1. Introduction
It has been known since the first identified SARS epidemic that the receptor critical for SARS-CoV entry into host cells is the angiotensin-converting enzyme 2 (ACE2), an important actor on renin-angiotensin system (RAS) [Citation1,Citation2]. Similarly, with the novel coronavirus (SARS-CoV-2), the S1 domain of the spike protein (SARS-2-S) attaches the virus to its cellular receptor ACE2 on the host cells. The subsequent step is the ACE2 downregulation by primary enzyme TACE and TMPRSS2, which culminate on the receptor cleavage [Citation3,Citation4]. The partial lack of ACE2 expression has protagonist post on the severe lung injury by SARS-CoV [Citation5,Citation6].
2. The role of the ACE2 in the lung and the SARS infection damage
ACE2 expression occurs in alveoli-type 2 pneumocytes and macrophages present into the pulmonary parenchyma [Citation7]. Indeed, ACE2 displays advantageous function in several tissues including the lungs. Abundant expression in pulmonary tissue can be attributed to its defensive feature against several diseases. Recent studies have demonstrated that ACE2 protects murine lungs from acute lung injury as well as SARS-spike protein-mediated lung injury, suggesting an important role of ACE2 in SARS infections and protection from ARDS [Citation8]. Based on these SARS effects, it could be thought initially that the RAS blockers might increase the risk of developing a notorious and sometimes fatal severe acute respiratory syndrome coronavirus infection since ACE2 can be triggered by clinical RAS inhibition. However, Meng and coworkers, in a brilliant discussion, showed the first clinical evidence that RAS inhibitors improve the clinical outcomes of Covid-19 patients with hypertension [Citation9]. In another study, it was proposed that there are no data supporting that ACE inhibitors (ACEi) or angiotensin II type 1 receptor blockers facilitate coronavirus entry by increasing ACE2 expression and the authors suggest that treatment with RAS blockers should not be discontinued because of concerns with coronavirus infection based on the currently available evidence [Citation10]. When it comes to assessing hyperinflammation in Covid-19, a clinical study compared IL-6, an inflammatory marker found in SARS-CoV-2 positive patients on ACEi versus non-ACEi therapy, revealing that the cytokine levels were reduced in the ACEi group [Citation9]. More recently, Khashkhusha and colleagues reported an interesting editorial emphasizing the fact that we do not yet know exactly the clinical benefit of using ACEi. Indeed, they suggested that large studies are needed to delineate the role of ACEi in treating Covid-19, ideally both in patients naïve to ACEi and chronic users of ACEi [Citation11].
Wang and Cheng reported that SARS-CoV and MERS-CoV upregulate the expression of ACE2 in lung tissue, a process that could accelerate their replication and spread. Indeed, based on a causative course, this is expected [Citation12]. Nevertheless, this event of increased ACE2 expression by PCR techniques could reveal compensatory effect due to the virus entry mechanism. Thereby, how could be the pattern of ACE2 expression not only its related mRNA as Wang and Cheng showed? Kuba and colleagues published an interesting experimental model injecting SARS-spike protein to mimic the inflammatory response on lung tissue. Indeed, this event may explain that PCR was not the best perspective to evaluate the enzyme expression since they detected by blot analysis the ACE2 downregulation associated to increased Ang II, a proinflammatory peptide when binds to AT1 receptors [Citation13]. Recently, our research group raised a putative connection between ACE2 downregulation and harmful role of its imbalance on Covid-19. The medical hypothesis speculates that ACE2 when downregulated promotes bradykinin (BK) and des-Arg9-BK excess culminating in cytokine storm owing to BK-receptors type 1 binding [Citation14]
It is noteworthy that ACE2 expression is not restricted to the lung, but also extra pulmonary spread of SARS-CoV-2 in ACE2 positive tissues was observed such as heart, liver, kidney, and GI tract [Citation15,Citation16]. We thought that it is timely to explain the connection between the ACE2 in late stages of SARS-CoV-2 and the association with rationale use of ACE2 activators as a potential therapy. Yang and colleagues showed in an experimental murine model of SARS-CoV infection that overexpression of human ACE2 enhanced disease severity, demonstrating that viral entry into cells is a critical step [Citation17]. Nevertheless, up to our knowledge, no study has shown if reestablishment of ACE2 on pneumocytes and lung parenchyma could led to an improvement of pulmonary function and attenuation of inflammatory response in SARS-CoV-2 during late phase, which includes specially cytokine storm and failure in gas exchange, as well as extra lung damage such as heart tissue and gut repercussions. So, based in all these findings, we believe that the ACE2 specific activators could achieve useful properties in severe acute respiratory syndrome induced by SARS-CoV-2 infection.
3. Therapy for Covid-19 is still required
As an acute viral disease, Covid-19 has some phases already observed and suggestively proposed by epidemiologists. Chakraborty and colleagues (2020) published a very organized compilation which brings these phases of coronavirus outbreak with SARS-CoV-2 in (i) incubation period (up to 5 days), (ii) symptoms appear (6–7 days); (iii) painful breathing (8 day); (iv) respiratory distress syndrome – acute phase (9 days), and (v) severe case – patient admitted to ICU (more than 10 days). In early stages, before acute phase, the appropriate recommendation in the scope of medical virology is the use of antivirals to mitigate viral replication. In Covid-19, there is not a specific antiviral although a plenty of clinical studies are ongoing with off-label medications such as chloroquine, hydroxychloroquine, lopinavir, and remdesivir [Citation18,Citation19]. However, in late phases, patients with Covid-19 have been minor benefits with antiviral therapies. Recently, preliminary study results suggested dexamethasone, a commonly used steroid, to reduce risk of death in sickest Covid-19 patients [Citation20]. Clinical pharmacology is yet seeking effective options for treating Covid-19 due to the high nonresponsive patients to suggested drugs up to this date.
In terms of massive control of a viral pandemic, the most expected is the intervention of an effective vaccine to achieve herd immunity. There are many speculations and advanced studies regarding to vaccines based on known epitopes, notably focused on spike glycoprotein of SARS-CoV-2, which recognizes the gateway ACE2 and triggers an immune response characterized by hyperinflammation [Citation21]. Meanwhile, it’s noteworthy that the world is yet urging for therapeutic options in this pandemic scenario with emphasis on late stages.
4. Embarking effect of ACE2-angiotensin 1–7/Mas receptor Axis in SARS-CoV-2
Diminazene aceturate (Dize: C14H15N7 · 2C4H7NO3; Molecular Weight: 515.5 g/mol; PubChem CID: 5,284,544) is an old antiparasitic used primarily in animal clinical practice that activates ACE2. Dize is an aromatic diamidine that was first described in 1955 and has been originally developed for therapeutic approach in controlling trypanosomiasis, its IUPAC name is 2-acetamido acetic acid; 4-[2-(4-carbami midoylphenyl) iminohydrazinyl] benzene carboximidamide [Citation22]. Nevertheless, in recent years, this drug has been extensively studied with regard to its therapeutic potential and manifold effects; this pleiotropy for pharmacology development has consequently attracted palpable interest in drug repositioning [Citation22]. Actually, several studies have shown that Dize may influence positively other physiological conditions in different tissues ACE2+. In addition to activating ACE2, this drug stimulates the protective axis of the RAS, leading to the cleavage of Ang II. ACE2 metabolizes Ang II to Ang-(1–7) and thus counter regulates the deleterious effects of Ang II [Citation23].
Dize seems to be a daring candidate in experimental approaches of SARS-CoV-2 infection due to its (1) directly ACE2 activation, (2) anti-inflammatory profile, and (3) known tolerable use in humans, Berenil® is and FDA-approved drug since the last century [Citation22,Citation24–Citation26]. Recently, Fang and coworkers have demonstrated the beneficial effects of Dize in pulmonary disorders in animal models by regulating NF-κB and Nrf2 gene expression routes which encode proinflammatory cytokines that compromise lung function [Citation27]. Could the diminution in ACE2 levels explain partially the reasons for this damage? In the same study, the authors showed lower expression of ACE2/Ang-(1-7) in lung tissue and bronchoalveolar lavage fluid of mice with hyperoxia, while Dize restored ACE2 and this favorable phenomenon was aggravated by MLN-4476, an ACE2 inhibitor. Dize also diminished vascular permeability and parameters of pulmonary edema followed by controlled oxidative stress on induced-hyperoxia lung damage in mice. Another study developed by Imai and colleagues showed pivotal protective role of ACE2 on mice with severe acute lung injury induced by acid aspiration or sepsis [Citation28]. However, no studies have explored the off-label effects of Dize on epithelial and connective tissue and the role of the ACE2/Ang-(1-7)/Mas receptor pathway in healing of acute lung injury induced by SARS-CoV and SARS-CoV-2.
It is worth mentioning that although lung injury followed by cytokine storm is the major complication in Covid-19 on pnemocytes into the alveolar epithelial cells, but there is remote damage in other tissues ACE2+ such as heart, liver, and sometimes underestimated on intestine causing diarrhea [Citation15,Citation29–Citation34]. Dize also has shown that reestablishment of ACE2 in these tissues suggests advantageous guidance of thinking about its multi target fashion drug for SARS-CoV-2 far-off injuries [Citation35–Citation37].
5. Conclusion
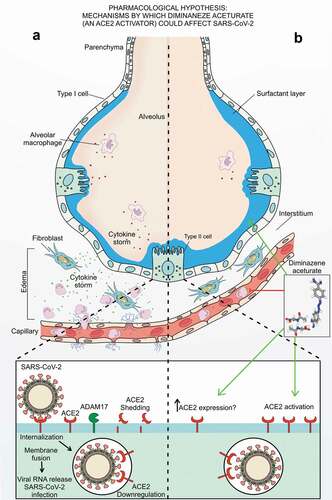
We hypothesize as novel therapeutic strategy for late stage mainly in pulmonary complications provoked by SARS-CoV-2 infection, but also remote damages, based on specific ACE2 activation, with diminazene aceturate, and that may important specially for nonresponsive patients to management protocols purposed by now (see ). If ACE2 levels are restored in tissues that SARS-CoV-2 reduced its expression, and this is the resumption of homeostasis, we can be close to the control of a pandemic through this therapeutic proposal.
Figure 1. Hypothetical scheme. (a) SARS-CoV-2 infection is characterized by the collapse of the alveoli orchestrated by the decrease in the surfactant layer, followed by the release of inflammatory mediators culminating in the cytokine storm and impaired by the downregulation in ACE2; while (b) diminazene aceturate, an ACE2 activator, could improve late clinical outcome due to its anti-inflammatory and tissue protectant profile by reduction of proinflammatory cytokines, by augmenting surfactant proteins ACE2 dependent followed by ACE2 activation and speculated upregulation of ACE2 expression in late stages of Covid-19.
Article highlights
In science, 2020 will be emblematically marked by the high flow of publications aimed at understanding, preventing, and treating Covid-19, but the pandemic scenario continues to advance with an opened avenue for therapeutic proposals.
Importantly, downregulation of ACE2 after SARS-CoV-2 infection has not an assumption of no experimental set by now. The benefit of ACE2 activating persists unknown until experimental approaches show its inefficacy in Covid-19 pathophysiology.
We are now eager to propose possible data on mechanisms of action of Dize in SARS-CoV-2 infection specially in late stages, and to show whether this ACE2 activator agents can be useful in the treatment of inflammatory response driven by ACE2 downregulation in SARS-CoV-2 infection.
Ongoing experimental approaches in SARS-CoV-2-infected mice should be addressed for these issues.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454.
- Prabakaran P, Xiao X, Dimitrov DS. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys Res Commun. 2004;314:235–241.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.
- Heurich A, Hofmann-Winkler H, Gierer S, et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307.
- Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARSCoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem. 2005;280:30113–30119.
- Haga S, Nagata N, Okamura T, et al. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010;85:551–555.
- Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134.
- Imai Y, Kuba K, Penninger JM. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci. 2007;64:2006–2012.
- Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757‐760.
- Danser JAH, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic - At present there is no evidence to abandon renin- angiotensin system blockers. Hypertension. 2020;75. DOI:https://doi.org/10.1161/HYPERTENSIONAHA.120.15082
- Khashkhusha TR, Chan JSK, Harky A. ACE inhibitors and COVID-19: we don’t know yet. J Card Surg. 2020;35(6):1172–1173.
- Wang PH, Cheng Y. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020;1–22. DOI:https://doi.org/10.1101/2020.02.24.963348
- Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875‐879. .
- Nicolau LAD, PJC M, Vale ML. What would Sérgio Ferreira say to your physician in this war against COVID-19: how about kallikrein/kinin system? Med Hypotheses. 2020;143(109886):1–7.
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637.
- Zaim S, Chong JH, Sankaranarayanan V, et al. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618.
- Yang XH, Deng W, Tong Z, et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57(5):450–459.
- Chakraborty C, Sharma AR, Sharma G, et al. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci. 2020;24(7):4016–4026.
- Saha A, Sharma AR, Bhattacharya M, et al. Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: need to Know More [published online ahead of print, 2020 May 12]. Arch Med Res. 2020;30699-8:S0188-4409(20).
- Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ. 2020;369:m2512.
- Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): immunoinformatics approach. J Med Virol. 2020;92(6):618–631.
- Oliveira GLS, Freitas RM. Diminazene aceturate - an antiparasitic drug of antiquity: advances in pharmacology & therapeutics. Pharmacol Res. 2015;102:138–157.
- Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin converting enzyme 2. J Biomol Screen. 2011;16:878–885.
- Peregrine AS, Mamman M. Pharmacology of diminazene: a review. Acta Trop. 1993;54:185–203.
- Rodrigues-Prestes TR, Rocha NP, Miranda AS, et al. The anti-inflammatory potential of ACE2/Angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets. 2017;18:1301–1313.
- Rajapaksha IG, Mak KY, Huang P, et al. The small molecule drug diminazene aceturate inhibits liver injury and biliary fibrosis in mice. Sci Rep. 2018;8(10175):1–14.
- Fang Y, Gao F, Liu Z Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM. 2019;112(12):914–924. https://doi.org/https://doi.org/10.1093/qjmed/hcz206
- Imai Y, Kuba Y, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116.
- Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630..
- Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;1–3. DOI:https://doi.org/10.1136/gutjnl-2020-320832.
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034.
- Strabelli TMV, Uip DE. COVID-19 and the heart. Arq Bras Cardiol. 2020. DOI:https://doi.org/10.36660/abc.20200209
- Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004.
- Velkoska E, Patel SK, Griggs K, et al. Diminazene aceturate improves cardiac fibrosis and diastolic dysfunction in rats with kidney disease. PLoS One. 2016;11:1–15.
- Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;1–5. DOI:https://doi.org/10.1002/jmv.25785
- Qaradakhi T, Gadanec LK, McSweeney KR, et al. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin Exp Pharmacol Physiol. 2020;47:751–758.
