatment of septic shock, and additional blood cultures were taken. She was transferred to the intensive care unit (ICU) at 18:10. At admission, she was somnolent with a Glasgow Coma Scale (GCS) of 13, hypotensive with a systolic BP of around 65 mmHg, tachycardia with sinus tachycardia of around 125/min, and hypoxemic with an oxygen saturation (SpO2) of 88% on a 40% venturi mask. Invasive hemodynamic monitoring was commenced, and vasopressor support with noradrenaline was immediately started with an initial dose of 0.30 mcg/kg/min. Bilateral pleural effusions with marked compressive atelectasis were seen on the ultrasound (). The inferior vena cava (IVC) was completely collapsed. Because of the clinical course, absence of a clear infectious cause, and history of recent immunotherapy application, a hematology fellow raised high suspicion of possible high-grade CRS. Blood was taken for laboratory testing and a methylprednisolone bolus of 1 mg/kg body weight (BW) was administered empirically at 18:45. Necessary noradrenaline dosage to maintain a mean arterial pressure (MAP) of 75 mmHg was rising; at 21:00 it was 0.50 mcg/kg/min, and vasopressin was added with a dose of 0.04 units (U)/min. Respiratory function was also deteriorating to the point where HFNC oxygenation with a flow of 40 l/min and a fraction of inspired oxygen (FiO2) of 50% was needed to reach an SpO2 of 95% (chest x-ray represented in ). Two hundred milliliter of 20% albumin was administered, with no increase in BP. At around 20:30, all laboratory testing came back with increased inflammatory markers (CRP 320, PCT 40), markedly elevated myoglobin, D-dimer, creatine kinase and elevated renal and liver tests, indicative of multiorgan failure. She was at this point anuric. Most prominently elevated was the level of IL-6, which was 108,878 pg/ml (reference <7). After additional consultation with hematology and oncology fellows, the diagnosis of CRS grade 4 [Citation20] was confirmed. An additional dose of methylprednisolone was administered to a cumulative dose of 1.5 mg/kg BW. With no hemodynamic improvement, anuria, the need for two-tier vasopressor support, marked capillary leak with collapsed IVC despite abundant fluid resuscitation, the indication for the treatment of high-grade CRS with tocilizumab was established. Because of the grave clinical condition, SLEDD with CytoSorb was started at 23:15, an hour before tocilizumab could be obtained and prepared. After the initialization of SLEDD with CytoSorb, a rise in mean BP was observed with no increase in vasopressor dosages. There was no significant improvement in heart rate, oxygenation or other vital signs.
Figure 1. The graph represents the timeline of the first 48 h in the intensive care unit with main therapeutic approaches, vasopressor infusion dosages and main vital parameters. The graph clearly depicts the rapidly decreasing necessary doses of vasopressor support after initiating CytoSorb hemadsorption and especially after tocilizumab infusion. The scale for mean blood pressure (mmHg) and heart rate (bpm) is on the left, and on the right for noradrenaline (mcg/kg/min) and vasopressin (U/min) dose. Main therapeutic modalities are also marked according to the timeline.
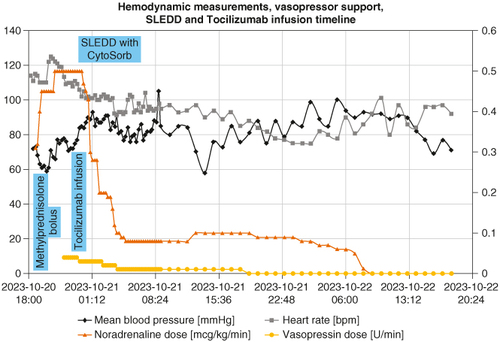
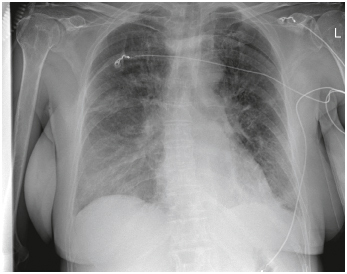
Figure 2. The chest x-ray at intensive care unit admission shows diffuse pulmonary edema consistent with prominent capillary leak, bilateral pleural effusions and the need for high-flow nasal cannula oxygenation.
On 21 October at 00:05, tocilizumab was infused at a dose of 8 mg/kg of BW over a 1 h period (after it was determined that it was safe to administer concurrently with CytoSorb [Citation21,Citation22]), finishing at 01:05. After 40 min of commencing the tocilizumab infusion, the heart rate started to decrease, and vasopressor dosages could be significantly decreased with a concomitant rise in mean BP. At the end of the infusion, at 01:05, the necessary dose of NA dropped from 0.50 to 0.30 mcg/kg/min, and vasopressin dropped from 0.04 to 0.03 U/min. In the next 6 h, an additional significant drop in vasopressor need was observed, with NA decreasing to 0.08 mcg/kg/min and vasopressin to 0.01 U/min. The heart rate dropped to normocardic range. SLEDD with CytoSorb was stopped at 07:15 in the morning; there was no observable significant deterioration after discontinuation. Additionally, kidney function improved with spontaneous diuresis. Mental status markedly improved from somnolence to complete alertness; the IVC became distended and noncollapsible. Only acute respiratory insufficiency persisted, with a need for HFNC.
Twenty-four hours after admission, vasopressin was discontinued, and noradrenaline was discontinued 12 h after that. One-sided pleural drainage was performed, respiratory insufficiency markedly improved on the 3rd day of ICU treatment. After obtaining urine cultures that were positive for proteus mirabilis urinary infection, flucloxacillin was discontinued. All other microbiology testing remained negative (). No additional tocilizumab dosages or SLEDD with CytoSorb were needed; only methylprednisolone was being administered to her at a daily dosage of 1 mg/kg BW. Markers of inflammation declined, and the kidney and liver function improved. Most markedly, the IL-6 level was 435 ng/l after two and a half days of ICU treatment. Lactate levels remained negative during whole ICU treatment.
Table 1. Microbiology testing.
On the 4th day, 24 October 2023, she was transferred to the pulmonary hospital to resume malignancy treatment. At transfer, she was hemodynamically stable without requiring vasopressor support and was normocardic. She was still mildly hypoxic, requiring 4 l/min of oxygen via binasal cannula, completely oriented, rehabilitated to walking, and all laboratory values showed marked improvement. She was transferred with methylprednisolone and ceftriaxone as previously described and discharged home after a total of 23 day hospitalization with tapering doses of methylprednisolone. Extensive microbiological testing did not confirm the diagnosis of sepsis, but urinary infection was confirmed. Graphical representation of the first 48 h of ICU treatment can be found in , and microbiological testing and main laboratory findings are listed in & , respectively. Comprehensive timeline is presented in .
Figure 3. Graphic timeline representation of main events before treatment of CRS in our hospital and a more detailed representation of the treatment of CRS. The left half represents events before admission to our hospital, and the right half represents the timeline in our hospital. Note the change in the time axis.
CRS: Cytokine release syndrome.
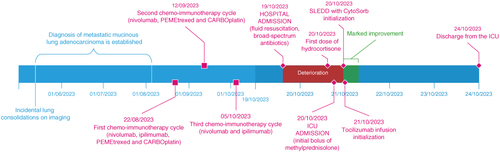
Table 2. Timeline of main laboratory findings.
3. Discussion
CRS is a recognized complication of novel immune checkpoint inhibitors (ICIs) and chimeric antigen receptor (CAR)-T cell therapy [Citation16,Citation23]. Most common ICIs associated with CRS are CTLA-4 blockers and PD-1 inhibitors [Citation24–26]. CRS is a systemic inflammatory syndrome mediated by several cytokines, mainly IL-6, TNF-α, IFN-γ, IL-2, IL-8, IL-10 and GM-CSF. Of all cytokines, IL-6 is believed to be the main protagonist [Citation9]. For CAR-T associated CRS, the American Society for Transplantation and Cellular Therapy grading system is used exclusively [Citation18]. CRS of all other causes is graded per Common Terminology Criteria for Adverse Events grading system. The most severe is grade 4, namely CRS with life-threatening consequences and in need of urgent intervention, hypoxia requiring ≥40 percent FiO2, and hypotension not adequately managed with one vasopressor [Citation20]. The most common presentation is mild (grades 1 and 2), presenting with fever, fatigue, headache, rash, diarrhea, arthralgia and myalgia. More severe forms (grades 3 and 4) progress to hypotension and uncontrolled systemic inflammatory response syndrome with circulatory collapse, vascular leakage, peripheral and/or pulmonary edema, renal failure, cardiac dysfunction and multiorgan system failure [Citation27,Citation28]. The timeline of presentation is dependent on the therapeutic approach for the primary disease and can vary from minutes to weeks after the last application. Laboratory findings depend on the underlying disease, but the main ones are electrolyte abnormalities and elevated values of acute phase markers (CRP, ferritin and IL-6) [Citation29]. In general, the degree of elevation of cytokines and markers of inflammation correlates with the severity of the clinical syndrome. IL-6 is also a marker of sepsis and septic shock. As per Song et al. the serum IL-6 value of 348.9 pg/ml was predictive of septic shock [Citation30]. In contrast, dramatic elevation of IL-6 is a supportive finding for the diagnosis of CRS, as reported by Lee et al., who found that IL-6 values exceeded 3000 pg/ml in grade 3 CRS [Citation7]. PCT was not widely studied as a CRS biomarker [Citation6,Citation31–35], but it was suggested to be associated with bacterial infection [Citation36,Citation37].
Multiple therapeutic options are recognized for CRS, depending on severity. It is paramount to first exclude possible and dangerous differential diagnostic options that might also be present simultaneously, most commonly sepsis, tumor progression, heart failure, thromboembolism or allergic reactions [Citation10]. Treatment of severe CRS is especially challenging, frequently requiring ICU level care. For CAR-T associated CRS, the tocilizumab and glucocorticoid combination is a recognized protocol of treatment [Citation38]. Bi-specific antibody-associated CRS is primarily treated with glucocorticoids as tocilizumab appeared less effective [Citation39]. As of this time, therapeutic approaches are still in development as immune related adverse events are being discovered and described in real time.
Our patient presented in the emergency department with symptoms and laboratory findings that were in line with septic shock and immune system dysregulation. She was initially treated for septic shock with hypovolemia and relative corticosteroid deficiency. Two-tier antibiotic therapy and fluid resuscitation were promptly initiated, and hydrocortisone was added the next day as her condition did not improve. Prominent vascular leakage was observed when, despite abundant crystalloid infusions, the IVC remained completely collapsed, and bilateral pleural effusions were observed, requiring the addition of oxygen.
With worsening of the clinical condition, despite appropriate therapy for septic shock, we proposed a working diagnosis of CRS due to the fact she received immunomodulatory therapy. We started treatment with high doses of glucocorticoids and sent blood samples to determine the level of IL-6. After receiving the IL-6 level, the diagnosis of grade 4 CRS was confirmed. We did not have tocilizumab readily available in our general hospital and had to wait for the transport of the drug from University Medical Centre Ljubljana. While waiting for tocilizumab to be transported, we settled on the rescue use of SLEDD with CytoSorb hemadsorption membrane. The decision was made according to reports, including one of our colleagues in the Medical Centre Ljubljana, of using CytoSorb in patients with systemic inflammatory response syndrome [Citation40] and the fact that IL-6 is adsorbable because of its molecular weight of 21–26 kilodaltons (kDa) [Citation22], nevertheless, the latest systematic review reported no significant benefits [Citation41]. The CytoSorb membrane is effective for small and mid-size hydrophobic molecules up to a size of approximately 60 kDa according to the manufacturer [Citation42] and studies [Citation43]. In the hour of SLEDD therapy, before we received tocilizumab, we observed a rise in mean BP. The fact that mean BP rose and stabilized is suggestive of the protective effect of hemadsorption on immune dysregulation in CRS. After the initial hour of SLEDD, we also received and started the infusion of tocilizumab that can be given simultaneously with CytoSorb as its molecular weight is 149 kDa [Citation21]. Her clinical status improved dramatically after infusing tocilizumab. We acknowledge that the time from the administration of glucocorticoids to the start of SLEDD was probably too short for steroids to provide full effect [Citation44]. Our approach with SLEDD with CytoSorb is the major difference regarding the case report of Rotz et al. [Citation45] that is otherwise similar to ours. They present a case of a 29-year-old woman with alveolar soft part sarcoma that presented with stage 4 CRS after nivolumab. The reaction in their case was early, starting 1 day after the second nivolumab infusion. She presented hemodynamically unstable, with high fever and disoriented. An important difference with our case is that her condition improved in 12 h after fluid resuscitation, and tocilizumab was administered only after stabilization. In our case, the condition of the patient was deteriorating rapidly, and we resorted to SLEDD with CytoSorb as a rescue therapy before possible tocilizumab infusion as specific therapy. At the moment of initiation, our patient was anuric, somnolent, and markedly hemodynamically unstable. Furthermore, the IL-6 value in our case was more than 2000-times higher, suggestive of a more fulminant CRS course.
Hemocultures remained sterile, further decreasing the possibility of a severe infection. Additional doses of SLEDD with CytoSorb and tocilizumab were not needed. The control value of serum IL-6 was much lower, consistent with clinical improvement. We observed no significant hemodynamic deterioration, thus suggesting a lasting effect of tocilizumab and delayed full effect of corticosteroid treatment, furthermore confirming CRS diagnosis. In contrast, some authors have completely different experiences in treating CRS, with their cases having long-lasting and recurrent episodes, requiring multiple prolonged hospitalizations [Citation24].
The main reason for the initial working diagnosis of sepsis and septic shock in our case was the high value of PCT, which is classically associated with severe systemic bacterial infection [Citation37]. Besides bacterial infection, reasons for elevated PCT values are numerous. In the recent pandemic, high values of PCT were observed in patients with presumed cytokine storm due to critical severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who also underwent hemadsorption with CytoSorb [Citation46]. Making parallels between CRS and SARS-CoV-2 induced immune system dysregulation is undoubtedly speculative. Prolonged hypovolemic and distributive shock can also produce elevated PCT values, as was described in patients after coronary bypass operations [Citation47], and also in other cases [Citation48–50]. Nevertheless, IL-6 and other proinflammatory cytokines are interconnected in the immune response. TNF-α, IL-1β and IL-6 were recognized as specific proximate stimuli to hyperprolactinemia [Citation48,Citation51–53]. As IL-6 in our case was extremely high and we did not prove bacterial systemic infection despite our best efforts, along with rapid improvement after the application of specific therapy, CRS being the sole and primary reason for elevated PCT is extremely likely.
As indications for the use of ICIs are expanding continuously, we suggest that the phenomena of immune system dysregulation are still not adequately understood as presentations vary individually, with primary therapeutic approaches, and with other factors. Our stance is that a wider array of laboratory testing should be used in patients on ICIs presenting with an acute severe illness, especially when they require ICU-level management. Besides testing standard biomarkers of inflammation (CRP, PCT and ferritin), we suggest testing IL-6, TNF-α, IL-1β and IFN-γ as biomarkers of life-threatening side effects of ICIs [Citation54]. Moreover, hemadsorption should be considered as rescue therapy, if available.
4. Conclusion
In conclusion, our case illustrates the complexity of diagnosing and managing CRS in patients undergoing ICI therapy. The patient's life-threatening grade 4 CRS presented similarly to septic shock, underscoring the need for differential diagnosis. Rapid initiation of treatments, including glucocorticoids, SLEDD with CytoSorb hemadsorption, and tocilizumab, were critical in stabilizing the patient. This case highlights the importance of recognizing CRS as a potential adverse effect of ICIs and suggests that comprehensive cytokine profiling and innovative treatments like hemadsorption can be crucial in managing severe cases. Continued research and case reporting are essential to refine therapeutic approaches and improve patient outcomes in ICI-associated CRS.
Author contributions
M Kurnik: Conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing – original draft, writing – review & editing. P Fazarinc : Resources, validation, writing – review & editing. M Podbregar: Methodology, project administration, supervision, writing – review & editing, funding acquisition.
Financial disclosure
This case report received funding from The Slovenian Research and Innovation Agency (ARIS), project number J3-50116. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
We have received verbal and written consent to publish this case report from the patient.
Acknowledgments
The authors would like to acknowledge the help of the Clinical department of hematology of University Medical Centre Ljubljana, Slovenia, specially to S Matjaž, MD, PhD, consultant hematologist, for the help of preparing tocilizumab for our use. Slovenian emergency medical services dispatch service and private ambulance transport company Pacient d.o.o. played an important role for urgently transporting the drug. Many thanks to all involved personnel, especially nurses and doctors of Pulmonology department and Department of Internal Intensive Medicine of General hospital Celje for all the care for the patient. Major contribution for the care of patient was also made by pulmonologist T Katja, MD that managed patient’s dire state on the floor and promptly notified intensive care of her deterioration. Finally, we would like to thank the personnel of University Clinic of Respiratory and Allergic Diseases Golnik, Slovenia for their collaboration and continued care.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Additional information
Funding
References
- Foran AE, Nadel HR, Lee AF, et al. Nivolumab in the treatment of refractory pediatric Hodgkin lymphoma. J Pediatr Hematol Oncol. 2017;39(5):e263–e266. doi:10.1097/MPH.0000000000000703
- Queirolo P, Boutros A, Tanda E, et al. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy. Semin Cancer Biol. 2019;59:290–297. doi:10.1016/j.semcancer.2019.08.001
- Hu Z, Li M, Chen Z, et al. Advances in clinical trials of targeted therapy and immunotherapy of lung cancer in 2018. Transl Lung Cancer Res. 2019;8(6):1091–1106. doi:10.21037/tlcr.2019.10.17
- Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17(3):137–150. doi:10.1038/s41585-020-0282-3
- June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. doi:10.1056/NEJMra1706169
- Tedesco VET, Mohan C. Biomarkers for predicting cytokine release syndrome following CD19-targeted CAR T cell therapy. J Immunol. 2021;206(7):1561–1568. doi:10.4049/jimmunol.2001249
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi:10.1182/blood-2014-05-552729
- Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. doi:10.1182/blood-2013-02-485623
- Gödel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2018;44(3):371–373. doi:10.1007/s00134-017-4943-5
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi:10.1186/s40425-018-0343-9
- Zhang X, Fu Z, Yan C. Cytokine release syndrome induced by pembrolizumab: a case report. Medicine (Baltimore). 2022;101(49):e31998. doi:10.1097/MD.0000000000031998
- Dimitriou F, Matter AV, Mangana J, et al. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J Immunother. 2019;42(1):29–32. doi:10.1097/CJI.0000000000000236
- Tay SH, Toh MMX, Thian YL, et al. Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: a case series of 25 patients and review of the literature. Front Immunol. 2022;13:807050. doi:10.3389/fimmu.2022.807050
- Liu LL, Skribek M, Harmenberg U, Gerling M. Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: case report and systematic review of the literature. J Immunother Cancer. 2023;11(3):e005841. doi:10.1136/jitc-2022-005841
- Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813–822. doi:10.1080/1744666X.2019.1629904
- Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T Cell therapy. Biol Blood Marrow Transplant. 2019;25(4):e123–e127. doi:10.1016/j.bbmt.2018.12.756
- Yomota M, Mirokuji K, Sakaguchi M, et al. Cytokine release syndrome induced by immune-checkpoint inhibitor therapy for non-small-cell lung cancer. Intern Med. 2021;60(21):3459–3462. doi:10.2169/internalmedicine.5922-20
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi:10.1016/j.bbmt.2018.12.758
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, Phase III trial. Lancet Oncol. 2021;22(2):198–211. doi:10.1016/S1470-2045(20)30641-0
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. U.S. Department of Health and Human Services, National Cancer Institute. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- ASSESSMENT REPORT FOR RoActemra. European Medicines Agency, evaluation of medicines for human use. 2009.
- Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi:10.2217/imt-2016-0020
- Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):567–572. doi:10.1182/asheducation-2016.1.567
- Ciner AT, Hochster HS, August DA, et al. Delayed cytokine release syndrome after neoadjuvant nivolumab: a case report and literature review. Immunotherapy. 2021;13(13):1071–1078. doi:10.2217/imt-2020-0329
- Oda H, Ishihara M, Miyahara Y, et al. First case of cytokine release syndrome after nivolumab for gastric cancer. Case Rep Oncol. 2019;12(1):147–156. doi:10.1159/000496933
- Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. doi:10.3389/fphar.2020.00557
- Hellwig Y, Yoo YE, Reß ML, et al. Fulminant skin GvHD with a cytokine pattern resemblant of cytokine release syndrome successfully treated with multimodal immunosuppression including tocilizumab. Pediatr Blood Cancer. 2015;62(11):2033–2035. doi:10.1002/pbc.25595
- Abboud R, Keller J, Slade M, et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is Associated with poor survival and Anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. 2016;22(10):1851–1860. doi:10.1016/j.bbmt.2016.06.010
- Gupta S, Seethapathy H, Strohbehn IA, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-Cell lymphoma. Am J Kidney Dis. 2020;76(1):63–71. doi:10.1053/j.ajkd.2019.10.011
- Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19(1):968. doi:10.1186/s12879-019-4618-7
- Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. doi:10.1158/2159-8290.CD-16-0040
- Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi:10.1182/blood-2017-06-793141
- Zhang Y, Zhang W, Dai H, et al. An analytical biomarker for treatment of patients with recurrent B-ALL after remission induced by infusion of anti-CD19 chimeric antigen receptor T (CAR-T) cells. Sci China Life Sci. 2016;59(4):379–385. doi:10.1007/s11427-016-5035-4
- Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi:10.1186/s40364-018-0116-0
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi:10.1126/scitranslmed.aac5415
- Powell MZ, Mara KC, Bansal R, et al. Procalcitonin as a biomarker for predicting bacterial infection in chimeric antigen receptor T-cell therapy recipients. Cancer Med. 2023;12(8):9228–9235. doi:10.1002/cam4.5665
- Arkader R, Troster EJ, Lopes MR, et al. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child. 2006;91(2):117–120. doi:10.1136/adc.2005.077446
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi:10.1126/scitranslmed.3008226
- Frey N. Cytokine release syndrome: who is at risk and how to treat. Best Pract Res Clin Haematol. 2017;30(4):336–340. doi:10.1016/j.beha.2017.09.002
- Persic V, Jerman A, Malgaj Vrecko M, et al. Effect of CytoSorb coupled with hemodialysis on interleukin-6 and hemodynamic parameters in patients with systemic inflammatory response syndrome: a retrospective cohort study. J Clin Med. 2022;11(24):7500. doi:10.3390/jcm11247500
- Becker S, Lang H, Vollmer Barbosa C, et al. Efficacy of CytoSorb(R): a systematic review and meta-analysis. Crit Care. 2023;27(1):215. doi:10.1186/s13054-023-04492-9
- Technical data CytoSorb 300 CytoSorbents Europe GmbH2021 [cited 2023 2023-11-11]. https://cytosorb-therapy.com/en/the-adsorber/technical-data/
- Scharf C, Liebchen U, Paal M, et al. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit Care. 2021;25(1):41. doi:10.1186/s13054-021-03468-x
- Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655–670. doi:10.4187/respcare.06314
- Rotz SJ, Leino D, Szabo S, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64(12):1–5. doi:10.1002/pbc.26642
- Nassiri AA, Hakemi MS, Miri MM, et al. Blood purification with CytoSorb in critically ill COVID-19 patients: a case series of 26 patients. Artif Organs. 2021;45(11):1338–1347. doi:10.1111/aor.14024
- Hensel M, Volk T, Docke WD, et al. Hyperprocalcitonemia in patients with noninfectious SIRS and pulmonary dysfunction associated with cardiopulmonary bypass. Anesthesiology. 1998;89(1):93–104. doi:10.1097/00000542-199807000-00016
- Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017;38(2):59–68.
- Vasishta S, Patel S. Elevated procalcitonin in acute pseudogout flare: a case report. Cureus. 2019;11(6):e4853. doi:10.7759/cureus.4853
- Rule JA, Hynan LS, Attar N, et al. Procalcitonin identifies cell injury, not bacterial infection, in acute liver failure. PLOS ONE. 2015;10(9):e0138566. doi:10.1371/journal.pone.0138566
- Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159(2):253–264. doi:10.1111/j.1476-5381.2009.00433.x
- Redl H, Schlag G, Togel E, et al. Procalcitonin release patterns in a baboon model of trauma and sepsis: relationship to cytokines and neopterin. Crit Care Med. 2000;28(11):3659–3663. doi:10.1097/00003246-200011000-00021
- Redl H, Schiesser A, Togel E, et al. Possible role of TNF on procalcitonin release in a baboon model of sepsis. Shock. 2001;16(1):25–27. doi:10.1097/00024382-200116010-00005
- Diorio C, Shaw PA, Pequignot E, et al. Diagnostic biomarkers to differentiate sepsis from cytokine release syndrome in critically ill children. Blood Adv. 2020;4(20):5174–5183. doi:10.1182/bloodadvances.2020002592
