ABSTRACT
Introduction: Chagas disease (CD) is one of the most neglected public health problems in the Americas, where <1% of the estimated 6 million people with the infection have been diagnosed and treated. The goal of treatment is to eliminate the parasite, decrease the probability of cardiomyopathy and other complications during the chronic stage of infection, and interrupt the cycle of disease transmission by preventing congenital infection. Currently, only benznidazole (BZN) and nifurtimox are recognized by the World Health Organization as effective drugs for treatment of CD. In this paper, we provide an overview of the clinical pharmacology of BZN.
Areas covered: This review covers the historical background, chemistry, mechanism of action, pharmacokinetics, preclinical research, resistance, clinical research, toxicology, adverse effects, and current regulatory status of BZN.
Expert commentary: Ongoing investigations aim to optimize BZN therapy by adjusting the current standard regimen or by combining BZN with new chemical entities. These studies are assessing alternatives that improve safety while maintaining or increasing the efficacy of BZN. Timely diagnosis and antitrypanosomal treatment are critical components of programs to eliminate CD as a public health problem, and can dramatically reduce the heavy burden of morbidity and mortality caused by the disease.
1. Introduction
Chagas disease (CD) is one of the most neglected public health problems in the Americas, where <1% of the estimated 6 million cases (including >300,000 in the United States) [Citation1,Citation2] have been diagnosed and treated. CD is also a significant public health challenge in Europe, where <10% of the estimated 68,000–122,000 cases have been diagnosed or treated [Citation3]. Globally, CD creates an annual burden exceeding 800,000 disability adjusted life years and $600,000,000 in healthcare costs [Citation4].
Caused by the protozoan Trypanosoma cruzi, CD is mainly spread by triatomine bugs and by mother-to-child transmission. The parasite is also transmitted through blood transfusions, organ donations, laboratory accidents, needle sharing among drug users, or orally through food and drink contaminated with triatomines or their feces [Citation5].
CD begins with an acute phase with a lack of defining features; it is often asymptomatic or resembles a common viral illness, although more serious outcomes such as myocarditis or meningoencephalitis are possible. During this time, T. cruzi trypomastigotes are directly observed in the bloodstream. After this comes an indeterminate chronic phase during which T. cruzi lodges in organ tissue in amastigote form, inducing a specific immune response. Although most remain asymptomatic, 30–40% of patients progress to an advanced disease stage, usually years to decades after the initial infection. The advanced chronic phase frequently involves damage to the heart’s conduction system and myocardium, which can result in heart failure and sudden death. In the Americas, myocarditis secondary to CD is the most common form of nonischemic cardiomyopathy [Citation6]. In other cases, CD produces gastrointestinal complications (especially megaesophagus and megacolon), or disorders of the central or peripheral nervous system, particularly in immunocompromised patients. Benznidazole (BZN) and nifurtimox (NFX) are the only drugs with proven effectiveness against T. cruzi. Both drugs are over 40 years old; NFX, developed by Bayer, was registered in 1967 and Roche registered benznidazole in 1971. The goal of etiological treatment of CD with BZN or NFX is to eliminate the parasite, thereby improving clinical outcomes in the patient and interrupting the cycle of disease transmission by blocking the congenital route of infection [Citation7].
Treatment recommendations have historically varied depending on disease phase and patient age. Two important shifts have occurred. Based on clinical trials in the late 1960s, investigators affirmed that NFX and BZN were effective for treating both acute and chronic CD [Citation8]. However, in 1983 an expert panel recommended against treatment in the chronic phase, based on the limited evidence of efficacy available and the belief that chronic CD symptoms were the product of an intense immune response unrelated to parasite presence, rendering etiological treatment fruitless [Citation9]. Subsequently, although acute or congenital cases and children were routinely treated, adults in the chronic phase (the vast majority of CD patients) could only hope for palliative care. Nonetheless, by the late 2000s, evidence mounted indicating that parasite persistence acted as a trigger for chronic CD pathology [Citation10]. Key studies demonstrated that etiological treatment significantly improved clinical outcomes in adults with chronic CD [Citation11–Citation13]. The indication for supporting treatment during the chronic phase of infection was finally reinstated, and the World Health Organization (WHO), Centers for Disease Control (CDC), Médecins sans Frontières/Doctors without Borders (MSF), and other health organizations, as well as national guidelines in endemic and non-endemic countries, recommended offering treatment to adults during the chronic indeterminate phase.
Currently, etiological treatment of CD using BZN and nifurtimox is indicated for the following: (a) the acute phase, including neonates or infants with congenital transmission; (b) reactivation of infection due to immune suppression; (c) the chronic phase in patients up to 18 years old, and (d) women of childbearing age with T. cruzi infection (contraception is recommended during treatment). Treatment may also be considered as a prophylactic measure for patients who are about to receive immunosuppressive therapy; however, there is not a complete consensus among experts on this recommendation, as some prefer to monitor patients closely and only treat in the event of reactivation.
There is agreement in international clinical guidelines that antiparasitic treatment should be offered to adults aged 19–50 years without advanced Chagas heart disease, and is optional for those older than 50 [Citation14–Citation17]. Nonetheless, such treatment has not been widely implemented due to a host of barriers, including the following: (1) low provider awareness of CD and its treatment options, (2) concerns about side effects, (3) low access to healthcare for many patients with CD, (4) lack of a straightforward test of cure, and (5) regulatory barriers [Citation18–Citation20].
BZN is the most commonly used treatment for CD. The recommended treatment course is 5–7 mg/kg orally, divided into two or three daily doses for 60 days for adults, and 5–10 mg/kg orally, divided into two or three daily doses for 60 days for children up to 12 [Citation16]. In this article, we provide an overview of the clinical and pharmacological properties of benznidazole.
2. Introduction to benznidazole (BZN)
2.1. Historical background
BZN is a member of a large group of nitroheterocyclic compounds, which comprises molecules containing one or more nitro-groups linked to an aromatic ring [Citation21]. Nitroheterocyclic compounds have a long history of human and veterinary use as antimicrobial and antiparasitic agents. Although the particular use of nitrofuran derivatives was discontinued in some parts of the world due to mutagenic potential, there has been a resurgence of R&D on nitroaromatic compounds as potential therapeutic candidates [Citation21–Citation25].
The identification in the 1950s of nitrofurazone as an active compound against Trypanosoma species pointed to the potential of nitroheterocyclic compounds as antiparasitic agents for treating human African trypanosomiasis and CD [Citation23]. Although nitrofurazone did not provide optimal efficacy and safety in humans, this earlier investigation led to the identification and development of BZN and NFX, both nitroaromatic compounds, for treatment of CD [Citation23–Citation27].
2.2. Chemistry
BZN () is a 2-nitroimidazole derivative whose chemical name is N-benzyl-2-(2-nitro-1H-imidazol-1-yl) acetamide. Its molecular formula is C12H12N4O3, with a molecular weight of 260.25 g/mol. BZN is provided in different tablet sizes containing the active pharmaceutical ingredient, formulated with common excipients approved by regulatory agencies for human drug products.
2.3. Mechanism of action
BZN is a prodrug that exerts trypanocidal activity after activation to produce reactive metabolites [Citation24,Citation25,Citation28,Citation29]. In T. cruzi, BZN is enzymatically activated by trypanosomal type I nitroreductases (NTR), a class of oxygen-insensitive enzymes present in several protozoan parasites, for which there is no mammalian homologue [Citation24].
In a series of reactions (see Patterson et al. 2014 for detailed information), Nicotinamide adenine dinucleotide (NADH)-dependent type I NTRs catalyze the reduction of the 2-nitroimidazole motif to a hydroxylamine, via a nitroso intermediate, in a two-step/two-electron transfer reaction [Citation24,Citation25]. This hydroxylamine is subsequently converted, via a series of non-enzymatic transformations, until the release of dialdehyde glyoxal, a highly reactive metabolite capable of forming adducts with proteins, DNA/RNA, and small molecules such as glutathione. These reactive metabolites are toxic, resulting in a rapid and localized trypanocidal effect against both intra- and extracellular forms of the parasite [Citation28].
However, glyoxal production is not likely to be the sole cytotoxic mechanism for BZN. A recent study, employing metabolomics approaches, showed that BZN is extensively metabolized to a number of reactive metabolites in epimastigotes, but the authors were unable to detect glyoxal and related adducts [Citation30]. Instead, they proposed that covalent binding of multiple reactive metabolites to low molecular weight thiols (important within glutathione and trypanothione pathways) is BZN’s primary mode of action against T. cruzi [Citation30]. Other studies suggest BZN possesses immunomodulatory effects in vitro, in vivo, and ex vivo, which could play an important role in CD pathogenesis in humans [Citation31,Citation32].
2.4. Pharmacokinetics
The non-clinical pharmacokinetics (PK) of BZN have been evaluated in multiple studies [Citation33–Citation36]. Overall findings suggest that BZN is almost fully bioavailable when administered orally, with a plasma half-life of 2–2.5 h, and plasma protein binding approximating 50% across species. After absorption, BZN distributes widely to tissues, including placental and fetal tissue, at levels similar to that in circulating blood. The metabolic and excretion pathways for this drug have not yet been fully elucidated (although nitroreductases are involved in the conversion of the nitroimidazole motif), but studies demonstrate minimal metabolism in hepatic microsomes and hepatocytes of different species, and no human-specific metabolites [Citation33,Citation36]. No inhibition or induction of CYP450 enzymes at clinically relevant concentrations were detected during in vitro studies using human hepatocytes, although BZN was shown to be a substrate and to increase the expression of glycoprotein-P (efflux pump) [Citation36,Citation37], and to inhibit the OAT3 receptor (with possible implications for drug–drug interactions) [Citation36].
2.5. Preclinical research
Preclinical research has yielded further insight on the susceptibility of different T. cruzi strains to BZN. Notable studies are briefly described below.
2.5.1. In vitro studies
In vitro activity of BZN has been evaluated against >50 laboratory strains and clinical isolates of T. cruzi belonging to different discrete typing units (DTUs) [Citation36,Citation38–Citation43]. A wide variety of experimental designs has been applied to the screening process, including different stages of the parasite, axenic or intracellular parasite cultures, varying duration of treatment, and different endpoints to measure activity. Limited interlaboratory comparability and lack of understanding of the clinical relevance of in vitro sensitivity testing are major drawbacks for CD drug discovery. Improved and evolving experimental designs are paramount to answering relevant questions and to helping translate experimental data into knowledge [Citation44].
Overall in vitro data suggest that susceptibility of different strains to BZN fluctuates, but the 50% inhibitory concentration (IC50) values are always ≤19.5 μg/mL (75 μM) and vary within a factor of 10-fold within the same assay. Activity against different forms of the parasite (epimastigotes, trypomastigotes, or amastigotes) also appears to vary within a relatively small range [Citation36,Citation41,Citation43]. Additionally, time-kill studies indicate that BZN’s trypanocidal effect is both time- and concentration dependent [Citation38,Citation41,Citation43]. Using multiple strains and a high-throughput screening platform, Moraes et al. demonstrated that BZN has a rapid trypanocidal effect and reaches 100% parasite clearance against multiple divergent T. cruzi genotypes,41 a rate superior to that for ergosterol biosynthesis inhibitors, for example.
2.5.2. In vivo studies
Several in vivo models mimic different aspects of human CD, and various formats have been widely used by the scientific community. However, current animal models have limitations, including variability of pathogen/host interactions, spontaneous clearance of parasites, and difficulties in identifying sterile parasitological cure and/or measuring drug treatment in real time, and thus have limited predictive value for development of new drug candidates [Citation45].
In vivo activity of BZN has been evaluated, in both acute and chronic stages, against approximately 64 strains/clones infecting (most frequently) mice, rabbits, and dogs [Citation36,Citation38,Citation39,Citation41,Citation46–Citation51]. Findings in animals suggest that BZN treatment is beneficial in both CD stages, but more efficacious in acute models of T. cruzi infection. BZN improves survival, decreases parasitaemia in blood and reservoir tissues including the heart and gastrointestinal tract, eventually achieving full parasite clearance, and reduces the immunological response to parasite antigens. In animal models, the prevention of severe chronic features of the disease is still a matter of debate [Citation36,Citation50,Citation51].
In dogs, an important reservoir of T. cruzi parasites providing an animal model where clinical findings and immunopathogenic mechanisms are similar to those reported in humans with CD, BZN was effective in reducing parasitic load during the acute or chronic phase of infection. However, while BZN effectively reduced systolic cardiac function alterations, it did not prevent development of cardiomyopathy in the chronic phase [Citation50,Citation52–Citation54].
Intriguingly, two recent studies suggested that BZN more readily cures chronic infections in mice, challenging the convention that it is more efficacious during the acute stage [Citation55,Citation56]. The investigators used a highly sensitive bioluminescence imaging system coupled with cyclophosphamide-mediated immunosuppression (to enhance the reactivation of any residual infection) for systematic and comparative studies of drug efficacy in vivo and ex vivo. Daily treatment of chronically infected mice with 100 mg/kg of BZN for 5, 10, or 20 days resulted in sterile cure. However, mice in the acute stage with the same treatment regimen for 5 or 10 days were not cured [Citation56]. These findings could have major implications for new research directions.
2.5.3. Safety, pharmacology, and toxicology
Non-clinical pharmacological and toxicological profiling of BZN has yielded multiple clinically relevant findings. Potential concerns include carcinogenicity, teratogenicity, effects on male fertility and female pregnancy, and in vitro and in vivo genotoxicity (positive mutagenicity and clastogenicity) [Citation34,Citation36,Citation47,Citation57,Citation58]. These results align with findings for other nitroimidazolic drugs used in the clinic, such as metronidazole. These toxic effects may be partially driven by reactive oxygen species arising from the reduction of the nitro group to an amino group by type II NTRs (oxygen-sensitive), which are present in mammalian organisms [Citation24,Citation30,Citation36].
2.6. Resistance
Several in vitro and in vivo studies suggest, via a comparison of parasite growth kinetics and cure rates in animal models, evaluation of shifts in IC50 values, and molecular approaches, that there is the potential for development of resistance to BZN [Citation36,Citation59–Citation62]. However, while there is clear evidence of resistance in T. cruzi under multiple experimental conditions, a direct link between in vitro/in vivo susceptibility and the clinical efficacy of BZN is still undefined.
The mechanism of generation of resistance is likely to be multifactorial, involving higher activity of efflux pumps, and mutation and/or modulation of parasite gene expression [Citation34,Citation36,Citation59–Citation62]. A recent whole genome sequencing study showed that mutagenic effects of BZN-reactive metabolites, combined with deficiencies in DNA repair mechanisms, could generate early-stage, extensive alterations in the T. cruzi genome, leading to the development of BZN resistance and other phenotypic changes [Citation63].
3. Clinical research on benznidazole
Clinical R&D for benznidazole (and CD more generally) has been hampered by a lack of investment. Only one large-scale, multicentric, randomized trial has taken place in the last half century [Citation64]. Another challenge involves the difficulty of measuring treatment success during the chronic phase. Detection of the parasite (or parasite DNA) is a probable indication of treatment failure. Early studies relied on xenodiagnosis, while more recently polymerase chain reaction (PCR) has been employed. Negative serology is an effective proxy for parasitological cure in acute, congenital, and early chronic cases. However, in adult patients with chronic CD, it can take over 20 years following treatment to revert to negative serology [Citation12]. There is an urgent need for development of a reliable test of cure to advance research and facilitate treatment.
3.1. Phase I studies
In the 1970s, multiple case series supported the safety of BZN [Citation65–Citation67].
Raaflaub et al. published the first study of BZN resembling a modern Phase I study [Citation68]. In this Roche-sponsored study, BZN’s PK was evaluated in six healthy adult female volunteers aged 22–24 years old, after administration of a single 100 mg BZN dose. The investigators reported quick absorption of the drug (Tmax 3 h), good distribution to tissues (volume of distribution 560 mL/kg) and moderately slow elimination (estimated average half-life of 12 h). No significant adverse events were observed. Despite the small sample, the investigators decided to proceed with a repeated-dose study (comparable to a modern Phase II study) of 14 patients with chronic CD, in which no adverse events were detected [Citation69].
Perhaps the most complete Phase I research to date assessed BZN for treatment of central nervous system cancers in adults (as an adjuvant therapy for CCNU) [Citation70,Citation71]. These studies, which enrolled a higher number of patients and exposed them to incremental BZN doses (up to 40 mg/kg, over four times higher than the doses for CD), yielded PK and safety results compatible with prior research [Citation68,Citation69]. Through biopsies, the investigators confirmed high tissue penetration of BZN, even in the central nervous system (CNS), an observation that later became relevant when treating reactivated CNS T. cruzi infections in immunosuppressed patients [Citation72].
A recent Phase I, open-label, nonrandomized pharmacokinetic study with eight healthy adult volunteers investigated the PK of a single 100 mg dose of BZN and used the calculated non-compartmental PK parameters to simulate two multiple-dose administration regimens: 100 mg administered every 8 h and 150 mg administered every 12 h. Observed PK parameters were similar to those in other studies, but the authors concluded that both simulated regimens reached steady-state concentrations above the minimum experimental therapeutic dose. Men had lower median Cmax and higher median volume of distribution than women, but the limited number of individuals (N = 4 per group) precludes definitive conclusions on the influence of sex on BZN PK [Citation73]. The BZN label mentions that food does not seem to affect absorption of the medication, according to comparative bioavailability studies performed for registration of the product in the USA [Citation36].
Studies in children and lactating women have also been conducted. Early pediatric studies highlighted BZN’s efficacy for treating CD, measured by persistent decreases in conventional antibody titers and seroreversion, compared to placebo, and noted a low frequency of adverse events in children [Citation74,Citation75]. Similarly, a case series and two prospective population pharmacokinetics studies confirmed BZN’s efficacy in infants and children using quantitative PCR (qPCR), which became universally negative after treatment, and conventional serological tests [Citation76,Citation77]. The frequency of adverse events was low, significantly below that reported for adults. Both studies showed a significantly shorter half-life due to higher clearance rates for BZN in younger children and infants, which was inversely proportional to body size. A systematic literature review with a Bayesian meta-analysis of basic pharmacokinetic properties of BZN showed consistency across studies; the authors provided improved estimates of the pharmacokinetic parameters under fasting conditions for a single 100 mg dose of BZN in adults. AUC and Cmax were 51.31 mg h/L (95% credible interval [CrI], 45.01–60.28 mg h/L) and 2.19 mg/L (95% CrI, 2.06, −2.33 mg/L) [Citation78].
4. Phase II studies
In an early study of 73 patients with acute CD treated with BZN, the cure rate (measured by xenodiagnosis) reached 88%. Additionally, three dosing regimens were tested in 33 patients with chronic infection: (a) 7–10 mg/kg/day for 60 days; (b) 7–10 mg/kg/day for 30 days; and (c) 4–5 mg/kg/day for 30 days. There were no statistical differences between treatment efficacy, with >90% of xenodiagnoses negative, but the higher dose produced more side effects [Citation8].
After decades of limited research, several recent studies have evaluated the safety and efficacy of BZN at lower, shorter, and/or intermittent dosing regimens, both as a monotherapy and in combination with other compounds.
4.1. E1224
The E1224 study, sponsored by the Drugs for Neglected Diseases initiative (DNDi) was a proof-of-concept, double-blinded, randomized Phase II clinical trial [Citation79]. E1224 (fosravuconazole), developed by Eisai, Ltd., had demonstrated promising activity against T. cruzi in animal models [Citation53]. Adult Bolivian patients (n = 231) with confirmed chronic indeterminate CD were randomly assigned to five oral treatment groups: high-dose E1224 (8 weeks, total dose 4,000 mg), low-dose E1224 (8 weeks, 2,000 mg), short-dose E1224 (4 weeks E1224 + 4 weeks placebo, 2,400 mg), BZN standard dose (60 days, 5 mg/kg per day), or placebo (8 weeks, E1224-matched tablets). The primary endpoint was parasite clearance. Although E1224 initially proved capable of eliminating the parasite, the effect was not sustained at 12-months follow-up. However, 82% of patients treated with BZN had negative PCR results at 12 months follow-up, underscoring the drug’s efficacy. The study served as a springboard for a currently ongoing DNDi-supported study of BZN at alternative dosing regimens and in combination with E1224 [Citation80].
4.2. CHAGASAZOL
Undertaken in Barcelona, Spain, CHAGASAZOL was a multicenter, randomized, open-label clinical trial assessing two schedules of posaconazole (100 mg/12 h and 400 mg/12 h for 60 days), compared to BZN (5 mg/kg/day for 60 days) in 78 chronic CD patients [Citation81]. During follow-up, a greater proportion of patients treated with posaconazole (81–92% vs. 38% for BZN) had treatment failure, as measured by positive real-time PCR for T. cruzi in peripheral blood.
4.3. STOP CHAGAS
STOP CHAGAS assessed posaconazole and BZN as monotherapies, posaconazole in combination with BZN, and placebo in a sample of 120 subjects from four Latin American countries and Spain [Citation82]. After 180 days of follow-up, only 13.3% of patients treated with posaconazole had negative PCR results, compared with 10% in the placebo arm. However, 80% of patients in the BZN+posaconazole group and 86.7% of patients who received BZN as a monotherapy had negative results. Similar to E1224, posaconazole showed some effectiveness during treatment, but negativization was not sustained in the follow-up period.
4.4. Intermittent regimen
A recent observational study assessed an intermittent dosage scheme of BZN at 5 mg/kg/day in two daily doses every 5 days for a total of 60 days. One patient showed detectable PCR at the end of treatment (1/17), corresponding to 6% treatment failure, compared with 11/17 (65%) patients pretreatment (P = 0.01). Adverse effects were present in 10/20 (50%) patients, but in only one case was treatment suspended [Citation83].
4.5. Ongoing studies
The aforementioned studies supported the effectiveness of BZN for eliminating the parasite during the chronic phase of CD, whereas E1224 and posaconazole demonstrated temporary but unsustained parasite-clearing potential. Consequently, new research is focusing on improved regimens of benznidazole, either as a monotherapy or in combination with E1224. These studies aim to ascertain whether the side-effect profile might be improved while maintaining or even improving the efficacy of the current standard dosing regimen.
Currently, two new proof-of-concept studies are underway. BENDITA (BEnznidazole New Doses Improved Treatment and Associations) is a Phase II randomized, multi-center study in Bolivia assessing benznidazole at shorter or intermittent dosing regimens and in combination with E1224, compared to placebo, in patients with chronic CD (ClinicalTrials.gov Identifier: NCT03378661). MULTIBENZ (Evaluation of Different Benznidazole Regimens for the Treatment of Chronic Chagas Disease) (BERENICE Project, ClinicalTrials.gov Identifier: NCT03191162) is testing a modified regimen of BZN for treatment of CD in the chronic phase compared to the standard scheme [Citation73].
4.6. Phase III studies
Phase III studies have evaluated BZN’s efficacy in eliminating the parasite and improving clinical outcomes. Antiparasitic effects of treatment are measured through detection of antibodies, parasites, and/or parasite DNA. Success of treatment is gauged by negative serology, while therapeutic failure is demonstrated by parasite detection. However, the reliability of these indicators hinges on the following: (a) the age of the patient at the moment he/she received the treatment; (b) the time elapsed between treatment and follow-up; and (c) the region where the patient was infected [Citation7].
Reversion to negative serology is much easier to demonstrate in patients who were more recently infected. Consequently, the efficacy of BZN for treatment of patients in the acute phase due to vector transmission or congenital infection has been clearly shown in several observational studies with a cure rate of consistently over 80% [Citation66,Citation84–Citation88].
Studies assessing the efficacy of BZN in chronically infected patients are summarized in . BZN efficacy in asymptomatic chronically infected children was demonstrated by two double-blinded, placebo-controlled trials in the 1990s. Cure rates, defined as conversion from positive to negative serology 3–4 years after treatment, were approximately 60% [Citation74,Citation75]. In one study, at 48 months follow-up, 51% of the placebo group had a positive xenodiagnosis compared to 4.1% of patients treated with BZN [Citation75]. These studies supported policy change in endemic countries toward treating children with indeterminate chronic CD.
Table 1. Clinical studies to assess efficacy against Trypanosoma cruzi infection in chronic phase. 1971–2018.
As patient age and length of infection increase, the time needed for seroreversion also increases. In one study, which defined treatment success as reversion to negative serology and treatment failure as a positive parasitological (xenodiagnosis) or molecular test (PCR), the rate of negativization was higher in children and adolescents than in adult patients (28.3% vs. 11.1%) in short term (<10 years) follow-up, and substantially higher (49.6% vs. 18.2%) in long-term (>10 years) follow-up ()). Similarly, rates of treatment failure were much lower for children/adolescents compared to adults in both long and short-term follow-up. Timely treatment of children during the early chronic phase can yield a cure rate of up to 80% [Citation89,Citation90], with higher rates observed when long-term follow-up is performed (). Furthermore, following treatment, antibody titer levels decrease much more quickly in children than in adults, even if they do not cross the cut-off to become non-reactive. A marked decrease in antibody titers in children is apparent three months after treatment by enzyme linked immunosorbent assay or immunofluorescence assay, and six months after treatment using indirect haemagglutination assay [Citation74,Citation75].
Figure 1. (a). Percentage of patients with non-reactive serological test (cure) after treatment. Children and adult patients with short- and long-term follow-up. (b). Percentage of patients with positive xenodiagnosis or PCR after treatment. Children and adult patients with short- and long-term follow-up.
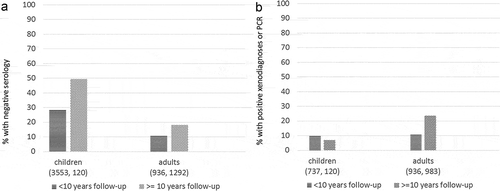
Despite the difficulty of measuring cure in chronically infected adults, most observational studies have demonstrated improved clinical outcomes in patients who are treated with BZN, compared to untreated patients (). In a sample of 566 Argentinian patients, only 4% of the treated group experienced progression of heart disease compared to 14% in the untreated group [Citation11]. Other studies in different settings have described similar results, with reduction in disease progression of up to 75% [Citation12,Citation91–Citation95]. However, other researchers did not observe differences between treated and untreated patients [Citation96,Citation97].
Table 2. Comparison of studies measuring long-term clinical outcomes after etiological treatment of chronic Chagas disease with benznidazole.
Some research indicates treatment with BZN eliminates maternal transmission of CD. In a multicenter, observational study of a cohort of mothers and children, trypanocidal treatment of women with CD proved effective at preventing congenital transmission of T. cruzi and halting disease progression [Citation92]. These results are consistent with other observational studies in which no congenital infection was detected in children born to T. cruzi-positive mothers who were treated before pregnancy [Citation98–Citation101].
A major randomized trial sought to determine whether treatment with BZN could improve outcomes for patients who had already developed heart disease [Citation64]. The BENEFIT trial (Benznidazole Evaluation for Interrupting Trypanosomiasis) was a multicenter, double-blind, placebo-controlled trial of trypanocidal treatment of BZN in 2,854 patients with chronic CD-related cardiomyopathy, conducted in 54 study centers in Argentina, Bolivia, Brazil, Colombia, and El Salvador. BZN was administered at a fixed daily dose of 300 mg for 40–80 days; the time period was adjusted according to body weight. The primary outcome was death, implantation of a pacemaker or defibrillator, transplant, heart failure, or other major cardiac event. Rates of conversion to negative PCR were also measured. After a mean follow-up of 5.4 years, there was not a significant difference in primary outcome between the placebo and BZN-treated group, even though parasite clearance was higher in the latter (66.2% vs. 33.5%). The mean patient age in the trial was 55, and most patients had New York Heart Association class I or II heart failure.
TRAENA (Treatment in Adult Patients, ClinicalTrials.gov Identifier: NCT02386358), another randomized trial, assessed the ability of BZN to prevent progression to chronic CD in a sample of patients that reflected the natural distribution of the disease (70% without demonstrable disease). The trial has recently concluded but results have not yet been published. Another ongoing trial, CHICAMOCHA 3, (ClinicalTrials.gov Identifier: NCT02369978) is evaluating both BZN and NFX versus placebo in a sample of patients with indeterminate CD. Both safety and parasite clearance will be assessed.
While BENEFIT, the only major clinical trial of the past decade, showed BZN had antiparasitic effect (measured by PCR), it was unable to demonstrate a corresponding improvement in clinical outcomes in patients treated with BZN. This likely reflects the advanced disease stage of the patients enrolled. In contrast, Viotti et al.’s observational study examined patients without advanced cardiomyopathy and included a much longer follow-up period (>20 vs. 5.4 years), finding significantly improved clinical outcomes. Therefore, the main lesson of the BENEFIT trial is that antiparasitic treatment should be offered as early as possible, before patients progress to an advanced stage of CD [Citation102].
5. Tolerability and pharmacovigilance
5.1. Adverse effects
Treatment discontinuation due to adverse events (AEs) typically ranges from 15% to 20%. AEs are more frequently observed in adolescents and adults than infants and children [Citation7,Citation76,Citation103]. Usually, treatment tolerance is satisfactory, and patients have not demonstrated serious side effects [Citation7,Citation11,Citation91,Citation103,Citation104]. Although cases with severe side effects are occasionally reported, these are generally associated with difficulties in seeking timely medical attention or receiving adequate care. The most commonly reported AEs from BZN include allergic dermopathy, nausea, and vomiting. Cutaneous reactions are the most common, and are positively associated with treatment withdrawal [Citation105,Citation106]. Less frequently, peripheral polyneuropathy and depression of bone marrow have been observed. Very rarely, cases with extensive lesions from allergic dermopathy, including Steven Johnson Syndrome, have been reported. No new AEs have been described in the literature in the past few decades.
Due to AEs, oral treatments sometimes have to be discontinued. Laboratory tests have typically shown normal bilirubin values and occasional elevation of transaminases. In all cases, AEs disappear when the dose is diminished or treatment is suspended. Children who underwent clinical examination 15 years after experiencing AEs did not show any pathological signs or symptoms associated with the adverse events [Citation107].
Several studies have attempted to identify predictors of adverse reactions to BZN. Two studies concluded that BZN serum concentrations are not correlated with the appearance of serious AEs [Citation108,Citation109]. A Colombian study retrospectively evaluated the safety profile of BZN in 224 adult patients to identify factors for definitive treatment interruption and development of severe reactions [Citation110]. A BZN dose ≥6 mg/kg/day, adverse event severity, eosinophilia, and female sex were the main predictors of treatment interruption.
Differences in patients’ genetic or immunological profile could drive susceptibility to AEs. A study of adult CD patients treated with BZN (100 mg, every 8 h, for 60 days) showed that patients with cutaneous drug reactions had a higher proportion of eosinophilia during treatment, and higher interleukin (IL)-5 and IL-10 serum concentrations at day 15 of treatment than those without cutaneous reactions [Citation111]. Treatment interruption (secondary to moderate–severe cutaneous reactions) was more frequent in patients carrying the HLA-B*3505 allele (45.5% vs. 15.4%, P = 0.033). No differences in BZN serum concentrations were found. The authors concluded that the BZN cutaneous reaction rate is high (38.5%), and related to a delayed hypersensitivity reaction with a Th2-specific immune response. Additionally, the HLA-B*3505 allele could be associated with moderate–severe cutaneous reactions.
5.2. Clinical management of adverse events
Despite the toxicological profile and documented side effects of BZN, data from clinical studies and the extensive use of the drug in Latin America indicate BZN can be used safely with proper management and monitoring. In the BENEFIT trial, only 8.3% of patients had to discontinue treatment secondary to a serious AE, despite the older mean age (55) in the sample [Citation64]. In over 2,000 patients treated with BZN by MSF in Bolivia, only 10.2% discontinued treatment. MSF used a system of weekly follow-up to minimize treatment suspension [Citation106].
Treatment of CD with BZN should ideally take place within primary health care facilities, which are much more accessible to the patient population. During treatment, patients should be continuously monitored. Prior to treatment initiation and biweekly, patients should receive a haemogram and tests of renal and hepatic function. Women of childbearing age should undergo a pregnancy test before initiation, and use contraception during treatment. Management of adverse events depends on their type and severity. Strategies for some of the main AEs for benznidazole are suggested in recent guidelines [Citation15], and may involve temporary reduction of daily dose or temporary suspension of treatment until AEs resolve. If the patient is near the end of treatment, discontinuation may be the best option. According to the Argentinian guidelines, > 30 days is sufficient to consider treatment complete [Citation16].
5.3. Benznidazole during pregnancy and breastfeeding
BZN is traditionally contraindicated in pregnancy because data that support its safety in the fetus are lacking [Citation112]. Nevertheless, when the clinical picture of the patient is severe, and given the known lower risks of fetal toxicity in the second and third trimesters of pregnancy [Citation113], treatment has been administered on a compassionate basis. There are some case reports in which it was necessary to prescribe treatment with BZN during pregnancy due to the risk to the mother’s life in the acute phase after vector/oral transmission [Citation114,Citation115] or reactivation of chronic infection for a patient with AIDS [Citation72]. All these cases showed a benefit for the mothers with a good response to the treatment; no congenital infection was detected in the children born, and no sequelae were described in the children during follow-up.
A prospective study analyzed BZN concentrations in blood samples and breastmilk from 12 lactating women receiving treatment for CD [Citation116]. Median observed breastmilk BZN concentrations were low; the expected exposure to BZN of breastfeeding infants from mothers receiving BZN treatment was estimated at 12% of the mother’s per kg dose, which is considered safe. The researchers concluded mothers’ treatment with BZN should not contraindicate breastfeeding in cases where treatment cannot be postponed.
6. Regulatory affairs
lists countries where BZN is registered. Recently (2017), the drug was registered in Mexico and the United States, the countries with the third and sixth highest global burdens, respectively. BZN is also on the WHO’s list of essential medicines. The Pan American Health Organization, through its strategic fund, purchases BZN and resells it to countries where it is not registered. In these countries, the BZN supply is managed by ministries of health.
Table 3. Global landscape of benznidazole registration, June 2018.
In the United States, BZN was previously only available through the Centers for Disease Control. Physicians were required to submit a special investigational protocol in order to receive a supply of BZN for each patient. In 2017, the FDA approved registration of benznidazole by Chemo Research (a subsidiary of Insud Pharma). The U.S. product is available through a central distributor (for more information see: http://www.benznidazoletablets.com/en).
However, registration alone is insufficient to assure patient access to BZN. Despite the proven benefits of etiological treatment of CD, health systems have struggled to make the drug available to patients. In the United States and other settings, fewer than 1% of expected CD cases have received treatment [Citation19,Citation27,Citation117]. Although 1,908 cases of CD have been identified through testing of U.S. blood donors from 2007 to 2013, only 422 courses of medication were solicited from the CDC, enough to cover just 22% of these cases [Citation19].
Several barriers have prevented patients in need from accessing BZN. Both patient and provider awareness of CD is low [Citation18,Citation118], which keeps down the demand for diagnosis. Furthermore, many providers still operate under the assumption that CD should not be treated in the chronic phase, despite the fact that there is now a consensus in international organizations and national guidelines that most adults with chronic CD should be offered treatment. In addition, CD disproportionately impacts socioeconomically vulnerable patients who have low access to healthcare.
In the United States and Europe, the majority of CD patients are Latin American immigrants, who may be excluded from health insurance coverage or be apprehensive about utilizing health services. A recent study indicated that transportation, lack of providers, low provider awareness, language barriers, and immigration status were potential barriers encountered by U.S. patients with CD [Citation20]. There is no routine screening for CD in the United States outside of blood and organ donations. Increasing access to BZN will require development of broad provider and patient education initiatives, and incorporation of treatment into healthcare services that are accessible to the population at risk.
7. Conclusion
BZN is a small molecule with a nitroheterocyclic structure that shows broad-spectrum trypanocidal activity against T. cruzi strains from different DTUs. It exhibits a time- and concentration-dependent effect against intra- and extracellular forms of T. cruzi. Its mechanism of action involves activation of the parent molecule by trypanosomal type I nitroreductases and generation of reactive metabolites.
BZN produces a clear trypanocidal effect in humans and plays an essential role in primary and secondary prevention. Although treatment with BZN is associated with side effects in some patients, it has a good adherence rate and tolerability, especially when treatment is carefully monitored. Because of the challenges involved in confirming a cure for CD, BZN’s benefit is more readily demonstrated during the acute phase, and for children, adolescents, and young adults with chronic indeterminate CD. Nonetheless, several observational studies suggest that BZN prevents morbimortality in adults. The BENEFIT trial was not able to show a similar effect in a sample of older adults who had already developed heart disease from CD. Therefore, every effort should be made to identify and treat patients early, before CD progresses to an advanced chronic form.
Another important benefit of BZN treatment is primary prevention. When T. cruzi-infected women are treated with BZN, congenital transmission is prevented in subsequent births. This has tremendous public health importance since congenital infection is a major transmission route. Consequently, treatment of women of gestational age should be an integral part of public health strategies. Finally, BZN plays a critical role in reducing the burden of CD and increasing the number of healthy, productive years of life of patients.
8. Expert commentary
Ongoing investigations aim to optimize BZN therapy by adjusting the current standard regimen [Citation73,Citation80,Citation83] or by combining BZN with new chemical entities [Citation80–Citation82]. These studies are assessing alternatives to improve safety while improving or at least maintaining the efficacy of BZN.
Infants and children respond well to BZN treatment, with relatively few, mostly mild adverse events. Treatment early in life produces consistently negative parasitological responses on qPCR, even years after treatment, and often causes reversion to negative serology. For insight on the role of treatment in prevention of chronic complications such as heart disease, studies of treated children with 3 or 4 decades of follow-up will be illustrative. The current absence of this type of long-term follow-up data has forced researchers to base treatment decisions and recommendations on surrogate markers such as antibody titers and parasitological clearance measured by qPCR, which may not have adequate sensitivity and specificity. Nevertheless, in pediatric studies, all the surrogate markers support BZN’s effectiveness. Additionally, monitoring of treated females who bore children later in life has not uncovered a single case of congenital transmission, which strongly supports the value of early treatment of CD.
Public health programs for CD must employ a two-pronged strategy, on the one hand interrupting transmission to prevent occurrence of new cases (these measures are cost-effective), while on the other hand providing timely diagnosis and treatment to infected individuals to prevent clinical evolution of the disease, reduce morbidity, and maximize healthy years of life. When these actions are implemented in a comprehensive, integrated manner, it will be possible to interrupt transmission of T. cruzi in a large territory and eliminate CD as a public health problem, with a dramatic reduction in the burden of the disease [Citation14].
9. Five-year view
Substantial progress has been made in the past decade, improving our understanding of BZN’s efficacy and safety. BZN is likely to remain the first-line treatment for CD in the coming years. Ongoing studies (BENDITA and MULTIBENZ) will provide further insight within the next five years, helping to determine whether intermittent dosage schemes, shorter regimens, and/or lower doses are capable of sustaining the efficacy of the standard regimen while improving the side effect profile. Moreover, the results of the BENDITA trial will indicate whether BZN and E1224 combination therapy can yield superior safety and/or efficacy than the standard regimen. Meanwhile, other compounds in the nitroheterocyclic class continue to be evaluated. Fexinidazole, recently proven effective against human African trypanosomiasis [Citation119], could be the next promising candidate for CD [Citation120–Citation122].
Clinical research continues to be hampered by the lack of a reliable measure of cure. New biomarkers with improved sensitivity to demonstrate failure and success in a timely fashion are needed. Identification of new biomarkers that more accurately measure efficacy could revolutionize clinical research and practice, and some initiatives are ongoing [Citation123].
The 2017 registration of benznidazole in Mexico and the United States removes regulatory barriers in two high-burden countries. Nonetheless, much work is needed to improve access for patients. Persistently low awareness among providers of current CD treatment guidelines, the absence of a gold standard for diagnosis, and a lack of widespread screening and treatment at the primary care level are key barriers. Providers capable of offering treatment are still too few, and often located too far from areas where patients live. Most importantly, CD predominantly impacts socioeconomically vulnerable groups who face significant challenges in accessing healthcare; these challenges are further magnified for immigrants in Europe and the United States who face legal, social, and linguistic barriers. Incorporation of etiological treatment with BZN and NFX into the primary health care system is still in its infancy, but successful models in Bolivia, Argentina, and elsewhere provide a template other programs can follow [Citation124–Citation126].
Key issues
BZN is a prodrug that requires activation by trypanosomal type I nitroreductases (NTRI). The group of reactive metabolites produced during the activation step is toxic, promoting a fast and concentration-dependent kill-off of the parasites.
In vitro, BZN has broad-spectrum trypanocidal activity against intra- and extracellular forms of T. cruzi strains belonging to discrete distinct typing units (DTUs). In several animal models, BZN improves survival, reduces parasite load in blood and key reservoir tissues, decreases antibody response to T. cruzi, and may prevent the development of severe clinical features of the disease.
BZN has a clear trypanocidal effect, which is more evident the more recent the infection. Furthermore, there is growing evidence from observational studies that etiological treatment with BZN reduces morbimortality when administered to adults with chronic CD.
Clinical research with new treatment schemes evaluating alternative doses and durations as well as combination therapies is ongoing, aiming primarily at improving or maintaining efficacy while bolstering safety.
There is absolute consensus that treatment with BZN and NFX should be offered within primary healthcare. Treatment adherence and safety is greatly enhanced when healthcare personnel engage in systematic monitoring of side effects.
BZN has been approved in nine countries in the Americas for the treatment of CD, including the United States and Mexico in August 2017.
Etiological treatment with BZN is recommended for acute and congenital cases, reactivations in immunocompromised patients, and children in the early chronic phase. Treatment should also be offered to adults with chronic infection because of evidence that it reduces morbimortality, and is sometimes considered as a prophylactic measure in patients about to undergo immunosuppressive therapy. Treatment appears to be more effective when administered before the onset of serious cardiac complications (Kuschnir class II or III).
There is a consensus that patient care involving antitrypanosomal treatment be integrated into primary prevention programs, particularly because of its demonstrated ability to eliminate congenital transmission. Preventing new cases is key to eliminating CD as a public health problem.
Box 1. Drug summary.
Declaration of interest
JM Kratz, CJ Forsyth and S Sosa-Estani are employees of DNDi. DNDi supported registration of benznidazole in the U.S. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
DNDi is grateful to its donors, public and private, who have provided funding for all DNDi activities since its inception in 2003.
DNDi received financial support from the following donors: Department for International Development (DFID), UK; Reconstruction Credit Institution-Federal Ministry of Education and Research (KfW-BMBF), Germany; Directorate-General for International Cooperation (DGIS), The Netherlands; Swiss Agency for Development and Cooperation (SDC), Switzerland; Médecins Sans Frontières (Doctors without Borders), International. The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Many thanks to Isabela Ribeiro and Eric Chatelain of DNDi for providing several thoughtful comments and suggestions on the article.
Additional information
Funding
References
- World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. In: Weekly epidemiological record. Geneva: World Health Organization; 2015. p. 33–40.
- Manne-Goehler J, Umeh CA, Montgomery SP, et al. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis. 2016;10(11):e0005033.
- Basile L, Jansa J, Salamanca D, et al. Chagas disease in European countries: the challenge of a surveillance system. Euresurveillance. 2011;16(37):9.
- Lee BY, Bacon KM, Bottazzi ME, et al. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13(4):342–348.
- Franco-Paredes C, Von A, Hidron A, et al. Chagas disease: an impediment in achieving the millennium development goals in Latin America. BMC Int Health Hum Rights. 2007;7(1):7.
- Bocchi EA. Heart failure in South America. Curr Cardiol Rev. 2013;9(2):147–156.
- Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr Opin Infect Dis. 2006;19(6):583–587.
- Cerisola JA. Chemotherapy of Chagas’ infection in man. In Chagas’ Disease Symposium Proceedings pp. 35–47. Pan American Health Organization; Washington, DC: 1977.
- Brener Z. Recent advances in the chemotherapy of Chagas’ disease. Memórias Do Instituto Oswaldo Cruz. 1984;79:149–155.
- Viotti R, Alarcón de Noya B, Araujo-Jorge T, et al. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother. 2014;58(2):635–639.
- Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic chagas disease with benznidazole versus no treatment: A nonrandomized trial. Ann Intern Med. 2006;144(10):724–734.
- Fabbro DL, Streiger ML, Arias ED, et al. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop. 2007;40(1):1–10.
- Tratamiento Etiológico de la Enfermedad de Chagas: conclusiones de una Consulta Técnica. Washington, DC: Pan American Health Organization; Rio de Janeiro, Brasil: Fundación Oswaldo Cruz; 1999.
- Sosa-Estani S, Segura EL. Integrated control of Chagas disease for its elimination as public health problem–a review. Mem Inst Oswaldo Cruz. 2015;110(3):289–298.
- Dias JCP, Ramos AN Jr., Gontijo ED, et al. Second Brazilian consensus on Chagas disease, 2015. Rev Soc Bras Med Trop. 2016;49:3–60.
- Chaben I, editor. Pautas para la atención al paciente infectado con Trypanosoma cruzi (Enfermedad de Chagas). Argentina, Buenos Aires: Ministerio de Salud; 2015.
- Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of chagas disease in the united states: a systematic review. JAMA. 2007;298(18):2171–2181.
- Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16(5):871–872.
- Manne-Goehler J, Reich MR, Wirtz VJ. Access to care for Chagas disease in the United States: a health systems analysis. Am J Trop Med Hyg. 2015;93(1):108–113.
- Forsyth CJ, Hernandez S, Flores CA, et al. “It’s like a phantom disease”: patient perspectives on access to treatment for Chagas disease in the United States. Am J Trop Med Hyg. 2018.
- Raether W, Hanel H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol Res. 2003;90(Supp 1):S19–S39.
- McCalla DR. Mutagenicity of nitrofuran derivatives: review. Environ Mutagen. 1983;5(5):745–765.
- Wilkinson SR, Bot C, Kelly JM, et al. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr Top Med Chem. 2011;11(16):2072–2084.
- Patterson S, Wyllie S. Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends Parasitol. 2014;30(6):289–298.
- Keenan M, Chaplin JH. A new era for chagas disease drug discovery? Prog Med Chem. 2015;54:185–230.
- Grunberg E, Beskid G, Cleeland R, et al. Antiprotozoan and antibacterial activity of 2-nitroimidazole derivatives. Antimicrob Agents Chemother (Bethesda). 1967;7:513–519.
- Cucunuba ZM, Manne-Goehler JM, Diaz D, et al. How universal is coverage and access to diagnosis and treatment for Chagas disease in Colombia? A health systems analysis. Soc Sci Med. 2017;175:187–198.
- Wilkinson SR, Kelly JM. Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev Mol Med. 2009;11:e31.
- Docampo R, Moreno SN. Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis. 1984;6(2):223–238.
- Trochine A, Creek DJ, Faral-Tello P, et al. Benznidazole biotransformation and multiple targets in Trypanosoma cruzi revealed by metabolomics. PLoS Negl Trop Dis. 2014;8(5):e2844.
- Murta SM, Ropert C, Alves RO, et al. In-vivo treatment with benznidazole enhances phagocytosis, parasite destruction and cytokine release by macrophages during infection with a drug-susceptible but not with a derived drug-resistant Trypansoma cruzi population. Parasite Immunol. 1999;21(10):535–544.
- Lambertucci F, Motino O, Villar S, et al. Benznidazole, the trypanocidal drug used for Chagas disease, induces hepatic NRF2 activation and attenuates the inflammatory response in a murine model of sepsis. Toxicol Appl Pharmacol. 2017;315:12–22.
- Perin L, Moreira Da Silva R, Fonseca KD, et al. Pharmacokinetics and tissue distribution of benznidazole after oral administration in mice. Antimicrob Agents Chemother. 2017;61(4).
- de Toranzo EG, Masana M, Castro JA. Administration of benznidazole, a chemotherapeutic agent against Chagas disease, to pregnant rats. Covalent binding of reactive metabolites to fetal and maternal proteins. Arch Int Pharmacodyn Ther. 1984;272(1):17–23.
- Workman P, White RA, Walton MI. Preclinical pharmacokinetics of benznidazole. Br J Cancer. 1984;50(3):291–303.
- Application 209570 (Benznidazole). Center for Drug Evaluation Research, U.S. Food and Drug Administration; Silver Spring, Maryland: 2017.
- Perdomo VG, Rigalli JP, Luquita MG, et al. Up-regulation of ATP-binding cassette transporters in the THP-1 human macrophage cell line by the antichagasic benznidazole. Mem Inst Oswaldo Cruz. 2016;111(11):707–711.
- Polak A, Richle R. Mode of action of the 2-nitroimidazole derivative benznidazole. Ann Trop Med Parasitol. 1978;72(1):45–54.
- Neal RA, van Bueren J. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans R Soc Trop Med Hyg. 1988;82(5):709–714.
- Revollo S, Oury B, Laurent JP, et al. Trypanosoma cruzi: impact of clonal evolution of the parasite on its biological and medical properties. Experimental Parasitology. 1998;89(1):30–39.
- Canavaci AM, Bustamante JM, Padilla AM, et al. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS Negl Trop Dis. 2010;4(7):e740.
- Moreno M, D’Avila DA, Silva MN, et al. Trypanosoma cruzi benznidazole susceptibility in vitro does not predict the therapeutic outcome of human Chagas disease. Mem Inst Oswaldo Cruz. 2010;105(7):918–924.
- Moraes CB, Giardini MA, Kim H, et al. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep. 2014;4:4703.
- Chatelain E. Chagas disease drug discovery: towarda New Era. J Biomol Screen. 2015;20(1):22–35.
- Chatelain E, Konar N. Translational challenges of animal models in Chagas disease drug development: a review. Drug Des Devel Ther. 2015;9:4807–4823.
- Teston AP, Monteiro WM, Reis D, et al. In vivo susceptibility to benznidazole of Trypanosoma cruzi strains from the western Brazilian Amazon. Trop Med Int Health. 2013;18(1):85–95.
- Teixeira AR, Calixto MA, Teixeira ML. Chagas’ disease: carcinogenic activity of the antitrypanosomal nitroarenes in mice. Mutat Res. 1994;305(2):189–196.
- Romanha AJ, Alves RO, Murta SM, et al. Experimental chemotherapy against Trypanosoma cruzi infection: essential role of endogenous interferon-gamma in mediating parasitologic cure. J Infect Dis. 2002;186(6):823–828.
- Bustamante JM, Craft JM, Crowe BD, et al. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis. 2014;209(1):150–162.
- Santos FM, Mazzeti AL, Caldas S, et al. Chagas cardiomyopathy: the potential effect of benznidazole treatment on diastolic dysfunction and cardiac damage in dogs chronically infected with Trypanosoma cruzi. Acta tropica. 2016;161:44–54.
- Garcia S, Ramos CO, Senra JF, et al. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother. 2005;49(4):1521–1528.
- Guedes P, Veloso VM, Tafuri WL, et al. The dog as model for chemotherapy of the Chagas’ disease. Acta tropica. 2002;84(1):9–17.
- Diniz L, Caldas IS, Guedes P, et al. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54(7):2979–2986.
- Santos FM, Lima WG, Gravel AS, et al. Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas’ disease. J Antimicrob Chemother. 2012;67(8):1987–1995.
- Lewis MD, Francisco AF, Taylor MC, et al. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J Biomol Screen. 2015;20(1):36–43.
- Francisco AF, Jayawardhana S, Lewis MD, et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci Rep. 2016;6:35351.
- Ferreira RC, Ferreira LC. Mutagenicity of nifurtimox and benznidazole in the Salmonella/microsome assay. Braz J Med Biol Res = Revista Brasileira de Pesquisas Medicas Biologicas. 1986;19(1):19–25.
- Gorla NB, Castro JA. Micronucleus formation in bone marrow of mice treated with nifurtimox or benznidazole. Toxicol Lett. 1985;25(3):259–263.
- Villarreal D, Nirde P, Hide M, et al. Differential gene expression in benznidazole-resistant Trypanosoma cruzi parasites. Antimicrob Agents Chemother. 2005;49(7):2701–2709.
- Campos MC, Castro-Pinto DB, Ribeiro GA, et al. P-glycoprotein efflux pump plays an important role in Trypanosoma cruzi drug resistance. Parasitol Res. 2013;112(6):2341–2351.
- Campos MC, Leon LL, Taylor MC, et al. Benznidazole-resistance in Trypanosoma cruzi: evidence that distinct mechanisms can act in concert. Mol Biochem Parasitol. 2014;193(1):17–19.
- Zingales B, Araujo RGA, Moreno M, et al. A novel ABCG-like transporter of Trypanosoma cruziis involved in natural resistance to benznidazole. Memórias Do Instituto Oswaldo Cruz. 2015;110:433–444.
- Campos MC, Phelan J, Francisco AF, et al. Genome-wide mutagenesis and multi-drug resistance in American trypanosomes induced by the front-line drug benznidazole. Sci Rep. 2017;7(1):14407.
- Morillo CA, Marin-Neto JA, Avezum A, et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. New England J Med. 2015;373(14):1295–1306.
- Ferreira HD. Clinico-therapeutic trial with benzonidazole in Chagas’ disease. Rev Inst Med Trop Sao Paulo. 1976;18(5):357–364.
- Barclay CA, Cerisola JA. Aspectos farmacológicos e resultados terapéuticos do benzonidazol novo agente quimioterapico para tratamiento da infeccao de Chagas. Prensa Med Argent. 1978;65:239–244.
- Schenone H, Concha L, Aranda R, et al. Chemotherapeutic activity of a nitroimidazolacetamide compound in chronic chagasic infection. Bol Chil Parasitol. 1975;30(3–4):91–94.
- Raaflaub J, Ziegler WH. Single-dose pharmacokinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung. 1979;29(10):1611–1614.
- Raaflaub J. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung. 1980;30(12):2192–2194.
- Roberts JT, Bleehen NM, Lee FY, et al. A phase I study of the combination of benznidazole and CCNU in man. Int J Radiat Oncol Biol Phys. 1984;10(9):1745–1748.
- Roberts JT, Bleehen NM. Benznidazole with CCNU: a clinical phase I toxicity study. Int J Radiat Oncol Biol Phys. 1985;11(2):331–334.
- Bisio M, Altcheh J, Lattner J, et al. Benznidazole treatment of chagasic encephalitis in pregnant woman with AIDS. Emerg Infect Dis. 2013;19(9):1490–1492.
- Molina I, Salvador F, Sanchez-Montalva A, et al. Pharmacokinetics of benznidazole in healthy volunteers and implications in future clinical trials. Antimicrob Agents Chemother. 2017;61(4).
- Sgambatti de Andrade ALS, Zicker F, de Oliveira RM, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348(9039):1407–1413.
- Sosa Estani S, Segura EL, Ruiz AM, et al. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas disease. Am J Trop Med Hyg. 1998;59(4):526–529.
- Altcheh J, Moscatelli G, Moroni S, et al. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics. 2011;127(1):e212–e218.
- Altcheh J, Ribeiro I, Alves F, et al. Population pharmacokinetics of benznidazole in children and adults with Chagas disease. In: American society of tropical medicine and hygiene. Annual Meeting, American Society of Tropical Medicine and Hygiene. November 13–17, Washington (DC); 2013.
- Wiens MO, Kanters S, Mills E, et al. Systematic review and meta-analysis of the pharmacokinetics of benznidazole in the treatment of Chagas disease. Antimicrob Agents Chemother. 2016;60(12)7035–7042.
- Torrico F, Gascon J, Ortiz L, et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis. 2018;18(4):419–430.
- Barreira F. Update on current clinical trials for improving etiological treatment of Chagas disease. In: American society of tropical medicine and hygiene. Annual Meeting, American Society of Tropical Medicine and Hygiene. November 5–9, Baltimore (MD); 2017.
- Molina I, Gómez I Prat J, Salvador F, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. New England J Med. 2014;370(20):1899–1908.
- Morillo CA, Waskin H, Sosa-Estani S, et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. Cruzi Carriers: the STOP-CHAGAS trial. J Am Coll Cardiol. 2017;69(8):939–947.
- Álvarez MG, Hernández Y, Bertocchi G, et al. New scheme of intermittent benznidazole administration in patients chronically infected with trypanosoma cruzi: a pilot short-term follow-up study with adult patients. Antimicrob Agents Chemother. 2016;60(2):833–837.
- Blanco SB, Segura EL, Cura EN, et al. Congenital transmission of Trypanosoma cruzi: an operational outline for detecting and treating infected infants in north-western Argentina. Trop Med Int Health. 2000;5(4):293–301.
- Schijman AG, Altcheh J, Burgos JM, et al. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother. 2003;52(3):441–449.
- Altcheh J, Corral R, Biancardi MA, et al. Anti-F2/3 antibodies as cure marker in children with congenital Trypanosoma cruzi infection. Medicina. 2003;63(1):37–40.
- Chippaux JP, Salas-Clavijo AN, Postigo JR, et al. Evaluation of compliance to congenital Chagas disease treatment: results of a randomised trial in Bolivia. Trans R Soc Trop Med Hyg. 2013;107(1):1–7.
- Alonso-Vega C, Billot C, Torrico F. Achievements and challenges upon the implementation of a program for national control of congenital Chagas in Bolivia: results 2004–2009. PLoS Negl Trop Dis. 2013;7(7):e2304.
- Sosa-Estani S, Viotti R, Segura EL. Therapy, diagnosis and prognosis of chronic Chagas disease: insight gained in Argentina. Memórias do Instituto Oswaldo Cruz. 2009;104:167–180.
- Sguassero Y, Cuesta CB, Roberts KN, et al. Course of chronic trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One. 2015;10(10):e0139363.
- Viotti R, Vigliano C, Armenti H, et al. Treatment of chronic Chagas’ disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127(1):151–162.
- Fabbro DL, Danesi E, Olivera V, et al. Trypanocide treatment of women infected with trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8(11):e3312.
- Fragata-Filho AA, França FF, Fragata C, et al. Evaluation of parasiticide treatment with benznidazol in the electrocardiographic, clinical, and serological evolution of Chagas disease. PLoS Negl Trop Dis. 2016;10(3):e0004508.
- Fragata Filho AA, Silva M, Boainain E. Ethiological treatment of acute and chronic Chagas’ heart disease. Sao Paulo Med J. 1995;113:867–872.
- Gallerano RR, Sosa RR. Interventional study in the natural evolution of Chagas disease. Evaluation of specific antiparasitic treatment. Retrospective-prospective study of antiparasitic therapy. Rev Fac Cien Med Univ Nac Cordoba. 2000;57(2):135–162.
- Lauria-Pires L, Braga MS, Vexenat AC, et al. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. Am J Trop Med Hyg. 2000;63(3–4):111–118.
- Storino R, Auger S, Wojdyla D, et al. Análisis descriptivo multivariado de la enfermedad de Chagas en 2,260 pacientes. Rev Argent Cardiol. 1998;66:17–39.
- Sosa-Estani S, Cura E, Velazquez E, et al. Etiological treatment of young women infected with Trypanosoma cruzi, and prevention of congenital transmission. Rev Soc Bras Med Trop. 2009;42(5):484–487.
- Moscatelli G, Moroni S, García-Bournissen F, et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz. 2015;110(4):507–509.
- Murcia L, Simón M, Carrilero B, et al. Treatment of infected women of childbearing age prevents congenital trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J Infect Dis. 2017;215(9):1452–1458.
- Alvarez MG, Vigliano C, Lococo B, et al. Prevention of congenital Chagas disease by Benznidazole treatment in reproductive-age women. An observational study. Acta Trop. 2017;174:149–152.
- Pecoul B, Batista C, Stobbaerts E, et al. The BENEFIT trial: where do we go from here? PLoS Negl Trop Dis. 2016;10(2):e0004343.
- Yun O, Lima MA, Ellman T, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of médecins Sans Frontières. PLoS Negl Trop Dis. 2009;3(7):e488.
- Sosa-Estani S, Armenti A, Araujo G, et al. Treatment of Chagas disease with benznidazole and thioctic acid. Medicina. 2004;64(1):1–6.
- Villar JC, Perez JG, Cortes OL, et al. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev. 2014;(5):CD003463.
- Sperandio da Silva GM, Mediano MFF, Hasslocher-Moreno AM, et al. Benznidazole treatment safety: the medecins sans frontieres experience in a large cohort of Bolivian patients with Chagas’ disease. J Antimicrob Chemother. 2017;72(9):2596–2601.
- Colantonio LD, Prado N, Segura EL, et al. electrocardiographic abnormalities and treatment with benznidazole among children with chronic infection by trypanosoma cruzi: a retrospective cohort study. PLoS Negl Trop Dis. 2016;10(5):e0004651.
- Pinazo MJ, Guerrero L, Posada E, et al. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic chagas disease. Antimicrob Agents Chemother. 2013;57(1):390–395.
- Soy D, Aldasoro E, Guerrero L, et al. Population pharmacokinetics of benznidazole in adult patients with Chagas disease. Antimicrob Agents Chemother. 2015;59(6):3342–3349.
- Olivera MJ, Cucunubá ZM, Valencia-Hernández CA, et al. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS One. 2017;12(9):e0185033.
- Salvador F, Sanchez-Montalva A, Martinez-Gallo M, et al. Evaluation of cytokine profile and HLA association in benznidazole related cutaneous reactions in patients with Chagas disease. Clin Infect Dis. 2015;61(11):1688–1694.
- Carlier Y, Torrico F, Sosa-Estani S, et al. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis. 2011;5(10):e1250.
- Model prescribing information: drugs used in parasitic diseases. Geneva: World Health Organization; 1995.
- Moretti E, Basso B, Castro I, et al. Chagas’ disease: study of congenital transmission in cases of acute maternal infection. Rev Soc Bras Med Trop. 2005;38(1):53–55.
- Correa VR, Barbosa FG, Melo Junior CA, et al. Uneventful benznidazole treatment of acute Chagas disease during pregnancy: a case report. Rev Soc Bras Med Trop. 2014;47(3):397–400.
- Garcia-Bournissen F, Moroni S, Marson ME, et al. Limited infant exposure to benznidazole through breast milk during maternal treatment for Chagas disease. Arch Dis Child. 2015;100(1):90–94.
- Manne JM, Snively CS, Ramsey JM, et al. Barriers to treatment access for Chagas disease in Mexico. PLoS Negl Trop Dis. 2013;7(10):e2488.
- Sanchez DR, Traina MI, Hernandez S, et al. Chagas disease awareness among Latin American immigrants living in Los Angeles, California. Am J Trop Med Hyg. 2014;91(5):915–919.
- Mesu VKBK, Kalonji WM, Bardonneau C, et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet. 2018;391(10116):144–154.
- Bahia MT, De Andrade IM, Martins TA, et al. Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Negl Trop Dis. 2012;6(11):e1870.
- Caldas S, Caldas IS, Cecilio AB, et al. Therapeutic responses to different anti-Trypanosoma cruzi drugs in experimental infection by benznidazole-resistant parasite stock. Parasitology. 2014;141(12):1628–1637. doi:10.1017/S0031182014000882.
- Bahia MT, Nascimento AF, Mazzeti AL, et al. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease. Antimicrob Agents Chemother. 2014;58(8):4362–4370.
- Pinazo M-J, Thomas MC, Bua J, et al. Biological markers for evaluating therapeutic efficacy in Chagas disease, a systematic review. Expert Rev Anti Infect Ther. 2014;12(4):479–496.
- Pinazo M-J, Pinto J, Ortiz L, et al. A strategy for scaling up access to comprehensive care in adults with Chagas disease in endemic countries: the Bolivian Chagas platform. PLoS Negl Trop Dis. 2017;11(8):e0005770.
- Sartor P, Colaianni I, Cardinal MV, et al. Improving access to Chagas disease diagnosis and etiologic treatment in remote rural communities of the Argentine Chaco through strengthened primary health care and broad social participation. PLoS Negl Trop Dis. 2017;11(2):e0005336.
- Marchiol A, Forsyth CJ, Bernal O, et al. Increasing access to comprehensive care for Chagas disease: development of a patient-centered model in Colombia. Rev Panam Salud Publica. 2017;41:e153.
