ABSTRACT
Introduction: Tamoxifen dominates the anti-estrogenic therapy in the early and metastatic breast cancer setting. Tamoxifen has a complex metabolism, being mainly metabolized by CYP2D6 into its 30–100 times more potent metabolite, endoxifen. Recently, a phase I study in which endoxifen as an orally z-endoxifen hydrochloride has been successfully evaluated.
Areas covered: the principal pharmacogenetic and non-genetic differences in the pharmacology of tamoxifen and endoxifen are evaluated. To this end, references from PubMed, Embase or Web of Science, among others, were reviewed As non-genetic factors, important differences and similarities such age, or adherence to tamoxifen therapy are comprehensively illustrated. Additionally, since CYP2D6 genotypes are considered the main limitation of tamoxifen, many studies have investigated the association between the worsened clinical outcomes in patients with non-functional CYP2D6 genotypes. In this review, an overview of the research on this field is presented. Also, a summary describing the literature about individualizing tamoxifen therapy with endoxifen concentrations and its limitations is listed.
Expert opinion: z-endoxifen hydrochloride is only investigated in the metastatic setting, still more research is required before its place in therapeutics is known. Similarly, monitoring tamoxifen efficacy based on endoxifen concentrations might not be overall recommended due to the limited evidence available.
1. Introduction
Breast cancer is a heterogeneous disease with disparate clinical outcomes. Globally, breast cancer is the most frequent cancer diagnosed among women, accounting for around 25% of the newly diagnosed cancers in female patients [Citation1]. According to the World Health Organization, approximately 570.000 deaths in 2015 were related to breast cancer worldwide, which represented around 15% of all cancer deaths in women [Citation2]. Nearly 60–75% of newly diagnosed breast cancer cases are estrogen-receptor positive (ER) [Citation3], and in these cases, endocrine therapy with, e.g. tamoxifen or aromatase inhibitors is prescribed.
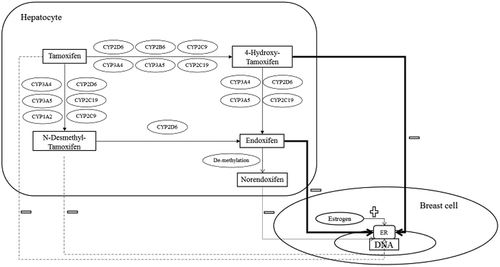
For more than 40 years, tamoxifen monopolized the antiestrogenic therapy in the early and metastatic breast cancer setting. Tamoxifen is a key element of endocrine therapy prescribed in breast cancer patients, whilst it also is a drug with a very complex metabolism. Tamoxifen is mainly metabolized by different cytochrome P-450 enzymes into its primary metabolites, N-desmethyl-tamoxifen (NDM-tamoxifen) and 4-hydroxy-tamoxifen. Thereafter, a second transformation from its primary metabolites, into the active metabolite endoxifen, occurs () [Citation4].
For pre-menopausal women, continuous tamoxifen is the preferred choice of treatment, whilst post-menopausal women can be switched to aromatase inhibitors after2 or 3 years [Citation5–Citation7]. In the adjuvant treatment setting tamoxifen therapy during 5 years has demonstrated to reduce mortality and disease recurrence [Citation8–Citation10]. Yet, as more studies with longer follow-up are published, it is apparent that extended duration of tamoxifen therapy up to 10 years is more beneficial in reducing mortality and disease recurrence in high-risk disease [Citation11]. In the metastatic setting, treatment with tamoxifen has also been investigated, showing longer survival rates and tumor reduction [Citation12], but still poorer results compared to the adjuvant practice. However, in early breast cancer roughly 30% of breast cancer patients will have a disease recurrence within 15 years after treatment, indicating a wide variability in clinical response to tamoxifen treatment [Citation10]. Both non-genetic (age [Citation13], gender [Citation14]) and genetic factors have been described to influence this high interpatient variability in response to tamoxifen. In this latter case, the most studied factor has been the variation in CYP2D6 gene encoding the CYP2D6 metabolic liver enzyme [Citation15,Citation16]. While it is almost present in all tamoxifen metabolic transformations, CYP2D6 is also the only enzyme which converts NDM-tamoxifen into endoxifen. Therefore, CYP2D6 is considered the critical enzyme of tamoxifen metabolism [Citation15]. At the same time, many researches have analyzed the clinical implications of CYP2D6 and its relationship with tamoxifen efficacy [Citation17]. While some studies describe the importance of CYP2D6 genotyping due to the poorer clinical outcome among patients with none or decreased CYP2D6 enzymatic activity [Citation18–Citation20], other authors have failed to find such an association [Citation21]. Consequently, the use of CYP2D6 genotyping for predicting tamoxifen efficacy has not generally been implemented in the daily clinical practice. In the search for an alternative in order to predict tamoxifen efficacy, monitoring endoxifen concentrations have been proposed [Citation22].
Recently, a phase I study in which endoxifen was orally administrated to hormone-resistant metastatic breast cancer patients was published [Citation23]. In this study, endoxifen presented an acceptable toxicity and a high anti-estrogenic exposure, whilst also clinical antitumor outcome was observed. According to the authors, the main advantage of endoxifen compared to tamoxifen is the fact that CYP2D6 metabolism is avoided, and consequently, the anti-estrogenic effect of endoxifen is unaffected by the CYP2D6 enzyme. Tamoxifen has been the preferred choice of oral anti-estrogenic therapy for pre- and post-menopausal women in the adjuvant setting, but endoxifen, its active metabolite, may be an interesting alternative of treatment in the future. In order to summarize these differences, a literature search in PubMed, Embase, Web of Science and Cochrane Library (until 30/11/2018) was performed. The aim of this review is to evaluate both the principal genetic and non-genetic differences in the pharmacology of tamoxifen and endoxifen.
2. Tamoxifen metabolic pathway
Generally, a description of tamoxifen metabolism mainly follows two pathways via 4-hydroxylation and N-de-methylation, into 4-hydroxy-tamoxifen and NDM-tamoxifen, respectively. Thereafter, both metabolites are finally transformed into the most potent secondary metabolite endoxifen () [Citation4]. Still, tamoxifen metabolic pathway is more complicated than only these two parallels pathways, since many newly tamoxifen metabolites have been discovered. Therefore, tamoxifen pathway is becoming more challenging to interpret. An example of the complexity of tamoxifen pathway was recently described by Johanning and colleagues, were estrogen-like tamoxifen metabolites were recently described. Authors suggest a broader metabolic pathway for tamoxifen where all these estrogen-like metabolites are included.
The transformation from tamoxifen into NDM-tamoxifen represents around 92% of tamoxifen metabolism, whilst the pathway through 4-hydroxy-tamoxifen accounts for 7% [Citation4]. Endoxifen and 4-hydroxy-tamoxifen are estimated to have comparable anti-estrogenic effect, which is around 30- to 100-fold more active in comparison to tamoxifen [Citation24]. However, since endoxifen is found in around 5 to 10 times higher concentrations compared to 4-hydroxy-tamoxifen [Citation25], endoxifen is considered the most relevant metabolite of tamoxifen.
Tamoxifen is largely metabolized to different, either active or inactive, metabolites, by many enzymes. For instance, several phase I enzymes, including different cytochrome P450 enzymes and flavin-monooxygenases (FMOs), and phase II enzymes, such as sulfotransferases (SULTs) and uridine-5ʹ-diphospho-glucuronosyl-transferases (UGTs).
Recently, a fifth and also active metabolite of tamoxifen, norendoxifen, has been identified [Citation26]. Norendoxifen is the product of the de-methylation of endoxifen or the product of the hydroxylation of di-desmethyltamoxifen [Citation27], and it slightly differs from the other tamoxifen metabolites since it also has the capacity to inhibit CYP19A1 (aromatase). Consequently, norendoxifen would have a dual mechanism of action: aromatase inhibition and estrogen receptor inhibition [Citation26,Citation28,Citation29]. Yet, to our best knowledge, this metabolite has not been commercialized at present [Citation28].
3. CYP2D6: limiting factor in tamoxifen metabolism
CYP2D6 enzymatic activity has been repeatedly reported as one of the essential elements of tamoxifen metabolism [Citation15,Citation17,Citation30–Citation32]. CYP2D6 is involved in the transformation of tamoxifen into 4-hydroxy-tamoxifen and NDM-tamoxifen, and in the conversion of 4-hydroxy-tamoxifen into endoxifen, and from NDM-tamoxifen into endoxifen. While many enzymes are involved in this complex metabolic pathway, CYP2D6 is the only enzyme responsible for the biotransformation from NDM-tamoxifen into endoxifen [Citation15].
The CYP2D6 gene is a highly polymorphic gene, for which currently more than 100 different polymorphisms have been described [Citation33]. All of these variations are mainly the result of differences in one single nucleotide polymorphisms (SNP), gene amplifications or deletions. While some of these variants encode a non-functional CYP2D6 enzyme, others encode for CYP2D6 with decreased enzymatic activity. Some typical examples of CYP2D6 inactive alleles are *3, *4,*5, *6, *7,*8 *11,*12, *13,*14A, *15, *19, *20,*40, whereas some cases of CYP2D6 alleles with decreased activity are *9,*10, *17, *29, *36, *41.
The most common allelic variant among Caucasians with non-functional CYP2D6 activity is CYP2D6*4 with an allele frequency of nearly 20% [Citation34,Citation35]. In contrast, CYP2D6*10 and its decreased activity is the most frequent allele among Asians, since it is found in almost 40% of this population [Citation35]. In the same manner, another relatively important variant with decreased reduced activity in the African-American population is CYP2D6*17 allele, with an allele frequency around 22% [Citation36]. According to the combination of these alleles, individuals can be categorized into four principal predicted phenotypes: ultra-rapid metabolizers (UM, duplication of fully active alleles), normal metabolizers (NM, with two fully active alleles, which also in the past used to be called extensive metabolizers, EM), intermediate metabolizers (IM, with two low activity alleles or a combination of one low activity allele and one inactive allele) and poor metabolizers (PM, with two non-functional alleles). In addition to this classification, some authors also contemplate a fifth phenotype named heterozygous extensive (hetEM), which consists of a combination of one fully functional active allele and an inactive allele [Citation32]. The enzymatic activity resulting from the hetEM genotype is in between the activity of IM and NM.
Another similar strategy for individualizing tamoxifen therapy is the classification of patients in five different groups according to the CYP2D6 gene activity score (AS) [Citation37]. The functionality of each allele is assigned an activity value as follows: 1 for fully functional alleles, 0.5 for alleles with decreased activity, and 0 for non-functional alleles. In the same manner, the combination of both alleles leads to five CYP2D6 phenotypes: PM (AS:0); IM (AS:0.5); IM or NM (AS:1.0); NM (AS:1.5–2.0); UM (AS:>2.0).
3.1. CYP2D6 genetics and tamoxifen outcomes
Since the publication of Goetz and colleagues [Citation38], the role of polymorphisms in CYP2D6 in clinical outcomes of women receiving tamoxifen as an adjuvant therapy has been an ongoing controversy due to the contradictory results of studies. Theoretically, patients with reduced and inactive CYP2D6 enzymatic activity and using, e.g. adjuvant tamoxifen, reach lower endoxifen concentrations, and consequently, lower exposure to anti-estrogenic therapy, with the consequence of a higher chance of relapse. Based on this hypothesis, PM and IM would be therefore more likely to have worsened clinical outcomes, since both phenotypes attain up to 74% and 60% lower endoxifen concentrations, respectively, compared to EM [Citation30]. This continuous discussion regarding poorer clinical outcomes for PM and IM receiving tamoxifen therapy may be difficult to clarify due to a great number of differences across studies. A few examples might be such the source of DNA for genotyping (tumour-tissue or blood), tamoxifen treatment duration (5 years or 10 years, or even shorter treatments), the study design (retrospective or prospective), the different endpoints analysed (relapse-free survival, disease-free survival, overall survival, among others), different quality of clinical cohorts, long-enough follow-up, and the number of CYP2D6 allelic variants which were analysed. Consequently, a comparison between studies is extremely challenging.
Many studies have been published since the study of Goetz and colleagues in 2005, with different outcomes. In 2007, another relevant study by Schroth and colleagues [Citation20] studied 1325 patients from Germany and the US who were diagnosed with breast cancer and receiving tamoxifen treatment between 1986 and 2005. According to the authors, hetEM, IM and PM patients had worsened disease-free survival (HR:1.29, 95% CI: 1.03–1.61), compared to EM.
In an attempt to uniformly investigate this potential relationship among patients with decreased enzymatic CYP2D6 activity and worsened clinical outcome, a meta-analysis performed by the International Tamoxifen Pharmacogenomics Consortium including 4973 patients treated with adjuvant tamoxifen was analyzed by using three beforehand defined inclusion criteria. In the primary analysis, in which the inclusion criteria were the most restricted, Province and colleagues reported a poorer survival outcome in PM patients (Hazard Ratio (HR): 1.25; 95% Confidence interval: 1.06–1.47; p-value: 0.009) among post-menopausal patients receiving 20 mg/day of adjuvant tamoxifen for 5 years. Because of the strict inclusion criteria, this first analysis included 1996 individuals. In contrast, in both criteria 2 and 3 analyses, where less rigorous inclusion conditions were applied, no differences in clinical outcomes between groups were observed [Citation39].
Still, this meta-analysis has been largely commented, since important studies with a large number of enrolled patients, e.g. ATAC [Citation40], BIG 1–98 [Citation41], or TEAM [Citation42] studies were not included. However, these studies also might have important limitations for the inclusion in this meta-analysis. For instance, the TEAM study might not have included in this meta-analysis due to the follow-up time of 2.5 years [Citation42], whilst the ATAC [Citation40] and BIG 1–98 [Citation41] trials analyzed tumor tissue, which could potentially lead to loss of heterozygosity.
Of note, the ATAC [Citation40] and BIG 1–98 [Citation41] studies have been criticized since potentially wrong outcomes were obtained. In these studies, formalin-fixed paraffin-embedded tissues were analyzed, but important deviations from Hardy Weinberg Equilibrium were not taken into consideration when interpreting their results. In this case, several reports analyzed this problem [Citation15,Citation43–Citation46], and the clinical implications and consequences of these results. If ‘loss of heterozygosity’ takes place, individuals could potentially be misclassified in ‘wrong’ CYP2D6 genotype and confusing outcomes could be expected. In contrast to these studies, the TEAM trial ruled out loss of heterozygosity by analyzing microsatellite markers with a high frequency of heterozygosity (D22S423, D22S276, and D22S2284) and found near to the CYP2D6 gene [Citation42]. In this case, authors did not find any statistically significant differences regarding disease-free survival among several CYP2D6 genotypes.
In the same line, a prospective specifically designed for investigating the clinical effect of CYP2D6 genotyping on 667 early-breast cancer female patients receiving adjuvant endocrine therapy with tamoxifen also did not find an association of poorer clinical outcome among patients with decreased or non-functional CYP2D6 genotype [Citation47] (Adjusted HR: 0.929; 95% CI 0.525–1.642; p = 0.799).
In an effort to uniformly interpret clinical evidence, a recent clinical guideline written by the Clinical Pharmacogenetics Implementation Consortium (CPIC) has been published in order to consistently give detailed recommendations based on the most current literature [Citation37]. Briefly, this CPIC guideline regarding tamoxifen therapy recommends to initiate tamoxifen therapy with the standard dose of 20 mg in UM (AS:>2.0) and NM (AS:1.5–2.0). In the case of NM or IM (AS:1.0) and IM (AS:0.5), this guideline suggests to consider alternative endocrine therapy, e.g. aromatase inhibitor for post-menopausal patients, and in pre-menopausal women, the use of aromatase inhibitor with ovarian function suppression. Yet, in these cases, if the use of aromatase inhibitor is contraindicated, the authors suggest to consider the use of 40 mg of tamoxifen. For PM (AS:0), alternative hormonal therapy, e.g. aromatase inhibitor should be preferably considered. Of note, no higher doses of tamoxifen (40 mg/day) are recommended in PM (AS:0), since endoxifen concentrations do not normalize (compare to NM).
Therefore, the potential association between decreased or lacking CYP2D6 enzyme activity and worsened clinical outcome still remains unclear. Consequently, CYP2D6 genotyping has not been commonly adopted in the clinical practice for tamoxifen treatment. Nonetheless, since tamoxifen metabolism is highly complex, it is debatable that tamoxifen therapy mainly depends on CYP2D6 genotypes. As a consequence, there seems to be a trend to take the focus off CYP2D6, since it only partly clarifies tamoxifen inter-patient variability, and paying more attention to other tamoxifen metabolites, especially endoxifen. As CYP2D6 is only partially predictive of endoxifen concentration levels, it is only logical to assume that endoxifen concentrations are better predictive of tamoxifen efficacy than solely CYP2D6 genotypes.
4. Tamoxifen and endoxifen
4.1. Tamoxifen and endoxifen pharmacokinetics
In , all these differences and comparisons are summarized. Tamoxifen is normally formulated as tamoxifen citrate, whilst endoxifen, is orally formulated as z-endoxifen hydrochloride. Elimination half-life of tamoxifen is 5–7 days on average. In contrast, endoxifen’s half-life varies between 49.0 and 68.1 h for the dose of 20 mg and 160 mg [Citation23], respectively. Also, minor difference in Tmax and Cmax have been reported. While tamoxifen has a longer Tmax (4–7 h), endoxifen as z-endoxifen hydrochloride requires a shorter time to reach Tmax (2–4 h). Also, differences in Cmax values are reported: when a 20 mg single dose of tamoxifen is administered a Cmax value of 40 ng/ml is reached [Citation48], whilst a single dose of 20 mg and 160 mg of z-endoxifen hydrochloride reaches Cmax of 64.8 and 635 ng/ml, respectively [Citation23].
Table 1. Pharmacokinetics parameters of tamoxifen and endoxifen.
4.1.1. Other minor enzymes in tamoxifen metabolism
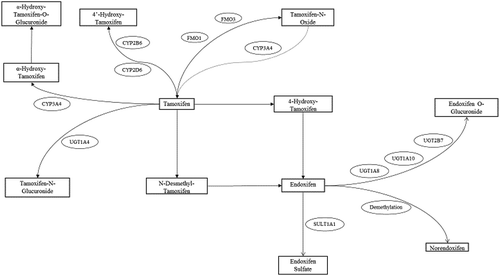
Besides the enzymes involved in the main transformations of tamoxifen into NDM-tamoxifen and 4-hydroxy-tamoxifen, other drug-metabolizing enzymes are also involved in the conversion of tamoxifen into active and inactive metabolites of tamoxifen ( and ).
In , an overview with all the minor enzymes in tamoxifen metabolism is listed. Genetic variants in the gene encoding the UGT1A4 enzyme, which catalyzes the transformation from tamoxifen into tamoxifen-N-glucuronide, include UGT1A4_48_Val and UGT1A4_48_Leu. To date, only the variant UGT1A4_48_Val has been associated with higher tamoxifen-N-glucuronide levels [Citation30,Citation49,Citation50]. Other phase I drug-metabolizing enzymes, FMO1 and FMO3, are also involved in tamoxifen metabolism. In this case, both enzymes transform tamoxifen into tamoxifen-N-oxide. Interestingly, the enzyme CYP3A4 enzyme can transform tamoxifen-N-oxide into tamoxifen in vivo, and it has been postulated that tamoxifen-N-oxide might behave as repository for tamoxifen, since the tamoxifen-N-oxide/tamoxifen ratio is reduced when higher tamoxifen dosages are used [Citation51–Citation53] ().
Table 2. Minor enzymes involved in tamoxifen metabolism.
Other subproducts of tamoxifen metabolism are 4ʹ-hydroxy-tamoxifen and α-hydroxy-tamoxifen. Both metabolites have been associated with the development of endometrial cancer [Citation54], different enzymes are found in these processes. While CYP2D6 and CYP2B6 are involved in the transformation of tamoxifen into 4ʹ-hydroxy-tamoxifen [Citation55–Citation57], CYP3A4 and CYP3A5 enzymes catalyze the conversion of tamoxifen into α-hydroxy-tamoxifen [Citation58,Citation59] ().
4.1.2. Other minor enzymes in endoxifen metabolism
In contrast to tamoxifen, a few endoxifen elimination and inactivation enzymes have been investigated and associated with clinical survival outcomes and tamoxifen active metabolites concentrations and metabolic ratios. In the same manner, summarizes all the minor enzymes in endoxifen metabolism.
Table 3. Minor enzymes involved in endoxifen metabolism.
Sulfotransferases (SULTs), UDP-glucuronosyltransferases (UGTs), and demethylases are the most significant enzymes participating in endoxifen elimination and inactivation (). Within all the SULTs enzymes, SUL1A1 enzyme is responsible for the inactivation of endoxifen into endoxifen sulfate. Genetic polymorphisms in the SULT1A1 enzyme have been analyzed for their association with different survival outcomes and drug concentrations. Nowell and colleagues reported a poorer overall survival for SULT1A1*2/*2 carriers, compared to SULT1A1*1/*1 or SULT1A1*1/*2 individuals [Citation61]. Later on, the same research group described again another statically significant poorer overall outcomes in SULT1A1*2/*2 and UGT2B15*2 patients [Citation62]. On the contrary, Wegman and colleagues found a better outcome for SULT1A1*1/*1 [Citation63]. However, none of these results were confirmed by other authors [Citation64,Citation65]. Interestingly, SULT1A1 copy number of variation has also been evaluated in these studies, but no significant differences in terms of survival were found [Citation64,Citation65]. Similarly, no statistically significant differences in endoxifen concentration levels were observed between when comparing patients carrying SULT1A1*1/*1, SULT1A1*1/*2, SULT1A1*2/*2 and SULT1A1 copy number of variation [Citation66–Citation68].
UGTs enzymes are also implicated in endoxifen metabolism, but only a few studies have examined their role in the conversion into endoxifen-O-glucuronide (). In an in vitro study, genetic polymorphisms in UGT1A10 were studied, but no significant differences in endoxifen concentration levels were observed [Citation69]. Another relevant variant allele is the non-functional allele UGT1A8*3, since it was suggested that oral bioavailability might be altered [Citation70]. Mizuma and colleagues studied the variant allele UGT1A8*3 in raloxifene-treated patients, and concluded that UGT1A8*3 carriers had higher oral bioavailability, which could be explained by the no functionality of glucuronidation of this variant, mainly at the intestinal level. However, these results have not been observed in tamoxifen-treated patients. In this case, when the non-functional variant UGT1A8*3 and the active allele UGT1A8*1 were compared, no differences in endoxifen levels [Citation69] or in survival outcomes [Citation71] were found. In line with these observations, UGT2B7*2 and its decreased activity were suggested to have an impact on endoxifen concentration levels, but no significant differences in concentrations [Citation30,Citation49,Citation69,Citation72,Citation73] or in clinical outcome [Citation40,Citation71] have been found (; ).
Another interesting transformation is the conversion of endoxifen into norendoxifen via N-de-methylation. Lim and colleagues described that CYP2C19*2 carriers were associated with lower norendoxifen concentration levels [Citation27].
5. Non-genetic factors affecting tamoxifen and endoxifen metabolism
All of the non-genetic factors are described and presented in .
Table 4. No genetic factors affecting tamoxifen and endoxifen concentrations.
5.1. Age
Among other non-genetic factors that could potentially influence metabolism, age has been described to affect both tamoxifen and endoxifen metabolism. Lien and colleagues reported significant higher endoxifen, tamoxifen NDM-tamoxifen concentration levels among patients in the oldest patient’s group (>69 years old) [Citation13] compared to younger patients. In the same way, Wu [Citation75] and Peyrade [Citation76] observed significantly higher concentrations of the four metabolites in older patients. In contrast, Antunes et al. found an inverse correlation between endoxifen and 4-hydroxy-tamoxifen concentration and age, whilst tamoxifen and NDM-tamoxifen concentrations were significantly higher among older patients () [Citation77]. Yet, these differences in the literature might be explained by natural physiological changes in humans due to the aging process, such as menopause [Citation78], lower metabolic hepatic enzyme activity [Citation79], due to poly-pharmacy [Citation80] or co-morbidities [Citation81].
5.2. Body mass index (BMI)
Different studies have demonstrated an inverse correlation between BMI and serum concentrations of tamoxifen and its metabolites [Citation75,Citation82–Citation84]. A simple explanation for this association is given by the larger volume of distribution in individuals with higher BMI, consequently leading to lower drug concentration levels. Despite of this association, there are no general recommendations of dose adjustments for tamoxifen in the clinical practice [Citation5,Citation84], whereas despite of the discrepancies in the current evidence, some clinicians tend to prescribe daily 40 mg of tamoxifen in patients with a BMI classified as overweight (25–30 kg/m2) or obese (>30 kg/m2) () [Citation85].
5.2.1. Food, circadian rhythm, and seasonal variation
Other non-genetic factors influencing tamoxifen and endoxifen metabolism are food, moment of drug intake, and seasonal variation. In the case of the effect of food on the pharmacokinetics, no clinically relevant effects have been described. Generally, tamoxifen is recommended to be administrated with or without food [Citation86].
In contrast to food, the time of drug intake influences tamoxifen and endoxifen metabolism. Binkhorst and colleagues reported 15% and 3% higher endoxifen and tamoxifen systemic exposure (AUC0-24) when tamoxifen was taken in the morning, whilst more balanced concentrations were found in the evening schedule [Citation87]. Regardless of these differences, the authors concluded this phenomenon would probably not be clinically important for tamoxifen efficacy.
Interestingly, seasonal variation may also influence tamoxifen and endoxifen pharmacokinetics. Teft et al. observed that during the winter season, significantly lower endoxifen concentrations were found that were detected during the other seasons [Citation88]. In addition, authors also found an association between lower vitamin D levels and lower endoxifen concentrations. In a study by Antunes and colleagues, 116 breast cancer patients who were receiving adjuvant tamoxifen were analyzed [Citation77]. In this research, comparable results to Teft and colleagues were found [Citation88], showing significant higher endoxifen concentration levels and vitamin D during the summertime, whilst a trend toward also lower tamoxifen concentrations was observed () [Citation77]. Yet, in both studies only a hypothetically relationship between vitamin D and endoxifen concentration levels are described, however, which of both elements is the cause and which the consequence, remains unclear. Still, no general recommendations for vitamin D supplements are given aimed at achieving higher anti-estrogenic exposure to tamoxifen or endoxifen.
5.2.2. Gender
The majority of breast cancer new cases are female individuals, but around 0.5–1% of the newly diagnosed patients are male subjects [Citation1,Citation89]. In these cases, the first choice of treatment as adjuvant endocrine therapy is tamoxifen, whilst aromatase inhibitors are not recommended, as they appear to be inferior compared to tamoxifen in this clinical setting [Citation90]. To date, only one study performed by Lenehan and colleagues has demonstrated that male patients reached significantly lower endoxifen concentration levels compared to female patients () [Citation91]. In general, female and male patients differ in response to drug treatments, and these variances are mainly explained by body differences [Citation92]. Normally, men tend to have higher BMI, total body water and plasma volume compared to women, which would lead to lower mean drug concentrations. However, it remains unknown if this difference is clinically relevant for the clinical efficacy of tamoxifen and endoxifen.
5.3. Smoking
Cigarette smoking has been associated with higher breast cancer risk [Citation93,Citation94], but to the best of our knowledge, no studies have been performed on the influence of smoking on tamoxifen and endoxifen pharmacokinetics. In one study, Persson and colleagues [Citation95]described that older patients (>50 years) who smoked during endocrine therapy with aromatase inhibitors did have higher probability of breast cancer events (HR: 2.97; 95%CI: 1.44–6.13), distant metastasis (HR: 4.19; 95% CI:1.81–9.72) and death (HR: 3.52; 95% CI: 1.59–7.81), whereas in the group treated with tamoxifen no association between tamoxifen efficacy and actively smoking during endocrine therapy was found (). However, Zhan and colleagues reported that smoking patients treated with tamoxifen presented higher probability of side effects like nausea, headaches, and depression in comparison with non-smokers [Citation96]. Although there is no clear evidence that tamoxifen efficacy is influenced by actively smoking, in daily practice all patients are recommended to quit smoking.
5.3.1. Triglycerides and cholesterol
In addition to its anti-estrogenic effect, tamoxifen use has been described to be beneficial in lowering the risk of cardiovascular diseases, since tamoxifen influences lipid metabolism by decreasing LDL- and HDL-cholesterol, and total cholesterol and triglycerides [Citation97,Citation98]. A study performed by Clarke and colleagues found a significant lowering effect of cholesterol and triglycerides in tamoxifen-treated male patients [Citation99]. These results were also observed by Shewmon et al. in healthy post-menopausal women [Citation100]. Goetz and colleagues observed these same lowering effects on cholesterol and triglycerides in endoxifen-treated patients () [Citation23]. These observations suggest a clinically relevant cardiovascular risk reduction in both endoxifen and tamoxifen users. Yet, no clinical guidelines recommend the use of tamoxifen or endoxifen in order to lower cholesterol or triglycerides.
5.3.2. Tamoxifen, endoxifen and cyp2d6-inhibitors
Drug–drug interactions are an important point of discussion, since interactions could have an important role on clinical efficacy and occurrence or worsening of adverse events. Over the last decade, few drug–drug interactions have been as contentious as the tamoxifen and selective serotonin reuptake inhibitors (SSRIs) or selective serotonin and norepinephrine reuptake inhibitors (SNRIs) interactions. Since around 10–25% of female breast cancer patients suffer from depression [Citation101,Citation102], these patients might require antidepressant treatment with SSRIs or SNRIs. In addition, these drugs are frequently prescribed for treating hot-flashes due to tamoxifen use, because in those cases estrogen or progesterone combinations are not an option to be used. Both specific SSRIs and SNRIs are recognized as CYP2D6-inhibitors [Citation103], and since CYP2D6 is the rate-limiting enzyme in tamoxifen activation into endoxifen, many studies have focused on this drug–drug interaction, with a wide range of controversial results.
Steams et al. observed an important decrease from a mean 12.4 ng/ml to 5.5 ng/ml of endoxifen concentrations among women who were treated with paroxetine [Citation25], and consecutive studies showed significantly lower concentrations of endoxifen among patients who were using potent CYP2D6-inhibitors, as paroxetine or fluoxetine [Citation66,Citation88,Citation104]. Over the last 10 years, there seems to be a change in trend co-prescribing tamoxifen and an SSRI. In a study in the U.S.A., it was observed that there was a significant reduction in the co-prescription of both drugs (tamoxifen and SSRI) (from 34% during the period between 2004 and 2006 compared 15% in 2010) [Citation105]. Likewise, a Belgian and a Dutch study demonstrated a dropping in the co-prescription of strong CYP2D6-inhibitors and tamoxifen, whilst a preference for a weak CYP2D6-inhibitor was observed [Citation106,Citation107]. To analyze the effect of switching from a strong to a weak CYP2D6-inhibitor, Binkhorst and colleagues, analyzed endoxifen concentration levels, before and after this switch. Interestingly, an improvement to higher endoxifen levels was reported after a switch from a strong inhibitor to escitalopram, a weak CYP2D6-inhibitor [Citation108].
In theory, tamoxifen efficacy may be affected by the concomitant use of tamoxifen and CYP2D6-inhibitors, due to a decrease in endoxifen exposure. However, a large discrepancy in studies investigating the effect of SSRIs on breast cancer survival outcomes using tamoxifen has been published [Citation25,Citation66,Citation88,Citation104,Citation109]. This high variance in the literature may be explained by the CYP2D6-inhibitor analyzed, the source of information and the lack of information on CYP2D6 genotype and compliance and the relative time in which patients had concomitantly used the CYP2D6 inhibitor. Still, one recent study with 16,887 patients concluded that no increased risk among the women who were using antidepressants and tamoxifen was observed [Citation110]. However, current guidelines with recommendations for endocrine therapy advise to avoid the co-prescription of CYP2D6-inhibitors in tamoxifen users due to the importance of drug–drug interactions [Citation7,Citation37,Citation111].
5.4. Adherence to tamoxifen therapy
Tamoxifen treatment adherence is an important problem in daily clinical practice. In the literature, reported tamoxifen adherence vary widely from 41% to 88% [Citation112–Citation115]. However, tamoxifen discontinuation is principally seen after the first year of treatment [Citation115]. In a recent prospective study analyzing tamoxifen adherence after the first year of endocrine treatment by quantifying tamoxifen concentrations, around 18.2% of the enrolled patients were classified as poor or no adherence [Citation116]. In the same manner, barely 50% of patients achieve to finish the suggested period of 5 years [Citation114,Citation115]. In a recent review analyzing this importance of endocrine therapy, Chlebowski and colleagues highlighted the necessity of good treatment adherence (defined as >80% use) in order to reach lower recurrence outcomes [Citation115,Citation117]. In this case, Chigwin and colleagues recently investigated the association of adherence to endocrine therapy and disease-free survival in the Breast Internation Group (BIG) 1–98 clinical trial. Authors reported worsened clinical outcome among the groups with poorer adherence to treatment (HR: 1.61; 95% CI 1.08–2.38; p-value: 0.02). Interestingly, sequential therapies (either switching from tamoxifen to letrozole or from letrozole to tamoxifen) were associated with higher percentages of non-adherence (20.8% and 20.3% for the switch from tamoxifen to letrozole and from letrozole to tamoxifen, respectively) compared with the monotherapies of letrozole (17.6%) and tamoxifen (16.9%). In most of the cases, side effects were the principal cause for poorer adherence. Also, other reported factors in the literature associated with lower or non-adherence are [Citation115]: medication cost, lack of network support, older age, absence or inadequate of doctor–patient relationship, among others. Consequently, treatment adherence is an important difficult, and therefore, strategies for detecting patients who could potentially discontinue endocrine therapy are extremely required.
6. Endoxifen: toward individualizing tamoxifen treatment?
6.1. Active metabolites and mechanism of action
Almost 30 years ago, endoxifen was characterized for the first time by Lien and colleagues [Citation24,Citation118]. In contrast to tamoxifen, endoxifen has a higher affinity for estrogen receptor, whilst it is also categorized as a selective estrogen receptor modulator [Citation119]. Initially, 4-hydroxy-tamoxifen was believed to be the principal active metabolite of tamoxifen, since it was found to be 30 to 100-times more potent as anti-estrogenic compared to tamoxifen [Citation24]. In an effort to find potential therapeutic alternatives, 4-hydroxy-tamoxifen was examined as a therapeutic drug, however, due to its unfavorable pharmacokinetics, it failed [Citation120].
Both endoxifen and 4-hydroxy-tamoxifen are chemically related molecules, with comparable anti-estrogenic effect [Citation25], although endoxifen reaches 5 to 10-fold larger concentrations [Citation25]. Additionally, in vitro studies have suggested that the mechanism of action of endoxifen might differ from 4-hydroxy-tamoxifen [Citation121,Citation122]. Initially, Wu et al. described that endoxifen targets the estrogen-receptor α by blocking its transcriptional activity and inhibiting estrogen breast cancer cell proliferation, only when high concentrations of endoxifen were used, in contrast to tamoxifen, NDM-tamoxifen and 4-hydroxy-tamoxifen [Citation122]. Later, Hawse and colleagues compared the capacity of endoxifen, 4-hydroxy-tamoxifen and ICI (a pure antiestrogen and estrogen receptor down regulator) [Citation121] to target estrogen receptor α for DNA binding in order to correctly identify differences in the gene expression profiles of MCF7 cells exposed to different concentrations of these drugs in the absence and presence of estrogen. Authors observed differences in gene expression profiles of MCF7 cells when high endoxifen concentrations were added, while when low concentrations were used, expression profiles barely varied. Based on these studies, the mechanism of action of endoxifen appears to be concentration dependent.
6.2. Approaches for predicting endoxifen concentrations
Since endoxifen is considered the most relevant metabolite of tamoxifen metabolism, many different strategies have been investigated in order to predict the exposure to this metabolite. The majority of these approaches have mainly focused on using different variables in order to improve the explained inter-patient variability of endoxifen concentrations. CYP2D6 polymorphisms are the main contributors to this inter-variability, yet they still only explain around 39–42.3% of the variability in concentrations of endoxifen [Citation30,Citation66]. For this reason, CYP2D6 genotyping in the current form might be a too simplistic strategy for personalizing tamoxifen treatment. Another analyzed approach is the use of 13C-dextromethorphan breath test for CYP2D6 phenotyping. This strategy allows to slightly improve the predictability of endoxifen concentrations to 47.5% [Citation123]. In the same way, Teft and colleagues also developed an algorithm, including demographic data, use of SSRIs, CYP2D6 genotypes and CYP3A4*22, among others, in order to predict endoxifen concentration levels [Citation88].
Both phenotyping approaches and the algorithm of Teft and colleagues are strategies for predict endoxifen concentrations. Still, these models only minimally improve the prediction of endoxifen concentrations, which is considered an important limitation. Consequently, other strategies are still being awaited.
6.3. Endoxifen concentrations and tamoxifen efficacy
Due to the current limitations of CYP2D6 genotyping, it has been hypothesized that monitoring endoxifen concentrations might be a better way for predicting tamoxifen efficacy. Based on this theory, endoxifen rather than CYP2D6 genotyping may be closer to the pharmacological effect, and consequently may be a better approach for personalizing tamoxifen efficacy [Citation22]. Also, a special consideration regarding endoxifen (Z-endoxifen), since it is the active isomer with the anti-estrogenic activity [Citation30]. In this case, it is important to properly quantify Z-endoxifen, separately from E-endoxifen or other hydroxylated metabolites, e.g. 4ʹhydroxy-desmethyltamoxifen [Citation30], in order to adequately obtain the concentration of the active metabolite (Z-endoxifen), and avoiding too high concentrations from a mix of all metabolites, which may lead to misinterpretations in further analysis.
Madlensky and colleagues were the first to study the relationship between endoxifen concentrations and clinical outcome [Citation82]. In this analysis, a threshold of 5.97 ng/ml for endoxifen concentrations was associated with a 26% lower chance of relapse (Adjusted HR: 0.76, 95% Confidence Interval: 0.55–1.00). In this case, 1370 patients were divided into five groups (quintiles) according to their concentrations of endoxifen, and the lowest quintile had a higher chance of relapse, compared to the other groups. However, this endoxifen threshold concentration of 5.97 ng/ml has been highly commended, since the event rate across the other groups or quintiles could be seen as comparable.
Another comparable limit-value for endoxifen concentration of 5.2 ng/ml was proposed for tamoxifen efficacy by Saladores and colleagues, but only in a cohort of 548 pre-menopausal women [Citation19]. Following this approach, Helland and colleagues suggested an even lower concentration of 3.3 ng/ml as a threshold for improved clinical outcomes [Citation124]. Interestingly, Helland used concentrations of 4-hydroxy-tamoxifen in his approach in order to also identify this low threshold concentration value of endoxifen.
Despite of the relevance of these findings of the aforementioned studies, none of them were specifically designed for investigating the association between endoxifen concentration and clinical outcome. In addition, these studies were performed in retrospective cohorts. Consequently, the general utility of these outcomes in the clinical practice are still awaiting for validation in studies with larger populations and specifically designed for this purpose. Of note, no statistical difference in relapse-free survival was observed between groups with endoxifen concentration levels below and above the 5.97 ng/ml threshold in a recent study using a cohort of 667 Caucasian women receiving adjuvant tamoxifen [Citation125]. In the same line, no association between endoxifen concentration and better clinical outcome, defined as progression-free survival, clinical benefit, and objective response rate, was found in the neoadjuvant and metastatic setting in a study population of 297 patients [Citation126].
In contrast, Love et al. proposed that there might exist a range of endoxifen concentrations for tamoxifen efficacy, instead of a minimal threshold concentration [Citation127]. In an exploratory analysis in a nested case-control including 48 patients, surprisingly high endoxifen concentrations (>70 ng/ml) were related with higher risk of recurrence. Additionally, they also observed a trend toward lower endoxifen concentrations (under 20 ng/ml) in the patients with relapse, but no clear threshold of efficacy was defined. In the same manner, a recent analysis by Groenland et al. found no differences in clinically relevant toxicities among patients with high endoxifen concentration levels (>25 ng/ml) [Citation128].
7. Expert opinion
For more than 40 years, tamoxifen has been a very successful and key element of the endocrine therapy of breast cancer. Yet, important variation in clinical response is still observed. Based on the current literature, predicting tamoxifen efficacy is still in its infancy. Initially, CYP2D6 genotyping seemed the best option, since a poorer clinical outcome might be expected in poor and probably also in intermediate CYP2D6 metabolizers, who are at least partly unable to metabolize tamoxifen to its active metabolite endoxifen. However, since CYP2D6 polymorphisms can only explain the interpatient variability of endoxifen concentrations to a limited extent, there is as yet not enough evidence to generally recommend CYP2D6 genotyping as a predictor for tamoxifen efficacy.
In a different approach, therapeutic drug monitoring (TDM) of endoxifen concentrations, might appear to have a potential role in individualizing tamoxifen treatment. However, there is no general agreement on the required endoxifen concentrations to be reached in order to predict tamoxifen efficacy. To the best of our knowledge, no study has specifically investigated how high or low should be the concentrations of endoxifen in order to predict tamoxifen efficacy. A potential explanation of why it is difficult to properly study this association between endoxifen concentrations and clinical outcome might be due to the mechanism of action of tamoxifen and its active metabolites. All of them block intracellularly the estrogen receptor, and therefore concentration levels in the blood of tamoxifen, or any of its active metabolites, might not be representative of the block of estrogen receptor. Interestingly, Lash and colleagues reported that active metabolites of tamoxifen occupied between 99.63% and 99.99% estrogen receptor [Citation129]. In theory, patients treated with adjuvant tamoxifen, normally receive the usual daily dose of tamoxifen is 20 mg. If concentrations of endoxifen are measured once steady-state is reached, normally after 2–3 months of treatment, and these endoxifen concentrations are lower than any of the abovementioned threshold for endoxifen concentration (5.97 ng/ml, 5.2 ng/ml or 3.3 ng/ml), applying a higher dose of tamoxifen will not always assure that the expected endoxifen concentrations would be reached, especially in CYP2D6 poor metabolizer patients. Indeed, applying an increased daily dose of tamoxifen in IM patients but not in PM individuals, resulted in endoxifen concentrations comparable to EM patients [Citation130,Citation131]. Yet, the long-term consequences of these higher doses are still unknown.
In order to adequately investigate the putative role of TDM of endoxifen concentrations in order to predict tamoxifen efficacy, a large study with a substantial number of patients and a very long follow-up would be required. In addition, since also other metabolites, e.g. 4-hydroxy-tramoxifen exert antiestrogenic activity, monitoring serum endoxifen concentration levels as a predictor for tamoxifen efficacy, might not be currently the best approach. Another limitation for such a study might be related to the applied tamoxifen regimens and the study populations. Tamoxifen monotherapy is mainly recommended for pre-menopausal women for 5 years, while for post-menopausal patients shorter tamoxifen regimens of 2–3 years is used [Citation5,Citation7]. Consequently, the ideal study population for such a study might not be possible to obtain, since in the daily clinical practice many diverse tamoxifen regimens and study populations might be difficult to investigate.
Although the concept of using TDM based on endoxifen concentrations as a manner for individualizing tamoxifen therapy is highly tempting, there is not enough evidence at present for using such an approach in routine care.
Another relevant point is the complexity of tamoxifen metabolism and the high number of described active metabolites, which also might be important for predicting tamoxifen efficacy. Endoxifen is considered the most crucial active metabolite of tamoxifen metabolite, but it has a comparable anti-estrogenic activity to 4-hydroxy-tamoxifen. At the same time, other active metabolites, e.g. norendoxifen could also potentially affect tamoxifen efficacy, since it has dual activity. As a consequence, the current approaches in which only a few elements of tamoxifen metabolism, such as CYP2D6 phenotypes, are used, might not be the best manner to predict tamoxifen efficacy. Since many other enzymes and tamoxifen active metabolites are involved, together with non-genetic determinants of response, a more complex analysis including all these key elements could be required in order to improve the prediction of tamoxifen efficacy and safety.
8. Five-year view
Since tamoxifen has such a complex metabolism, in which many enzymes and metabolites are involved, it is to be expected that prediction of tamoxifen efficacy in individual patients relies on more genes than the CYP2D6 gene alone. In addition, other non-genetic factors that may also alter tamoxifen pharmacokinetics and pharmacodynamics need to be considered. Therefore, models incorporating both genetic and non-genetic determinants of response may help to further improve the prediction of individual tamoxifen response.
Regarding the potential role of z-endoxifen hydrochloride, it is important to remark, that at present, z-endoxifen is mainly being investigated in the metastatic setting. In our opinion, z-endoxifen hydrochloride in the metastatic scenario may become an alternative therapy. However, more drug development research is needed before the role of z-endoxifen hydrochloride in breast cancer treatments becomes clear.
Article highlights
For more than 40 years, tamoxifen has been successfully prescribed in the adjuvant and metastatic setting, however, a great variation between patients is still observed
Tamoxifen also has a complicated metabolism, mainly metabolized by CYP2D6 into its 30-100 times more potent, endoxifen.
Endoxifen is currently investigated as an orally form of z-endoxifen hydrochloride in the metastatic scenario.
Therapeutic drug monitoring based on endoxifen concentration levels has been hypothesized to be the best approach for individualizing tamoxifen efficacy, still little evidence is currently available.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.
- World Health Organization. Breast cancer. [online]; 2019. [cited 2018 Nov 30]. Available from: https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/
- Huang B, Warner M, Gustafsson JA. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2015;418(Pt 3):240–244.
- Klein DJ, Thorn CF, Desta Z, et al. PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics. 2013;23(11):643–647.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–2269.
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol. 2016;34(25):3069–3103.
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30.
- Early Breast Cancer Trialists‘ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717.
- Early Breast Cancer Trialists‘ Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352.
- Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784.
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141.
- Thurlimann B, Robertson JF, Nabholtz JM, et al. Efficacy of tamoxifen following anastrozole (‘Arimidex‘) compared with anastrozole following tamoxifen as first-line treatment for advanced breast cancer in postmenopausal women. Eur J Cancer. 2003;39(16):2310–2317.
- Lien EA, Soiland H, Lundgren S, et al. Serum concentrations of tamoxifen and its metabolites increase with age during steady-state treatment. Breast Cancer Res Treat. 2013;141(2):243–248.
- Lenehan JG, Teft WA, Kim RB. Comparison of endoxifen levels between male and female breast cancer patients treated with tamoxifen. J clin oncol. 2016;34:578. Conference.
- Brauch H, Murdter TE, Eichelbaum M, et al. Pharmacogenomics of tamoxifen therapy. Clin Chem. 2009;55(10):1770–1782.
- Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther. 2008;83(1):160–166.
- Brauch H, Schwab M. Prediction of tamoxifen outcome by genetic variation of CYP2D6 in post-menopausal women with early breast cancer. Br J Clin Pharmacol. 2014;77(4):695–703.
- Knox SK, Ingle JN, Suman VJ, et al. Cytochrome P450 2D6 status predicts breast cancer relapse in women receiving adjuvant tamoxifen (Tam). J clin oncol. 2006;24(18):4S–4S.
- Saladores P, Murdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2015;15(1):84–94.
- Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302(13):1429–1436.
- Sanchez-Spitman A, Dezentje V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019;37(8):636–646.
- de Vries Schultink AHM, Huitema ADR, Beijnen JH. Therapeutic drug monitoring of endoxifen as an alternative for CYP2D6 genotyping in individualizing tamoxifen therapy. Breast. 2018;42:38–40.
- Goetz MP, Suman VJ, Reid JM, et al. First-in-human phase i study of the tamoxifen metabolite Z-Endoxifen in women with endocrine-refractory metastatic breast cancer. J Clin Oncol. 2017;35(30):3391–3400.
- Lien EA, Solheim E, Lea OA, et al. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49(8):2175–2183.
- Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764.
- Lu WJ, Xu C, Pei Z, et al. The tamoxifen metabolite norendoxifen is a potent and selective inhibitor of aromatase (CYP19) and a potential lead compound for novel therapeutic agents. Breast Cancer Res Treat. 2012;133(1):99–109.
- Lim JS, Sutiman N, Muerdter TE, et al. Association of CYP2C19*2 and associated haplotypes with lower norendoxifen concentrations in tamoxifen-treated Asian breast cancer patients. Br J Clin Pharmacol. 2016;81(6):1142–1152.
- Lv W, Liu J, Lu D, et al. Synthesis of mixed (E, Z)-, (E)-, and (Z)-norendoxifen with dual aromatase inhibitory and estrogen receptor modulatory activities. J Med Chem. 2013;56(11):4611–4618.
- Ma J, Chu Z, Lu JBL, et al. The cytochrome P450 enzyme responsible for the production of (Z)-norendoxifen in vitro. Chem Biodivers. 2018;15(1).
- Murdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708–717.
- Brauch H, Schroth W, Goetz MP, et al. Tamoxifen use in postmenopausal breast cancer: CYP2D6 matters. J Clin Oncol. 2013;31(2):176–180.
- Schroth W, Winter S, Murdter T, et al. Improved prediction of endoxifen metabolism by CYP2D6 genotype in breast cancer patients treated with tamoxifen. Front Pharmacol. 2017;8:582.
- Pharmvar.org. (2019). PharmVar [online]. Available at: https://www.pharmvar.org/gene/CYP2D6 [Accessed 28 Apr, 2019].
- Sachse C, Brockmoller J, Bauer S, et al. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60(2):284–295.
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3(2):229–243.
- Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: implications for the use of neuroleptics and antidepressants. Brain Res Bull. 1997;44(5):561–571.
- Goetz MP, Sangkuhl K, Guchelaar HJ, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen Therapy. Clin Pharmacol Ther. 2018;103(5):770–777.
- Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318.
- Province MA, Goetz MP, Brauch H, et al. CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther. 2014;95(2):216–227.
- Rae JM, Drury S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452–460.
- Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst. 2012;104(6):441–451.
- Dezentje VO, van Schaik RH, Vletter-Bogaartz JM, et al. CYP2D6 genotype in relation to tamoxifen efficacy in a Dutch cohort of the tamoxifen exemestane adjuvant multinational (TEAM) trial. Breast Cancer Res Treat. 2013;140(2):363–373.
- Stanton V Jr. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst. 2012;104(16):1265–1266. author reply 1266-1268.
- Pharoah PD, Abraham J, Caldas C. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial and Re: CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(16):1263–1264. author reply 1266-1268.
- Nakamura Y, Ratain MJ, Cox NJ, et al. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104(16):1264. author reply 1266-1268.
- Goetz MP, Sun JX, Suman VJ, et al. Loss of heterozygosity at the CYP2D6 locus in breast cancer: implications for germline pharmacogenetic studies. J Natl Cancer Inst. 2014 Dec 8;107(2). doi:10.1093/jnci/dju401
- Sanchez-Spitman A, Dezentje V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019;37(8):636–646.
- Accessdata.fda.gov. (2019). Drug Approval Package: Nolvadex (Tamoxifen Citrate) NDA #21-109 [online]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21109_Nolvadex.cfm [Accessed 28 Apr, 2019].
- Romero-Lorca A, Novillo A, Gaibar M, et al. Impacts of the glucuronidase genotypes UGT1A4, UGT2B7, UGT2B15 and UGT2B17 on tamoxifen metabolism in breast cancer patients. PLoS One. 2015;10(7):e0132269.
- Sun D, Chen G, Dellinger RW, et al. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res. 2006;8(4):R50.
- Parte P, Kupfer D. Oxidation of tamoxifen by human flavin-containing monooxygenase (FMO) 1 and FMO3 to tamoxifen-N-oxide and its novel reduction back to tamoxifen by human cytochromes P450 and hemoglobin. Drug Metab Dispos. 2005;33(10):1446–1452.
- Krueger SK, Vandyke JE, Williams DE, et al. The role of flavin-containing monooxygenase (FMO) in the metabolism of tamoxifen and other tertiary amines. Drug Metab Rev. 2006;38(1–2):139–147.
- Gjerde J, Gandini S, Guerrieri-Gonzaga A, et al. Tissue distribution of 4-hydroxy-N-desmethyltamoxifen and tamoxifen-N-oxide. Breast Cancer Res Treat. 2012;134(2):693–700.
- Kim SY, Suzuki N, Laxmi YR, et al. Genotoxic mechanism of tamoxifen in developing endometrial cancer. Drug Metab Rev. 2004;36(2):199–218.
- Dahmane E, Mercier T, Zanolari B, et al. An ultra performance liquid chromatography-tandem MS assay for tamoxifen metabolites profiling in plasma: first evidence of 4‘-hydroxylated metabolites in breast cancer patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(32):3402–3414.
- Crewe HK, Notley LM, Wunsch RM, et al. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4‘-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos. 2002;30(8):869–874.
- Cuevas ME, Lindeman TE. In vitro cytotoxicity of 4‘-OH-tamoxifen and estradiol in human endometrial adenocarcinoma cells HEC-1A and HEC-1B. Oncol Rep. 2015;33(1):464–470.
- Mugundu GM, Sallans L, Guo Y, et al. Assessment of the impact of CYP3A polymorphisms on the formation of alpha-hydroxytamoxifen and N-desmethyltamoxifen in human liver microsomes. Drug Metab Dispos. 2012;40(2):389–396.
- Notley LM, Crewe KH, Taylor PJ, et al. Characterization of the human cytochrome P450 forms involved in metabolism of tamoxifen to its alpha-hydroxy and alpha,4-dihydroxy derivatives. Chem Res Toxicol. 2005;18(10):1611–1618.
- Sutiman N, Lim JS, Muerdter TE, et al. Pharmacogenetics of UGT1A4, UGT2B7 and UGT2B15 and their influence on tamoxifen disposition in Asian breast cancer patients. Clin Pharmacokinet. 2016;55(10):1239–1250.
- Nowell S, Sweeney C, Winters M, et al. Association between sulfotransferase 1A1 genotype and survival of breast cancer patients receiving tamoxifen therapy. J Natl Cancer Inst. 2002;94(21):1635–1640.
- Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91(3):249–258.
- Wegman P, Vainikka L, Stal O, et al. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005;7(3):R284–R290.
- Moyer AM, Suman VJ, Weinshilboum RM, et al. SULT1A1, CYP2C19 and disease-free survival in early breast cancer patients receiving tamoxifen. Pharmacogenomics. 2011;12(11):1535–1543.
- Wegman P, Elingarami S, Carstensen J, et al. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9(1):R7.
- Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39.
- Gjerde J, Hauglid M, Breilid H, et al. Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol. 2008;19(1):56–61.
- Fernandez-Santander A, Gaibar M, Novillo A, et al. Relationship between genotypes Sult1a2 and Cyp2d6 and tamoxifen metabolism in breast cancer patients. PLoS One. 2013;8(7):e70183.
- Blevins-Primeau AS, Sun D, Chen G, et al. Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res. 2009;69(5):1892–1900.
- Mizuma T. Intestinal glucuronidation metabolism may have a greater impact on oral bioavailability than hepatic glucuronidation metabolism in humans: a study with raloxifene, substrate for UGT1A1, 1A8, 1A9, and 1A10. Int J Pharm. 2009;378(1–2):140–141.
- Ahern TP, Christensen M, Cronin-Fenton DP, et al. Functional polymorphisms in UDP-glucuronosyl transferases and recurrence in tamoxifen-treated breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1937–1943.
- Areepium N, Panomvana D, Rungwanonchai P, et al. Effects of CYP2D6 and UGT2B7 polymorphisms on pharmacokinetics of tamoxifen in Thai breast cancer patients. Breast Cancer (Dove Med Press). 2013;5:73–78.
- Sutiman N, Lim JSL, Muerdter TE, et al. Pharmacogenetics of UGT1A4, UGT2B7 and UGT2B15 and their influence on tamoxifen disposition in Asian breast cancer patients. Clin Pharmacokinet. 2016;55(10):1239–1250.
- Sahebkar A, Serban MC, Penson P, et al. The effects of tamoxifen on plasma lipoprotein(a) concentrations: systematic review and meta-analysis. Drugs. 2017;77(11):1187–1197.
- Wu AH, Pike MC, Williams LD, et al. Tamoxifen, soy, and lifestyle factors in Asian American women with breast cancer. J Clin Oncol. 2007;25(21):3024–3030.
- Peyrade F, Frenay M, Etienne MC, et al. Age-related difference in tamoxifen disposition. Clin Pharmacol Ther. 1996;59(4):401–410.
- Antunes MV, Timm TA, de Oliveira V, et al. Influence of CYP2D6 and CYP3A4 phenotypes, drug interactions, and vitamin D status on tamoxifen biotransformation. Ther Drug Monit. 2015;37(6):733–744.
- Davis SR, Lambrinoudaki I, Lumsden M, et al. Menopause. Nat Rev Dis Primers. 2015;1:15004.
- Tan JL, Eastment JG, Poudel A, et al. Age-related changes in hepatic function: an update on implications for drug therapy. Drugs Aging. 2015;32(12):999–1008.
- Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65.
- Piccirillo JF, Vlahiotis A, Barrett LB, et al. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67(2):124–132.
- Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–725.
- Antunes MV, de Oliveira V, Raymundo S, et al. CYP3A4*22 is related to increased plasma levels of 4-hydroxytamoxifen and partially compensates for reduced CYP2D6 activation of tamoxifen. Pharmacogenomics. 2015;16(6):601–617.
- Sendur MA, Aksoy S, Ozdemir NY, et al. Effect of body mass index on the efficacy of adjuvant tamoxifen in premenopausal patients with hormone receptor-positive breast cancer. J BUON. 2016;21(1):27–34.
- Goodwin PJ, Pritchard KI. Obesity and hormone therapy in breast cancer: an unfinished puzzle. J Clin Oncol. 2010;28(21):3405–3407.
- Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–1156.
- Binkhorst L, Kloth JSL, de Wit AS, et al. Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients. Breast Cancer Res Treat. 2015;152(1):119–128.
- Teft WA, Gong IY, Dingle B, et al. CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res Treat. 2013;139(1):95–105.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193.
- Eggemann H, Ignatov A, Smith BJ, et al. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat. 2013;137(2):465–470.
- Lenehan JG, Teft WA, Kim RB. Comparison of endoxifen levels between male and female breast cancer patients treated with tamoxifen. J Clin Oncol. 2016;34(15_suppl):578–578.
- Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–157.
- Catsburg C, Kirsh VA, Soskolne CL, et al. Active cigarette smoking and the risk of breast cancer: a cohort study. Cancer Epidemiol. 2014;38(4):376–381.
- Catsburg C, Miller AB, Rohan TE. Active cigarette smoking and risk of breast cancer. Int J Cancer. 2015;136(9):2204–2209.
- Persson M, Simonsson M, Markkula A, et al. Impacts of smoking on endocrine treatment response in a prospective breast cancer cohort. Br J Cancer. 2016;115(3):382–390.
- Zhan M, Flaws JA, Gallicchio L, et al. Profiles of tamoxifen-related side effects by race and smoking status in women with breast cancer. Cancer Detect Prev. 2007;31(5):384–390.
- Imperato F, Marziani R, Perniola G, et al. Effects of tamoxifen and estrogen replacement therapy on lipid metabolism and some other cardiovascular risk factors. A prospective study in hysterectomised women. Minerva Ginecol. 2003;55(1):87–93.
- Nordenskjold B, Rosell J, Rutqvist LE, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: results from a randomized trial. J Natl Cancer Inst. 2005;97(21):1609–1610.
- Clarke SC, Schofield PM, Grace AA, et al. Tamoxifen effects on endothelial function and cardiovascular risk factors in men with advanced atherosclerosis. Circulation. 2001;103(11):1497–1502.
- Shewmon DA, Stock JL, Rosen CJ, et al. Tamoxifen and estrogen lower circulating lipoprotein(a) concentrations in healthy postmenopausal women. Arterioscler Thromb. 1994;14(10):1586–1593.
- Zainal NZ, Nik-Jaafar NR, Baharudin A, et al. Prevalence of depression in breast cancer survivors: a systematic review of observational studies. Asian Pac J Cancer Prev. 2013;14(4):2649–2656.
- Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004;32:32–39.
- Jeppesen U, Gram LF, Vistisen K, et al. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51(1):73–78.
- Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74.
- Dusetzina SB, Alexander GC, Freedman RA, et al. Trends in co-prescribing of antidepressants and tamoxifen among women with breast cancer, 2004–2010. Breast Cancer Res Treat. 2013;137(1):285–296.
- Dieudonne AS, De Nys K, Casteels M, et al. How often did Belgian physicians co-prescribe tamoxifen with strong CYP2D6 inhibitors over the last 6 years? Acta Clin Belg. 2014;69(1):47–52.
- Binkhorst L, Mathijssen RH, van Herk-Sukel MP, et al. Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen. Breast Cancer Res Treat. 2013;139(3):923–929.
- Binkhorst L, Bannink M, de Bruijn P, et al. Augmentation of endoxifen exposure in tamoxifen-treated women following SSRI switch. Clin Pharmacokinet. 2016;55(2):249–255.
- Dezentje VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28(14):2423–2429.
- Haque R, Shi J, Schottinger JE, et al. Tamoxifen and antidepressant drug interaction in a Cohort of 16,887 breast cancer survivors. J Natl Cancer Inst. 2016;108(3).
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438.
- Pagani O, Gelber S, Colleoni M, et al. Impact of SERM adherence on treatment effect: international breast cancer study group trials 13-93 and 14-93. Breast Cancer Res Treat. 2013;142(2):455–459.
- Wigertz A, Ahlgren J, Holmqvist M, et al. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat. 2012;133(1):367–373.
- Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220.
- Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila). 2014;7(4):378–387.
- Pistilli B, Paci A, Michiels S, et al. 185O_PR. Serum assessment of non-adherence to adjuvant endocrine therapy (ET) among premenopausal patients in the prospective multicenter CANTO cohort. Ann Oncol. 2018 Oct 1;29(suppl_8). Available from: https://doi.org/10.1093/annonc/mdy424.004.
- Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452–2459.
- Lien EA, Solheim E, Kvinnsland S, et al. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988;48(8):2304–2308.
- Ahmad A, Ali SM, Ahmad MU, et al. Orally administered endoxifen is a new therapeutic agent for breast cancer. Breast Cancer Res Treat. 2010;122(2):579–584.
- Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16(2):239–251.
- Hawse JR, Subramaniam M, Cicek M, et al. Endoxifen‘s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS One. 2013;8(1):e54613.
- Wu X, Hawse JR, Subramaniam M, et al. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69(5):1722–1727.
- Opdam FL, Dezentje VO, Den HJ, et al. The use of the 13C-dextromethorphan breath test for phenotyping CYP2D6 in breast cancer patients using tamoxifen: association with CYP2D6 genotype and serum endoxifen levels. Cancer Chemother Pharmacol. 2013;71(3):593–601.
- Helland T, Henne N, Bifulco E, et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017;19(1):125.
- Sanchez-Spitman AB, Dezentjé VO, Swen JJ, et al. A prospective study on the effect of endoxifen concentration and CYP2D6 phenotypes on clinical outcome in early stage breast cancer patients receiving adjuvant tamoxifen. J clin oncol. 2018;36(15_suppl):523.
- Neven P, Jongen L, Lintermans A, et al. Tamoxifen metabolism and efficacy in breast cancer: a prospective multicenter trial. Clin Cancer Res. 2018;24(10):2312–2318.
- Love RR, Desta Z, Flockhart D, et al. CYP2D6 genotypes, endoxifen levels, and disease recurrence in 224 Filipino and Vietnamese women receiving adjuvant tamoxifen for operable breast cancer. Springerplus. 2013;2(1):52.
- Groenland SL, Sanchez-Spitman AB, Moes DJAR, et al. 258PIncidence of clinically significant toxicities in patients with high endoxifen concentrations. Ann Oncol. 2018;29(suppl_8):mdy270.252-mdy270.252.
- Lash TL, Lien EA, Sorensen HT, et al. Genotype-guided tamoxifen therapy: time to pause for reflection? Lancet Oncol. 2009;10(8):825–833.
- de Martinez DE, Ochoa AE, Blancas Lopez-Barajas I, et al. Adjusting the dose of tamoxifen in patients with early breast cancer and CYP2D6 poor metabolizer phenotype. Breast. 2014;23(4):400–406.
- Dezentje VO, Opdam FL, Gelderblom H, et al. CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res Treat. 2015;153(3):583–590.