KEYWORDS:
1. Introduction
From birth, children may need to take medication for acute or chronic diseases. Antibiotics, anti-asthmatic drugs, antihistamines, analgesics, gastrointestinal drugs and attention-deficit/hyperactivity disorder medications are widely prescribed in the pediatric population. In some cases, anticancer drugs are also needed. Prescribing drugs in children is however often difficult for physicians because of limitations in pharmacological research in children due to safety concerns and ethical challenges [Citation1]. Therefore, the use of off-label medicines is common in pediatrics because of the lack of clinical trials in this population. Direct extrapolation of clinical adult data to children requires careful assessment of the differences between these two populations, including the influence of the developmental aspects of pathogenesis and ontogeny [Citation1]. Indeed, age-related differences in drug absorption, distribution, metabolism and elimination must be considered when administering drugs to pediatric patients to ensure safe and effective drug therapy [Citation2]. The determinants of each of these steps are prone to intra- and inter-individual pharmacokinetic variability.
Particularly, since the activity of drug‐metabolizing enzymes (DME) may vary with age, comprehension of the ontogeny of these enzymes is essential for the prediction of pediatric pharmacokinetics (PK) [Citation2]. DME include the cytochrome P450 (CYP450) enzymes, especially important in catalyzing the oxidative metabolism of several drugs [Citation3]. The expression of CYP450 enzymes varies significantly during child’s growth and development [Citation2]. For instance, CYP3A4 levels increase gradually with age, approaching 50% of adult activity by one year of age. In contrast, CYP3A7 activity shows increased activity in the fetus, followed by a rapid decrease during the first week of life.
CYP450 genotyping, including copy-number-variation analysis, is extensively used in clinical settings. It allows the identification of single nucleotide polymorphisms, gene deletion and gene duplication or multiplication. It requires DNA extraction from buccal swabs, saliva or blood and is therefore easily applicable in pediatrics. When available, it is then possible to translate the genotype of an isoenzyme into a phenotype using a standardized method as developed, for example, for the CYP2D6 enzyme by the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group [Citation4]. Less standardized systems for translating genotype into phenotype also exist for CYP2B6, CYP2C9, CYP2C19 and CYP3A5 [Citation4]. However, phenotype-genotype relationships are poorly described for CYP1A2 and CYP3A4 [Citation3]. In addition, genotyping does not take into account the influence of environmental factors such as concomitant medication or smoking on the enzymatic activity. For these reasons, CYP450 phenotyping (i.e. real-time measurement of CYP450 activity) is therefore preferred to genotyping. In addition, it allows the evolution of these enzymes over time and age to be captured [Citation2,Citation5].
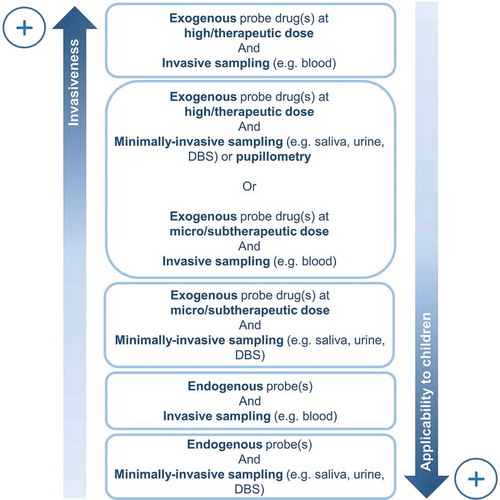
In the adult population, phenotyping is commonly applied in clinical settings to detect changes in CYP450 enzymatic activities. CYP450 phenotyping requires the administration of a probe drug specifically metabolized by a given isoenzyme and the analysis of the metabolized product in bio-fluids. For example, midazolam, a benzodiazepine, is considered as a reference probe drug for measuring CYP3A activity in adulthood and the plasma metabolic ratio 1ʹ-hydroxymidazolam/midazolam is often used as a phenotyping metric. Due to the necessary ingestion of an exogenous compound, the majority of current phenotyping tools are relatively invasive and difficult to apply to children, resulting in a lack of phenotyping resources for this population [Citation5]. In addition, phenotyping usually requires the collection of urine or blood, which may be challenging in pediatrics [Citation5]. The aim of this editorial is therefore to give a brief overview of less invasive phenotyping methods compared to conventional methods using high-dose probe drugs and invasive blood sampling that are difficult to apply in pediatrics ().
2. Alternative and minimally invasive phenotyping methods
2.1. Pupillometry
The study of changes in pupil diameter, i.e. pupillometry, is a promising tool that could be used as a safe and rapid method for CYP2D6 phenotyping in pediatrics following opioid analgesics intake as part of a therapeutic treatment. Indeed, opioids have a miotic effect through their action on µ-receptors [Citation6]. Therefore, the degree of alteration of pupillary response upon exposure to these drugs may provide an indication of the opioid-mediated pharmacological effect. For instance, the active metabolite of tramadaol, O-desmethyltramadol, whose formation is catalyzed by CYP2D6, causes pupillary constriction through its action on µ-receptors. Thus, the extent of pupillary constriction following tramadol administration is dependent on the function of CYP2D6 and could serve as an indirect biomarker to determine the phenotype of this enzyme rather than bio-fluid collection [Citation6]. According to Connelly et al., pupillometry is noninvasive, user-friendly and provides robust measurements of pupillary function in pediatric patients [Citation7]. However, further studies are needed to assess the validity and feasibility of CYP2D6 phenotyping by pupillometry after opioid intake in children.
This technique is only for a specific class of opioid analgesics and cannot be generalized to tailored pediatric therapy. Moreover, since the ingestion of an exogenous compound, such as an opioid, is relatively invasive and difficult to apply to children, this method can only be applied during therapy.
2.2. Low- or microdoses of probe drugs
In children, the administration of low- or microdosed probe drugs rather than therapeutic doses, in order to avoid any toxicity, may be considered for CYP450 phenotyping. Microdosing is generally defined as the administration of a dose substantially reduced (usually at least 100 times) compared to the therapeutic dose [Citation8]. In order to replace the use of therapeutic doses of probe drugs with lower or microdoses, it is essential to first assess the linearity of the pharmacokinetic parameters between the two dosages [Citation8]. For example, van Groen et al. showed recently that an intravenous [14 C]midazolam microdose (37.6 ng kg−1) can be an alternative to midazolam administered at therapeutic doses in children after having demonstrated linearity [Citation9]. It is important to note that formulation of low- or microdosed probe drugs is often not available and may require specific and internal manufacturing or reconditioning unless a liquid formulation is already marketed for instance [Citation8]. In addition, it requires a more sensitive quantification method than probe drugs used at therapeutic doses [Citation8]. Despite these constraints, low-dose or microdosed probe drugs may offer a great opportunity to improve knowledge of CYP450 activity in children after clinical validation. However, even if it is technically feasible, it is still largely unexploited.
2.3. Micro- and saliva and sampling
Collecting bio-fluids, especially blood and urine, from infants can be a difficult task. Less invasive and more convenient sampling methods exist and may be used in this particular population for CYP450 phenotyping. For instance, saliva has been successfully used in a study performed by El-Yazigi et al. to measure caffeine clearance in order to phenotype CYP1A2 activity in children [Citation10]. In adulthood, the utilization of saliva to phenotype CYP2D6 and CYP3A has also been demonstrated using dextromethorphan and midazolam, respectively [Citation11,Citation12]. However, it requires relatively high doses of probe drugs or a very sensitive analytical method and, to our knowledge, has not yet been tested in pediatrics. The use of dried blood spot (DBS) is also an alternative to conventional blood collection as it requires smaller amounts of blood. DBS is performed by pricking the tip of a finger or even the heel in newborns in order to draw a drop of capillary blood on blotting paper. It may remain however painful in some individuals, particularly in children [Citation5].
2.4. Cocktail approach
In order to avoid successive and separate administration of various probe drugs to phenotype different isoenzymes (i.e. individual phenotyping), it is possible to administer concomitantly multiple probes (i.e. simultaneous phenotyping), namely phenotyping cocktail. When phenotyping of several isoenzymes is required, cocktail approaches are considered less invasive because they reduce the patient’s participation time, as a single experiment is necessary to evaluate multiple pathways simultaneously. Li et al. administered a phenotyping cocktail in children aged 12–21 years composed of four probe drugs (caffeine 100 mg, omeprazole 20 mg, losartan 25 mg and midazolam 2 mg) [Citation13]. Venous blood and urine were collected during the study to assess the activity of the isoenzymes. As described in the previous paragraphs, a preferred strategy for CYP450 phenotyping in children may be the integration of low- or microdoses of probe drugs, micro- or saliva sampling and cocktail approaches. In adulthood, this has already been successfully developed (e.g Geneva Cocktail) using DBS as a sampling method and subtherapeutic doses of the probe drugs [Citation14]. Further scientific research needs to be carried out in this context for the pediatric population. For example, the potential translation of the results obtained in adults for the Geneva cocktail using subtherapeutic probe drugs to children may need to be clinically validated before implementation due to clear pharmacokinetic differences between these two populations [Citation2].
2.5. Phenotyping using endogenous biomarkers
Going even further, CYP450 phenotyping using endogenous compounds may be considered in child population [Citation15]. This approach would prevent the intake of exogenous compounds intake and the resulting safety problems after their administration (e.g. dosage errors, unexpected reactions to probe drugs) [Citation15]. This area has already been explored by Tay-Sontheimer et al. in a clinical trial performed in children diagnosed with attention-deficit hyperactivity disorder [Citation16]. They highlighted, using metabolomics approach, an unknown urinary biomarker capable of differentiating CYP2D6 poor metabolizers from other metabolizers but this compound has not yet been structurally characterized.
3. Expert opinion
Research on CYP450 phenotyping in pediatrics is expected to develop further in the future. The use of endobiotics to phenotype CYP450 activity is a very promising area, especially if it can be combined with a noninvasive sampling method.
Personalized medicine in children can then go deeper with the use of mechanistic physiologically based pharmacokinetic (PBPK) modeling integrating population-specific (e.g. CYP450 phenotypes, organ volumes) and drug-specific (e.g. lipophilicity, volume of distribution) parameters [Citation17]. Indeed, PBPK models could help to predict the PK of drugs to support prescribing decision-making or to provide data for pediatric clinical trials by integrating growth and biological maturation processes that are known to affect the disposition of drugs [Citation17]. To obtain the data needed for PBPK modeling, in particular CYP450 phenotypes, the use of endobiotics and minimally or noninvasive sampling method (e.g. saliva, DBS) appears theoretically to be the best option as this would be the least invasive procedure. However, further research in this promising field is needed as very few studies are being conducted.
Finally, it is also important to note that drug metabolism is only one element of pharmacokinetic variability and that the measurement of drug metabolism phenotypes is not sufficient on its own to predict individual variations in drug concentrations and exposure. The field of phenomics, that is ‘the acquisition of high-dimensional phenotypic data on an organism-wide scale’ [Citation18], would allow for advanced real-time and real-life phenotyping of drug outcomes [Citation19]. Technologies for high-throughput phenotyping are becoming increasingly available and is a promising area for personalized medicine.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128:e1242-1249.
- Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167.
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141.
- Caudle KE, Sangkuhl K, Whirl‐Carrillo M, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch pharmacogenetics working group. Clin Transl Sci. 2020;13:116–124.
- Magliocco G, Rodieux F, Desmeules J, et al. Toward precision medicine in pediatric population using cytochrome P450 phenotyping approaches and physiologically based pharmacokinetic modeling. Pediatr Res. 2020;87:441–449.
- Slanar O, Nobilis M, Kvetina J, et al. Miotic action of tramadol is determined by CYP2D6 genotype. Physiol Res. 2007;56:129–136.
- Connelly MA, Brown JT, Kearns GL, et al. Pupillometry: a non-invasive technique for pain assessment in paediatric patients. Arch Dis Child. 2014;99:1125–1131.
- Hohmann N, Haefeli WE, Mikus G. Use of microdose phenotyping to individualise dosing of patients. Clin Pharmacokinet. 2015;54:893–900.
- van Groen BD, Vaes WH, Park BK, et al. Dose‐linearity of the pharmacokinetics of an intravenous [14C]midazolam microdose in children. Br J Clin Pharmacol. 2019;85:2332–2340.
- el-Yazigi A, Shabib S, al-Rawithi S, et al. Salivary clearance and urinary metabolic pattern of caffeine in healthy children and in pediatric patients with hepatocellular diseases. J Clin Pharmacol. 1999;39:366–372.
- Chen R, Zheng X, Hu P. CYP2D6 phenotyping using urine, plasma, and saliva metabolic ratios to assess the impact of CYP2D6*10 on interindividual variation in a Chinese population. Front Pharmacol. 2017;8:239.
- Link B, Haschke M, Grignaschi N, et al. Pharmacokinetics of intravenous and oral midazolam in plasma and saliva in humans: usefulness of saliva as matrix for CYP3A phenotyping. Br J Clin Pharmacol. 2008;66:473–484.
- Li H, Canet MJ, Clarke JD, et al. Pediatric cytochrome P450 activity alterations in nonalcoholic steatohepatitis. Drug Metab Dispos. 2017;45:1317–1325.
- Bosilkovska M, Samer CF, Déglon J, et al. Geneva cocktail for cytochrome p450 and P-glycoprotein activity assessment using dried blood spots. Clin Pharmacol Ther. 2014;96:349–359.
- Magliocco G, Thomas A, Desmeules J, et al. Phenotyping of human CYP450 enzymes by endobiotics: current knowledge and methodological approaches. Clin Pharmacokinet. 2019;58:1373–1391.
- Tay-Sontheimer J, Shireman LM, Beyer RP, et al. Detection of an endogenous urinary biomarker associated with CYP2D6 activity using global metabolomics. Pharmacogenomics. 2014;15:1947–1962.
- Ince I, Solodenko J, Frechen S, et al. Predictive pediatric modeling and simulation using ontogeny information. J Clin Pharmacol. 2019;59:S95–S103.
- Houle D, Govindaraju DR, Omholt S. Phenomics: the next challenge. Nat Rev Genet. 2010;11:855–866.
- Özdemir V. Phenomics 2.0: real-world real-time patient outcomes measured by the internet of pharmaceutical things. OMICS. 2020;24:119–121.