ABSTRACT
Introduction: Caplacizumab is a humanized anti-von Willebrand Factor (vWF) Nanobody® for the treatment of acquired Thrombotic Thrombocytopenic Purpura (aTTP). Caplacizumab targets the A1-domain of vWF, inhibiting the interaction between vWF and platelets. Clinical studies conducted in aTTP patients confirmed the rapid and sustained complete suppression of the vWF activity using an initial intravenous dose of 10 mg, and a maintenance subcutaneous 10 mg daily dosing regimen, with corresponding favorable efficacy and safety profiles.
Areas covered: The pharmacokinetics of caplacizumab are non-linear, characterized by a target-mediated disposition and the exposure is dependent upon drug and target concentration over time. The pharmacokinetics of caplacizumab are predictable when considering the turn-over of the circulating vWF and its modulation by the drug over time. Renal and hepatic impairment are not expected to influence the exposure to the drug, and no direct or indirect drug–drug pharmacokinetic interactions are anticipated based on the mechanism of action and the specificity of the pharmacodynamic effect of caplacizumab.
Expert opinion: Caplacizumab prevents the interaction between vWF and platelets, offering a direct and rapid therapeutic intervention to stop microthrombosis. The combination of caplacizumab with plasma exchange and immunosuppression represents an important, potentially life-saving advance in the treatment of aTTP patients.
1. Introduction
Acquired thrombotic thrombocytopenic purpura (aTTP) is an ultra-rare life-threatening autoimmune disorder characterized by thrombotic microangiopathy manifested by microvascular occlusions and consequent organ ischemia, hemolytic anemia, and thrombocytopenia. It is an ultra-orphan disease with an annual incidence of six cases per million per year in the United Kingdom [Citation1], between 3 and 11 per million people in the U.S.A. [Citation2,Citation3], and is more common in women (2:1 vs. men). The incidence in children (<18 years) is much lower, about 3% of that in adults [Citation3].
aTTP is caused by a severe deficiency of the von Willebrand factor (vWF) cleaving enzyme ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, number 13) due to the presence of inhibitory autoantibodies to ADAMTS-13 [Citation4]. As a result, ultra-large multimers of vWF (ULvWF) are not cleaved appropriately, the ULvWF binds to platelets inducing formation of microthrombi. The consumption of platelets in the formation of thrombi results in thrombocytopenia, a major sign of aTTP, which can present as symptoms epistaxis, bruising, petechiae, hematuria, and gastrointestinal bleeding [Citation5–Citation7]. Red blood cells get mechanically damaged when passing through obliterated small vessels, resulting in the production of fragmented erythrocytes (schistocytes) and microangiopathic hemolytic anemia (MAHA). The formation of the microthrombi may lead to obstruction of vessels, causing tissue ischemia, and organ dysfunction, predominantly in the brain, heart, and kidneys, resulting in long term complications or even early death.
1.1. Treatment approaches
Plasma exchange (PE) and immunosuppression currently constitute the standard of care treatment for aTTP. The therapeutic plasma acts as a replacement therapy and replenishes functional ADAMTS13, while the exchange procedure removes the anti-ADAMTS13 antibodies and ULvWF multimers [Citation8]. Glucocorticoids, vincristine, cyclophosphamide, splenectomy, and rituximab have been used as immunomodulating treatments. The current treatments do not directly address the microthrombosis that is characteristic for TTP [Citation9].
Despite recent advances in understanding the disease, and the replacement and immunosuppressive therapies, episodes of aTTP are still reported to be associated with an acute mortality of up to 20% [Citation10,Citation11]. In addition, patients remain at risk for refractory disease [Citation12–Citation14], which has been identified as an indicator of a poor prognosis for survival, and disease exacerbations after stopping daily PE [Citation15]. In addition to the acute risks of disease, patients experiencing an episode of aTTP may suffer long-term consequences, such as cognitive deficits, depression, and arterial hypertension [Citation16]. They are also at risk for recurrence that has been reported to occur in up to 84% of patients with aTTP [Citation17,Citation18]. Caplacizumab prevents the interaction of vWF with platelets, offering a direct and rapid therapeutic intervention to stop microthrombosis. The combination of caplacizumab with PE and immunosuppression represents an important, potentially life-saving advance in the treatment of aTTP patients.
1.2. Chemistry
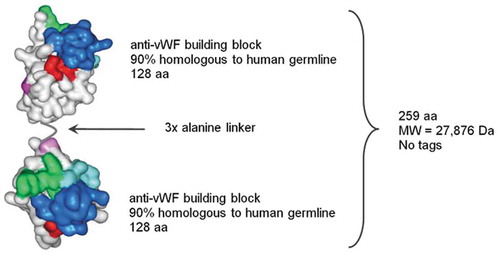
Caplacizumab is a bivalent Nanobody® produced in E. coli and consists of two identical, humanized building blocks, joined by a tri-alanine linker (). Caplacizumab binds to the A1 domain of vWF and specifically inhibits the interaction between vWF and the glycoprotein (GP)1b-IX-V receptor on platelets [Citation19]. The molecular weight (MW) of caplacizumab is approximately 28 kDa.
1.3. Introduction to vWF and caplacizumab
vWF, a key protein in hemostasis, is an adhesive, multimeric plasma glycoprotein with a pivotal role in the recruitment of platelets to sites of vascular injury. More than 90% of circulating vWF is expressed by endothelial cells and secreted into the systemic circulation as multimers [Citation20]. The vWF multimers exist in a globular, conformationally flexible form which unfolds under high shear stress conditions, such as in arterioles and capillaries [Citation21]. In elongated ULvWF multimers, the platelet-binding A1 domain is constitutively active, and able to interact spontaneously with the GP1b-IX-V receptor on platelets [Citation22,Citation23].
In healthy individuals [Citation24], ULvWF multimers are immediately cleaved into smaller, regular-sized multimers by the vWF-cleaving protease ADAMTS13 [Citation25,Citation26]. In patients with aTTP, autoantibodies have developed to ADAMTS13, thus impairing the normal enzyme activity, leading to impaired processing and accumulation of ULvWF multimers [Citation4]. Consequently, platelets spontaneously adhere to these ULvWF multimers leading to microvascular platelet-rich thrombi in high-shear blood vessels () [Citation7,Citation9,Citation27,Citation28].
Figure 2. Mechanism of action of caplacizumab in aTTP [Citation28]. Reprinted from transfus apher sci, volume 46, Holz JB, The TITAN trial–assessing the efficacy and safety of an anti-von Willebrand factor Nanobody in patients with acquired thrombotic thrombocytopenic purpura, pages 343–346, Copyright (2012), with permission from Elsevier.
![Figure 2. Mechanism of action of caplacizumab in aTTP [Citation28]. Reprinted from transfus apher sci, volume 46, Holz JB, The TITAN trial–assessing the efficacy and safety of an anti-von Willebrand factor Nanobody in patients with acquired thrombotic thrombocytopenic purpura, pages 343–346, Copyright (2012), with permission from Elsevier.](/cms/asset/b90c2d65-2ac5-442e-bf02-d199da3e3d58/ierj_a_1607293_f0002_oc.jpg)
Caplacizumab binds to vWF in both its active (i.e. ULvWF multimers or normal multimers activated through immobilization or shear stress) and inactive forms (i.e. multimers prior to conformational change of A1 domain), thereby immediately blocking the interaction of vWF with platelets and preventing further platelet consumption and new microthrombotic damage ().
The interaction of caplacizumab with vWF is highly specific, and binding of the Nanobody® to the vWF A1 domain does not affect the capacity of vWF to interact with coagulation factor VIII (FVIII), for which vWF has a carrier function [Citation29]. Similarly, the selective binding of caplacizumab does not affect the capacity of vWF to interact with fibrillar collagens, collagen type VI or ADAMTS13. The Nanobody® does not cross react with erythrocytes or platelets. Due to this high specificity, off-target effects are not expected and have not been observed in preclinical studies and clinical trials.
In this review, we aimed at providing an integrated summary of the clinical pharmacology of caplacizumab.
2. Pharmacodynamics
Levels of total (free + drug-complexed) vWF, ristocetin cofactor activity (RICO), and FVIII levels were measured in healthy volunteers after single ascending intravenous or subcutaneous administration and multiple 10 mg daily subcutaneous administration [Citation30,Citation31], and in aTTP patients after multiple 10 mg once daily subcutaneous doses or placebo following an initial 10 mg intravenous bolus or placebo [Citation32,Citation33].
2.1. vWF antigen (vWF:Ag)
At baseline, vWF:Ag levels are higher in aTTP patients than in healthy subjects. In clinical studies conducted with caplacizumab, the mean (± standard deviation [SD]) plasma vWF:Ag levels were 38.5 ± 10.9 nM in healthy subjects and 70.5 ± 30.0 nM in aTTP patients. Caplacizumab treatment impacts vWF disposition, resulting in a transient reduction of total circulating vWF:Ag levels. On average, this effect is reversed within 2–7 days after final dose administration in healthy volunteers and aTTP patients () [Citation31,Citation33,Citation34]. The transient decrease of total vWF:Ag levels is attributed to a faster elimination of the drug–target complex compared to the free target.
Figure 3. Mean vWF:Ag levels vs time profiles during a 7 days repeated subcutaneous 10 mg daily dosing of caplacizumab in healthy volunteers (a) [Citation31], and during repeated subcutaneous daily administration of 10 mg caplacizumab in aTTP patients (b) [Citation33,Citation34]. aTTP: acquired thrombotic thrombocytopenic purpura; PE: plasma exchange; FU: follow-up; vWF: von Willebrand factor.
![Figure 3. Mean vWF:Ag levels vs time profiles during a 7 days repeated subcutaneous 10 mg daily dosing of caplacizumab in healthy volunteers (a) [Citation31], and during repeated subcutaneous daily administration of 10 mg caplacizumab in aTTP patients (b) [Citation33,Citation34]. aTTP: acquired thrombotic thrombocytopenic purpura; PE: plasma exchange; FU: follow-up; vWF: von Willebrand factor.](/cms/asset/714a22da-4003-4ca2-833c-4ecdff125218/ierj_a_1607293_f0003_oc.jpg)
2.2. RIPA/RICO
The ristocetin platelet aggregation (RIPA) and RICO assays are in vitro assays to evaluate the platelet-binding capacity of vWF present in the blood. The RIPA and RICO assay methodology is based on addition of ristocetin to plasma in the presence of platelets that causes platelet agglutination [Citation35]. The antibiotic ristocetin activates vWF to a similar extent as high shear blood flow conditions, and consequently modulates the binding of vWF to the platelet receptor GP1b. As in vitro platelet aggregation can be blocked by the interaction of caplacizumab with vWF, these methods were selected to evaluate the activity of caplacizumab during treatment in the clinical development program. Full inhibition of vWF mediated platelet adhesion by caplacizumab is indicated by RIPA or RICO activity decreasing below 10% or 20%, respectively. Complete stable target inhibition for at least 24 h was observed after a single subcutaneous dose of ≥10 mg in healthy volunteers. This 10 mg subcutaneous dose, given daily, also elicited full inhibition of vWF-mediated platelet adhesion in patients with aTTP throughout the complete treatment period () [Citation32,Citation33]. In all clinical studies, the RICO activity recovered to baseline values within 7 days upon discontinuation of the study drug.
Figure 4. Mean (± SD) RICO activity in the phase II (left panel) [Citation32] and the phase III (right panel) [Citation33]. RICO values of <20% represent the threshold for pharmacological activity of caplacizumab. Graphs show mean ± standard error of the mean. PE: plasma exchange; FU: follow-up; RICO: ristocetin cofactor; SD: standard deviation; vWF: von Willebrand factor.
![Figure 4. Mean (± SD) RICO activity in the phase II (left panel) [Citation32] and the phase III (right panel) [Citation33]. RICO values of <20% represent the threshold for pharmacological activity of caplacizumab. Graphs show mean ± standard error of the mean. PE: plasma exchange; FU: follow-up; RICO: ristocetin cofactor; SD: standard deviation; vWF: von Willebrand factor.](/cms/asset/8f57d2e2-b56a-49a9-b5bb-e87291843468/ierj_a_1607293_f0004_oc.jpg)
2.3. FVIII
vWF acts as a carrier for FVIII. The modulation of total vWF levels by caplacizumab results also in a transient reduction of the levels of FVIII, and as for the vWF, the recovery of FVIII to normal ranges is observed within 2–7 days after final dose administration in healthy volunteers and aTTP patients.
2.4. QT/QTC studies
No cardiovascular effect was observed in nonclinical studies. There was no evidence for clinically relevant ECG findings in the completed human studies. Given the molecular structure and size of caplacizumab, its target specificity, and the absence of in vivo cardiovascular liability, caplacizumab is not expected to prolong the QT interval.
3. Pharmacokinetics
Levels of total (free + target-bound) caplacizumab concentrations have been measured in plasma of healthy volunteers and aTTP patients. Full pharmacokinetic profiles were obtained in healthy subjects after single ascending intravenous infusions, and single and repeated subcutaneous dose administration () [Citation31]. Sparse plasma samples were obtained in aTTP patients in the phase II [Citation32,Citation36] and phase III [Citation33,Citation34] trials. The pharmacokinetic profile of caplacizumab has been investigated through standard non-compartmental analysis (NCA) in healthy volunteers, and in population pharmacokinetic analyses in healthy volunteers and aTTP patients [Citation37]. After repeated 10 mg daily subcutaneous administration, steady-state is rapidly attained as of the second drug administration, with a limited accumulation of caplacizumab, dependent upon the expression of the target vWF:Ag.
Figure 5. Caplacizumab plasma concentration versus time profile after administration of single ascending subcutaneous doses (a), and after single (Day 1 solid line) and repeated (Day 7 dotted line) administration of 10 mg daily subcutaneous doses for seven days (b) in healthy volunteers [Citation31]. sc: subcutaneous.
![Figure 5. Caplacizumab plasma concentration versus time profile after administration of single ascending subcutaneous doses (a), and after single (Day 1 solid line) and repeated (Day 7 dotted line) administration of 10 mg daily subcutaneous doses for seven days (b) in healthy volunteers [Citation31]. sc: subcutaneous.](/cms/asset/d079ce84-0e94-4ad5-bef8-8f6e6439f171/ierj_a_1607293_f0005_oc.jpg)
3.1. Absorption
Main exposure parameters, estimated by standard non-compartmental methods after single dose in healthy volunteers or model-predicted at steady-state in aTTP patients, are reported in and .
Table 1. Pharmacokinetic parameters following single 10 mg intravenous or subcutaneous administration of caplacizumab in healthy volunteers.
Table 2. Simulated mean steady-state pharmacokinetic exposure parameters (AUC, Cmax, Cmin, and Cavg) and corresponding pharmacodynamic effect (vWF:Ag change from baseline) following 40 days of 10 mg subcutaneous daily administration of caplacizumab in aTTP patients.
After single dose administration, the extent (area under the curve; AUC) and rate of exposure (maximal concentrations; Cmax) increase with the administered dose, but not proportionally. After subcutaneous administration of 10 mg, caplacizumab Cmax are attained 4 h post-dose. The absolute bioavailability determined in the population pharmacokinetic analysis was estimated to 90% in aTTP patients, and close to 100% in healthy volunteers.
3.2. Distribution and metabolism
Caplacizumab pharmacokinetics present a biphasic plasma profile. A central volume of distribution of 5.35 and 6.33 L was estimated in healthy volunteers and aTTP patients, respectively, by the population pharmacokinetic model, while the peripheral volume of distribution was estimated to 27 L.
Preclinical studies in cynomolgus monkeys indicate that in the systemic circulation caplacizumab binds and neutralizes its vWF activity within five minutes. The caplacizumab-vWF complex, the major circulating caplacizumab form, distributes to the liver, where similarly to the unbound vWF [Citation24,Citation38] it is rapidly catabolized by the reticuloendothelial system. Preclinical studies suggest that the excess of unbound caplacizumab distributes to other well-perfused organs/tissues, where it is degraded by high capacity aspecific catabolism. Free caplacizumab, with a MW of 28 kDa can be filtered through the glomerulus, although not freely. The renal contribution to the overall elimination of small proteins depends on the proteolytic activity in other body regions [Citation39]. For caplacizumab the fraction of the administered dose recovered in urine is negligible (<0.5%) [Citation31].
3.3. Elimination
The apparent clearance of caplacizumab varies with the administered dose. After a single intravenous dose of 10 mg in healthy volunteers a mean clearance of 769 ± 343 mL/h and a mean terminal half-life of 19.2 ± 7.5 h were estimated using a model-independent method [Citation31]. The pharmacokinetics of caplacizumab after subcutaneous dosing seem absorption controlled. After a single subcutaneous dose of 10 mg, the mean apparent clearance is 386 ± 160 mL/h and the mean terminal half-life 38.5 ± 22.2 h in healthy volunteers [Citation31].
3.4. Dose proportionality
With increasing caplacizumab subcutaneous doses from 2 to 16 mg in healthy subjects, the increase in Cmax and AUC was less than dose proportional. For a dose increase ratio of 1.0:2.0:4.0:5.0:8.0, the mean Cmax ratio was 1.0:1.8:2.0:2.4:2.8 and the mean AUC increased in a ratio of 1.0:3.9:4.1:5.3:6.1. The total clearance of caplacizumab depends on the drug and target levels and is the sum of a linear (catabolic) and non-linear (target-mediated) clearance. The terminal half-life of caplacizumab is also drug- and target-level dependent. In healthy volunteers, the mean apparent terminal half-life increased from 13 to 40 h after single intravenous dose (0.5–12 mg), and from 11 to 53 h after single subcutaneous administration (2–16 mg).
3.5. Effect of demographic factors and body size
The population pharmacokinetic analysis in aTTP patients showed that age, gender, race, and blood group do not affect the pharmacokinetics of caplacizumab. Body weight and renal function, as expressed by the creatinine clearance (CrCL), have a statistically significant effect on the pharmacokinetics, with an expected higher exposure in patients with a lower bodyweight and CrCL. However, the expected exposure range in patient populations with extreme values of these covariates are largely overlapping, as shown in , and no specific dose-adjustment is deemed necessary. Baseline vWF levels have a statistically significant effect on drug exposure, but the increased drug exposure for patients with elevated vWF does not result in a different pharmacodynamic effect (change from baseline vWF level), and no individual dose-adjustment is deemed necessary [Citation37].
Table 3. Simulated median steady-state pharmacokinetic exposure parameters (AUC, Cmax, Cmin) and corresponding pharmacodynamic effect (vWF:Ag change from baseline) following 40 days of 10 mg subcutaneous daily administration of caplacizumab in aTTP patients with low bodyweight, high baseline vWF levels, and low CrCL.
3.6. Pharmacokinetic in special populations
No formal study with caplacizumab has been conducted in patients with severe acute or chronic hepatic impairment and no data regarding the use of caplacizumab in these populations are available. The risk associated with the use of caplacizumab in mild and moderate hepatically impaired patients is considered similar to that of the overall patient population, as the caplacizumab–vWF complex is expected to be cleared through the activated Kupffer cells rather than through the damaged hepatocellular parenchyma. Caplacizumab should be used with caution in patients with severe hepatic impairment, presenting an increased risk of bleeding.
No formal study of the effect of renal impairment on the pharmacokinetics of caplacizumab has been conducted. In the population pharmacokinetic/pharmacodynamic model, renal function (baseline CrCL, range: 11.9 to >120 mL/min) had a statistically significant effect resulting in a limited increase in predicted exposure (AUCss) in severe renal impairment [Citation37]. In the clinical studies of patients with aTTP, those with decreased renal function, including those on hemodialysis for acute renal failure, did not show an additional risk of adverse events.
No pediatric patient was ultimately recruited in the clinical trials. To support the use of caplacizumab in pediatrics, simulations were performed based on the pharmacokinetic/pharmacodynamic model developed in adults to establish a suitable dosing regimen in adolescents and children above 2 years, under the assumption of similar vWF:Ag levels and disease effect as in adults [Citation40,Citation41]. The recommended dose in adolescents 12–18 years with a body weight ≥40 kg is 10 mg, and 5 mg if <40 kg. Since no differences are expected based on differences in age, the same dosing recommendation applies for children 2–12 years, 10 mg if the body weight is ≥40 kg and 5 mg if <40 kg.
3.7. Drug–drug interactions
No in vitro drug–drug interaction study was conducted for caplacizumab, since Nanobodies, as single-variable domain antibody fragments, are expected to be catabolized by ubiquitous proteolytic enzymes, and do not interact directly with cytochrome P450 isoforms or other metabolizing enzymes or transporters. While cytokine modulation may be an indirect mechanism through which Nanobodies could alter CYP expression, cytokine mediated CYP-related drug–drug interactions are unlikely for caplacizumab, as it selectively targets vWF and is not expected to have immune-modulatory properties.
Treatment of aTTP often involves the use of corticosteroids and rituximab, with aspirin and low MW heparin sometimes used as thromboprophylaxis. More rarely, vincristine, cyclophosphamide, or cyclosporine, have been used in refractory disease [Citation42]. Except for rituximab, all these compounds are known to be extensively cleared by the liver through CYP-mediated pathways while the renal clearance pathway is only a minor route, and no drug-nanobody interaction is anticipated in case these treatments are initiated in combination with caplacizumab. As for other monoclonal antibodies, rituximab degradation occurs by non-specific high-capacity proteolysis, with no expected drug–drug interaction potential.
The standard of care in aTTP is PE. The latter has an effect on the elimination of free caplacizumab, free vWF, and caplacizumab–vWF complex. The effect of PE has been characterized in the population pharmacokinetic model. After the first intravenous administration of 10 mg caplacizumab, the 10 mg daily subcutaneous dosing maintains the suppression of vWF activity during and after the PE period without need for any specific further dosing adjustment.
Caplacizumab selectively and specifically inhibits the A1 domain of vWF. In vitro, caplacizumab only partially competes with the binding of heparin to vWF. In vivo, pharmacodynamic interactions between co-administered heparin and caplacizumab are not expected to be clinically meaningful, since the main target effect of heparin is mediated by binding to the enzyme inhibitor antithrombin.
3.8. Dose–response relationship
Initial confirmation of the expected pharmacological response and safety of caplacizumab was obtained in studies in healthy volunteers after single ascending intravenous and single and repeated sub-cutaneous dose administration.
In the first in human study, caplacizumab was administered as a single intravenous infusion over 60 min, at doses ranging from 0.5 to 12 mg or placebo. Caplacizumab was safe over the whole tested dose range, with only mild and transient adverse events, and a reduction of FVIII and vWF:Ag plasma levels, which were fully reversible. The pharmacological activity of caplacizumab, measured by RIPA as biomarker, started at 2 mg and reached a maximum duration of 12 h at 12 mg total dose.
The single subcutaneous dosing study in healthy volunteers consisted of a single ascending dose (doses 2–16 mg) and a multiple dose part, at a daily dose level of 10 mg, selected based on the pharmacodynamic response (complete RICO suppression for 24 h) observed in the single dose part of the study. The target pharmacodynamic effect was maintained during the whole caplacizumab treatment period.
A treatment regimen including an initial intravenous 10 mg dose, enables a fast and complete inhibition of vWF, followed by a daily 10 mg subcutaneous dosing to maintain this effect, as has been tested in the phase II [Citation32,Citation36] and phase III [Citation33,Citation34] trials in aTTP patients. Results from these studies confirmed the desired pharmacodynamic effect (), along with clinical efficacy in terms of time to platelet count normalization and favorable safety profile, with mild to moderate bleeding-related adverse events.
3.9. Exposure–response relationship
To assess the exposure–response relationship of caplacizumab treatment, clinical data from the phase II and III studies (TITAN [Citation32,Citation36] and HERCULES [Citation33,Citation34]) were pooled, and efficacy and safety profiles were compared after stratification of the patients by quartile of observed drug level at the end of PE (efficacy and safety) and at the last visit post-PE (safety). At the end of the PE period, concentrations of caplacizumab increased by 2.7-fold from quartile 1 (223.33 ± 41.34 ng/mL) to quartile 4 (611.95 ± 94.65 ng/mL). At the last treatment visit, levels of caplacizumab in quartile 4 (770.67 ± 214.93 ng/mL) were 2.7-fold greater than in quartile 1 (284.75 ± 58.57 ng/mL).
Time to confirmed platelet response appears similar across the four quartiles () suggesting the exposures were at plateau of the exposure–response curve.
Table 4. Efficacy endpoints by pharmacokinetic quartile at the end of Plasma Exchange and end of the study drug (caplacizumab) treatment period in aTTP patients from the phase II and III studies.
There was no trend towards a higher incidence of treatment emergent adverse events (TEAEs) or bleeding events with higher exposure to caplacizumab from quartile 1 to quartile 4, either at the end of the PE period or at the end of study drug treatment period ().
Table 5. Percentage of patients reporting adverse events by body system and preferred term at end of Plasma Exchange and end of study drug (caplacizumab) treatment by quartile of exposure from the phase II and III studies in aTTP patients.
Data available from in vitro studies demonstrate that a similar target occupancy is required to neutralize vWF activity as measured by RICO in healthy volunteers and aTTP patients. The exposure-response relationship of caplacizumab has therefore been characterized based on the biomarker vWF [Citation37]. Simulations based on the available pharmacokinetic/pharmacodynamic model confirm the adequacy of the proposed caplacizumab dosing regimen to attain the target pharmacokinetic/pharmacodynamic effect.
4. Conclusion
Caplacizumab, by binding to the A1 domain of vWF, suppresses its platelet adhesion activity as evidenced by the complete and sustained RICO suppression obtained in clinical trials. As such, it prevents the subsequent formation of microthrombi in aTTP patients, with a favorable safety profile. The combination of caplacizumab with PE and immunosuppression represents an important, potentially life-saving advance in the treatment of aTTP patients.
5. Expert opinion
The understanding of the pathophysiology of aTTP has significantly increased in the last decades. The introduction of PE for the treatment of aTTP decreased the mortality from an acute episode of aTTP from nearly 100% to 15–20% [Citation10,Citation43]. Subsequently, the immunologic nature of the disease was unraveled [Citation44] and the combination of immunosuppressives (such as corticosteroids and, increasingly, rituximab) with PE are currently the mainstay of therapy. However, while PE and immunosuppression is effective, it takes time to attain its full effect and leaves the patients at risk for major thrombotic complications and even acute death. The use of therapeutic agents with a direct effect to prevent the formation of microthrombi are therefore deemed necessary to protect patients until there is a complete resolution of the underlying immunological disease. As indicated by the successful phase II and phase III trials conducted in patients with aTTP, caplacizumab immediately halts vWF-mediated platelet adhesion and platelet consumption in microthrombi and protects patients with aTTP from recurrences until immunosuppression has reached its full effect, representing an important, potentially life-saving advance in the treatment of patients with aTTP.
In terms of real-life clinical practice, no drug–drug interaction potential is anticipated with the use of caplacizumab, and based on current knowledge no dose-adjustment is deemed necessary in special populations. Although dosing recommendations exist for the pediatric population, caplacizumab has not yet been used in this patient population. Caplacizumab is well tolerated and has a favorable safety profile, with mucocutaneous bleeding reported as the most common adverse effect, consistent with its mechanism of action. Like other biological medicinal products, caplacizumab has the potential to trigger immune reactions. No severe immune reactions have been attributed to caplacizumab in the clinical trials supporting its approval, with both the phase II and phase III trials reporting very consistent results with low overall immunogenicity. The long-term follow-up study of the phase III trial will collect information on immunogenicity and hypersensitivity reactions, which will add to the current body of evidence.
Looking to the future for aTTP is somewhat challenging, as the introduction of caplacizumab already represents a substantial shift in the current treatment paradigm and helps to fill an important unmet need for patients with aTTP. While the ultimate goal of treatment is ever prevention or cure, here, it could be considered to be rapid resolution of an episode of aTTP including normalization of the underlying immunological components of the disease, thereby providing patients with an effective and durable treatment option. In this respect, clearly much progress has been made as the addition of caplacizumab to PE plus immunosuppression gives patients and physicians the time they need for the immunosuppressive components to control the auto-immune disease effectively. Beyond this, areas of research and attention are the further understanding of the processes leading to acquired ADAMTS13 deficiency, faster diagnosis and initiation of therapy, and prevention of life-threatening recurrent episodes of aTTP.
Article Highlights
Caplacizumab is a humanized anti-vWF Nanobody® that blocks the vWF-mediated platelet adhesion and microthrombi formation
Caplacizumab is administered as an initial 10 mg intravenous dose, followed by a 10 mg daily subcutaneous dosing for at least 30 days after end of daily PE
The proposed dosing regimen produces a sustained complete blockade of the vWF mediated platelet adhesion in patients with aTTP
Phase II (NCT01151423) and III (NCT02553317) clinical trials demonstrated the safety and efficacy of caplacizumab used in conjunction with standard of care in adult patients suffering from aTTP
Caplacizumab is the first drug approved for the treatment of aTTP
Declaration of interest
All authors are employees of Ablynx NV. C Tersteeg was an employee of Ablynx NV at the time the studies were conducted. She is now Researcher at VIVES University of Applied Sciences, Belgium. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Scully M, Yarranton H, Liesner R, et al. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142:819–826.
- Rajan SK Thrombotic thrombocytopenic purpura. BMJ best practice topics. 2017. [cited 2019 Apr]. Available from: https://bestpractice.bmj.com/topics/en-gb/715
- Reese JA, Muthurajah DS, Kremer Hovinga JA, et al. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60:1676–1682.
- Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594.
- Moake JL. Thrombotic thrombocytopenic purpura: the systemic clumping “plague”. Annu Rev Med. 2002;53:75–88.
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129:2836–2846.
- Kremer Hovinga JA, Coppo P, Lammle B, et al. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020.
- Coppo P. Management of thrombotic thrombocytopenic purpura. Transfus Clin Biol. 2017;24:148–153.
- Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: toward targeted therapy and precision medicine. Res Pract Thromb Haemost. 2019;3:26–37.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med. 1991;325:393–397.
- Han B, Page EE, Stewart LM, et al. Depression and cognitive impairment following recovery from thrombotic thrombocytopenic purpura. Am J Hematol. 2015;90:709–714.
- Benhamou Y, Boelle PY, Baudin B, et al. Cardiac troponin-I on diagnosis predicts early death and refractoriness in acquired thrombotic thrombocytopenic purpura. experience of the French thrombotic microangiopathies reference center. J Thromb Haemost. 2015;13:293–302.
- Chemnitz JM, Uener J, Hallek M, et al. Long-term follow-up of idiopathic thrombotic thrombocytopenic purpura treated with rituximab. Ann Hematol. 2010;89:1029–1033.
- Sayani FA, Abrams CS. How I treat refractory thrombotic thrombocytopenic purpura. Blood. 2015;125:3860–3867.
- Coppo P, Veyradier A. Current management and therapeutical perspectives in thrombotic thrombocytopenic purpura. Presse Med. 2012;41:e163–176.
- Deford CC, Reese JA, Schwartz LH, et al. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood. 2013;122:2023–2029, quiz 2142.
- Falter T, Alber KJ, Scharrer I. Long term outcome and sequelae in patients after acute thrombotic thrombocytopenic purpura episodes. Hamostaseologie. 2013;33:113–120.
- Thejeel B, Garg AX, Clark WF, et al. Long-term outcomes of thrombotic microangiopathy treated with plasma exchange: A systematic review. Am J Hematol. 2016;91:623–630.
- Ulrichts H, Silence K, Schoolmeester A, et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood. 2011;118:757–765.
- Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91:1–19.
- Springer TA. von Willebrand factor, jedi knight of the bloodstream. Blood. 2014;124:1412–1425.
- Luo GP, Ni B, Yang X, et al. von Willebrand factor: more than a regulator of hemostasis and thrombosis. Acta Haematol. 2012;128:158–169.
- Huizinga EG, Tsuji S, Romijn RA, et al. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179.
- Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019–2028.
- Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494.
- Crawley JT, de Groot R, Xiang Y, et al. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118:3212–3221.
- Knöbl P. Thrombotic thrombocytopenic purpura. Memo. 2018;11:220–226.
- Holz JB. The TITAN trial–assessing the efficacy and safety of an anti-von Willebrand factor nanobody in patients with acquired thrombotic thrombocytopenic purpura. Transfus Apher Sci. 2012;46:343–346.
- Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92:3983–3996.
- Bioequivalence of liquid and reconstituted lyophilized subcutaneous formulations of caplacizumab. [cited 2019 Apr]. Available from: https://ClinicalTrials.gov/show/NCT02189733.
- Caplacizumab single and multiple dose study in healthy Japanese and white subjects. [cited 2019 Apr]. Available from: https://ClinicalTrials.gov/show/NCT03172208.
- Study to assess efficacy and safety of anti-von Willebrand factor nanobody in patients with acquired Thrombotic Thrombocytopenic Purpura (TTP). [cited 2019 Apr]. Available from: https://ClinicalTrials.gov/show/NCT01151423.
- Phase III trial with caplacizumab in patients with acquired thrombotic thrombocytopenic purpura. [cited 2019 Apr]. Available from: https://ClinicalTrials.gov/show/NCT02553317.
- Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380:335–346.
- Howard MA, Firkin BG. Ristocetin: a new tool in the investigation of platelet aggregation. Thromb Diath Haemorrh. 1971;26:362–369.
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374:511–522.
- Bergstrand M, Hansson E, Sargentini-Maier M. Caplacizumab dosing rational in aTTP patients supported by mechanism based PKPD modelling. Page. 2019;2019(Abstract):8800.
- van Schooten CJ, Shahbazi S, Groot E, et al. Macrophages contribute to the cellular uptake of von Willebrand factor and factor VIII in vivo. Blood. 2008;112:1704–1712.
- Meibohm B, Zhou H. Characterizing the impact of renal impairment on the clinical pharmacology of biologics. J Clin Pharmacol. 2012;52:54S–62S.
- Flanders MM, Crist RA, Roberts WL, et al. Pediatric reference intervals for seven common coagulation assays. Clin Chem. 2005;51:1738–1742.
- Sosothikul D, Seksarn P, Lusher JM. Pediatric reference values for molecular markers in hemostasis. J Pediatr Hematol Oncol. 2007;29:19–22.
- Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–335.
- Masias C, Cataland SR. Novel therapies in thrombotic thrombocytopenic purpura. Res Pract Thromb Haemost. 2018;2:19–26.
- Furlan M. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and hemolytic-uremic syndrome. Adv Nephrol Necker Hosp. 2000;30:71–81.