1. Introduction
In the United States clozapine was approved in 1989 for treatment-refractory schizophrenia (TRS) and in 2002 for reducing suicide risk in schizophrenia [Citation1]. National registry databases and naturalistic studies suggest that patients with TRS who take clozapine have lower mortality and higher life expectancy than those not taking clozapine [Citation1].
2. Clozapine toxicity
Lower mortality is remarkable because clozapine is the second-generation antipsychotic with the narrowest therapeutic window index [Citation2] and is usually considered a drug prone to cause multiple adverse drug reactions (ADRs). Thus, the US package insert has warnings for agranulocytosis, syncope, myocarditis, seizures and increased mortality in the elderly with dementia. According to a pharmacovigilance study of ADRs reported between 1998 and 2005 to the Food and Drug Administration (FDA), clozapine was the third most toxic medication with 3277 deaths or serious non-fatal outcomes, lower only than oxycodone and fentanyl [Citation3].
In July 2019 [Citation4] we reviewed the ADR reports sent from drug agencies all over the world to the World Health Organization (WHO). After combining lethal clozapine ADRs in broad categories, agranulocytosis was associated with 550 deaths, myocarditis with 539, syncope with 299, and seizures with 308. These death totals were much lower than the 2077 deaths associated with pneumonia. Moreover, there were 6893 broad pneumonia cases with a lethality of 30% versus a lethality of 2% in broad agranulocytosis or 12% in broad myocarditis.
The history of the association between clozapine and pneumonia is complex and protracted [Citation5,Citation6].
In 2005, using postmarketing surveillance, the FDA described the association between antipsychotics and deaths in dementia patients who rarely take clozapine [Citation5]. After 15 years it is clear that, when compared with other antipsychotics, clozapine carries higher risks of 1) pneumonia and 2) lethality during pneumonia [Citation5]. In the WHO database, around 0.9% of ADR reports for any and all drugs are on pneumonia (using a narrow definition); clozapine had significantly more than this 0.9% expected while risperidone, quetiapine and olanzapine had lower than expected. Moreover, the number of deaths during pneumonia was 10 times higher in clozapine than in 3 other antipsychotics which are much more frequently prescribed [Citation7]. In a better controlled study using a mirror-image design in the Danish registry [Citation8], risperidone was also significantly associated with pneumonia.
3. The role of TRS in the pneumonia of clozapine patients
A reanalysis of the mirror-image design of the Danish registry study has led us to conclude that TRS may be more important than expected in the pneumonia of patients taking clozapine. Clozapine appeared to explain less than one-third of the increased risk of pneumonia (0.19/0.64 = 0.30) while TRS explained more than two-thirds (0.45/0.64 = 0.70) [Citation9]. In the Taiwanese registry, schizophrenia was associated with increased morbidity in pneumonia [Citation10], but the role of TRS was not explored.
represents the complex relationship between TRS, clozapine and pneumonia [Citation5,Citation6]. TRS contributes to pneumonia by at least three mediating factors: 1) smoking, 2) medication issues, and 3) obesity.
Figure 1. Pneumonia in CLO patients
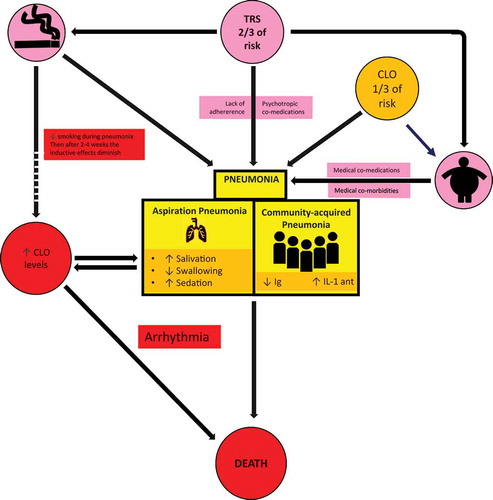
Schizophrenia, and particularly TRS, is associated with smoking and heavy smoking [Citation11], which in the general population is a major risk factor for community-acquired pneumonia [Citation12] and probably for aspiration pneumonia [Citation13]. In a retrospective study in a Japanese hospital, smoking also increased the risk of pneumonia in schizophrenia patients [Citation14].
Regarding medication issues, TRS is associated with poor adherence not only to psychiatric treatment but also to all medical treatment [Citation1]. In patients with TRS, psychiatric polypharmacy is frequent. Other psychiatric medications can also increase the risk of pneumonia. Specifically, benzodiazepines were independently associated with pneumonia in patients with schizophrenia in the Taiwan registry [Citation15]. In high doses benzodiazepines can interfere with swallowing and cause sedation. In the same Taiwanese database, another analysis suggested that co-prescription of benzodiazepines increased the risk of pneumonia in clozapine-treated patients [Citation16]
In the general population obesity is a risk factor for pneumonia [Citation17]. The relationship between TRS and obesity has not been studied, but in a survey of psychiatrists, obesity was reported as one of the main medical burdens in TRS [Citation18]. Obesity can lead to more medical comorbidities and co-medications that also may contribute to pneumonia. As the prescription of clozapine in TRS varies extremely widely from country to country [Citation19], it is difficult to estimate the contribution of clozapine to obesity in patients with TRS. Clozapine definitively contributes to obesity in some patients with TRS, particularly in those who were not obese before starting clozapine [Citation20]. Moreover, the progressive weight gain patients may experience after clozapine treatment may be associated with a decrease in clozapine metabolism [Citation21].
4. The role of clozapine in the pneumonia of clozapine patients
There are two main types of pneumonia: community-acquired and aspiration pneumonia, but most articles in psychiatric journals do not specify the type of pneumonia studied [Citation5]. graphically summarizes how clozapine can contribute to community-acquired pneumonia by decreasing immunoglobulin levels and increasing the interleukin-1 receptor antagonist [Citation5,Citation6]. All antipsychotics may interfere with swallowing which, in some cases, can be explained by parkinsonism or tardive dyskinesia [Citation5]. Increased salivation and sedation also can contribute to swallowing disturbances in these patients and probably to aspiration pneumonia. Clozapine is probably the antipsychotic most frequently associated with hypersalivation and sedation which may partly explain how it increases the risk of pneumonia [Citation5]. Clozapine has a high affinity for muscarinic receptors that may contribute to hypersalivation and its high affinity for histamine 1 receptors that may contribute to sedation [Citation5,Citation22,Citation23].
5. The role of clozapine in high lethality among clozapine patients with pneumonia
Clozapine is mainly metabolized by the cytochrome P450 (CYP) 1A2 (CYP1A2), but other CYPs (CYP2C19, CYP3A4 and CYP2D6) may also be minor metabolic pathways. Pneumonia, as in any systemic infection, releases cytokines which inhibit CYP1A2 and other CYPs (including CYP3A4) [Citation1,Citation5]. Thus, unless the clozapine dose is decreased once pneumonia has developed, the patient is at risk of increased serum clozapine concentration [Citation24]. A positive feedback loop then ensues, as the increased serum concentration increases the risk of clozapine’s detrimental effects including more swallowing disturbances, sedation and hypersalivation [Citation25], which increases the risk of further aspiration ().
In smokers, smoking cessation during pneumonia can lead to additional increases in serum clozapine concentrations. The polycyclic aromatic hydrocarbons from tobacco smoke are inducers of CYP1A2 [Citation1]. Thus, when smokers stop smoking during pneumonia, serum clozapine concentrations increase; studies suggest that it may take 2–4 weeks to reach maximum effect [Citation26]. Pharmacokinetic principles provide similar estimations: 1) 7 half-lives of CYP1A2 are, on average, 11 days (range 8 to 16) [Citation27] since 7 half-lives may be required for eliminating almost all (99%) extra CYP1A2 from induction, and 2) an extra week may be needed (after all induction disappears, 7 clozapine half-lives are needed for reaching maximum clozapine concentrations).
High serum clozapine concentrations during pneumonia, by blocking heart potassium channels, may prolong the QTc interval and increase the risk of arrhythmia. In the Taiwanese registry, arrhythmias were associated with pneumonia mortality, but clozapine did not appear to increase that risk [Citation28].
6. Limitations
All studies on the association of clozapine and pneumonia are naturalistic studies using pharmacoepidemiological databases [Citation4,Citation5,Citation7–10,Citation14–16,Citation22,Citation23,Citation28,Citation29] or clinical samples [Citation4,Citation5,Citation24], which cannot completely eliminate confounders. The mirror-image pharmacoepidemiological study from the Danish registry that compared pneumonia cases in the same patients on antipsychotics and then when they were switched to clozapine had the best design to control for confounders. Unfortunately, better-controlled studies are not possible in this area; it is not possible to randomize patients to pneumonia vs placebo after stratifying for confounders. The WHO database contains ADR data from >30 countries and 4 continents [Citation4]. Thus, the increased risk of pneumonia in clozapine patients, when compared with other antipsychotics, appears reliable since it was significant across all age groups and on 4 continents [Citation7]. More importantly, the dramatic fourfold-higher number of deaths during pneumonia compared to clozapine-induced agranulocytosis was shocking. In summary, there is no doubt in our minds that patients on clozapine have an increased risk of pneumonia and increased lethality once pneumonia develops.
is developed by combining information from multiple sources [Citation5,Citation6]. The elegant design of the mirror-image study from the Danish registry led to an independent verification of the increased risk of pneumonia in clozapine patients [Citation8] and allowed us to perform a re-analysis which suggests that TRS may be more important than clozapine for pneumonia in clozapine patients [Citation9]. Independent replication is needed. There is no way of knowing which biases are introduced by reanalyzing the small, but overlapping, numbers of patients with pneumonia in multiple analyses from the Taiwan registry [Citation10,Citation15,Citation16,Citation23,Citation28]. Those results need to be replicated in other countries. Likewise, a few relatively small clinical studies in other countries have also associated clozapine and pneumonia; these studies were described in prior reviews [Citation4,Citation5].
7. Expert Opinion
Clozapine appears to attenuate the defensive mechanisms against infection and aspiration and maybe an aggravating factor by increasing the lethality of pneumonia. On the other hand, clozapine is an excellent treatment for TRS and has been associated with increased life expectancy when patients can tolerate it as maintenance treatment for years. This is why it is very important to increase the safe use of clozapine worldwide and decrease its toxicity. To decrease clozapine toxicity in a country like Denmark, where there were no deaths during the first 2 months of clozapine treatment due to myocarditis or agranulocytosis [Citation29], Danish psychiatrists should focus on preventing and decreasing the lethality of pneumonia during maintenance treatment. In a country like Australia with 2% myocarditis [Citation30], besides focusing on pneumonia, Australian psychiatrists may also need to improve titration through slower personalized dosing [Citation1]. Finally, prior review articles discuss additional practical aspects such as the diagnosis of pneumonia in clozapine patients versus other clozapine ADRs [Citation31] and its management [Citation1].
Declaration of interest
E. Spina has participated in speakers/advisory boards and lectures supported by AstraZeneca, Bristol-Myers, Eli Lilly & Co., Janssen Pharmaceuticals, Lundbeck and Pfizer. J. de Leon personally develops his presentations for lecturing, has never lectured using any pharmaceutical or pharmacogenetic company presentation, and has never been a consultant for pharmacogenetic or pharmaceutical companies. In the past, J. de Leon has received researcher-initiated grants from Eli Lilly (one ended in 2003 and the other, as co-investigator, ended in 2007); from Roche Molecular Systems, Inc. (ended in 2007); and, in a collaboration with Genomas, Inc., from the NIH Small Business Innovation Research program (ended in 2010). He was on the advisory boards of Bristol-Myers Squibb (2003/04) and AstraZeneca (2003). Roche Molecular Systems supported one of his educational presentations, which was published in a peer-reviewed journal (2005). His lectures have been supported once by Sandoz (1997), twice by Lundbeck (1999 and 1999), twice by Pfizer (2001 and 2001), three times by Eli Lilly (2003, 2006, and 2006), twice by Janssen (2000 and 2006), once by Bristol-Myers Squibb (2006), and seven times by Roche Molecular Systems, Inc. (once in 2005 and six times in 2006). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
J. de Leon started his research on clozapine and pneumonia in 2002. Since then, he has further developed his ideas in this topic by collaborating with C.J. Ruan in the studies focused on serum clozapine concentrations, with C. Rohde in the study using the Danish registry, with C. De las Cuevas in the study using the database of the World Health Organization and with G. Schoretsanitis, H. Verdoux, and E. Spina in review articles. The first draft was written by G. Schoretsanitis and J. de Leon. All the authors have approved the final version. All the authors agree to be accountable for all aspects of this article review.
Acknowledgments
The authors acknowledge Lorraine Maw at the Mental Health Research Center at Eastern State Hospital, Lexington, KY, USA, who helped in editing the article. Can-Jun Ruan is supported by a 2019 NARSAD Young Investigator Award from the Brain & Behavior Research Foundation. The authors are grateful to the reviewers for providing useful suggestions for improving the article.
Additional information
Funding
References
- de Leon J, Ruan CJ, Schoretsanitis G, et al. A rational use of clozapine based on adverse drug reactions, pharmacokinetics, and clinical pharmacopsychology. Psychother Psychosom. 2020;89(4):200–214.
- Spina E, Hiemke C, de Leon J. Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2016;12(4):407–422.
- Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the food and drug administration, 1998-2005. Arch Intern Med. 2007;167:1752–1759. .
- De Leon J, Sanz EJ, De Las Cuevas C. Data from the world health organization’s pharmacovigilance database supports the prominent role of pneumonia in mortality associated with clozapine adverse drug reactions. Schizophr Bull. 2020;46(1):1–3.
- Cicala G, Barbieri MA, Spina E, et al., A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev Clin Pharmacol. 2019;12(3):219–234.
- de Leon J, Ruan CJ, Verdoux H, et al. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen Psychiatr. 2020;33(2):e100183.
- de Leon J, Sanz EJ, Norén GN, et al. Pneumonia may be more frequent and have more fatal outcomes with clozapine than with other second-generation antipsychotics. World Psychiatry. 2020;19(1):120–121.
- Rohde C, Siskind D, de Leon J, et al., Antipsychotic medication exposure, clozapine, and pneumonia: results from a self-controlled study. Acta Psychiatr Scand. 2020;142(2):78–86.
- Villasante-Tezanos AG, Rohde C, Nielsen J, et al., Pneumonia risk: approximately one-third is due to clozapine and two-thirds is due to treatment-resistant schizophrenia. Acta Psychiatr Scand. 2020;142(1):66–67.
- Chou FH, Tsai KY, Chou YM. The incidence and all-cause mortality of pneumonia in patients with schizophrenia: a nine-year follow-up study. J Psychiatr Res. 2013;47(4):460–466.
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2–3):135–157.
- Baskaran V, Murray RL, Hunter A, et al. Effect of tobacco smoking on the risk of developing community acquired pneumonia: A systematic review and meta-analysis. PLoS One. 2019;14(7):e0220204.
- Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13(2):69–81.
- Haga T, Ito K, Sakashita K, et al. Risk factors for pneumonia in patients with schizophrenia. Neuropsychopharmacol Rep. 2018;38(4):204–209.
- Cheng SY, Chen WY, Liu HC, et al. Benzodiazepine and risk of pneumonia in schizophrenia: a nationwide case-control study. Psychopharmacology (Berl). 2018;235(11):3329–3338.
- Wu CS, Chen TY, Tsai SY, et al. Estimating the risk of pneumonia in patients with schizophrenia newly receiving clozapine: a nationwide cohort study. J Clin Psychopharmacol. 2019;39(4):297–304.
- Phung DT, Wang Z, Rutherford S, et al. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14(10):839–857.
- Correll CU, Brevig T, Brain C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry. 2019;19(1):362.
- Bachmann CJ, Aagaard L, Bernardo M, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136(1):37–51.
- de Leon J, Diaz FJ, Josiassen RC, et al. Weight gain during a double-blind multidosage clozapine study. J Clin Psychopharmacol. 2007;27(1):22–27.
- Diaz FJ, Josiassen RC, de Leon J. The effect of body weight changes on total plasma clozapine concentrations determined by applying a statistical model to the data from a double-blind trial. J Clin Psychopharmacol. 2018;38(5):442–446.
- Knol W, van Marum RJ, Jansen PA, et al. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56(4):661–666.
- Kuo CJ, Yang SY, Liao YT, et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull. 2013;39(3):648–657.
- Ruan CJ, Zang YN, Cheng YH, et al., Around 3% of 1,300 levels were elevated during infections in a retrospective review of 131 Beijing hospital in-patients with more than 24,000 days of clozapine treatment. Psychother Psychosom. 2020;89(4):255–257.
- Schoretsanitis G, Kuzin M, Kane JM, et al. Elevated clozapine concentrations in clozapine-treated patients with hypersalivation. Clin Pharmacokinetics. 2020 Oct 1. Online ahead of print. DOI:10.1007/s40262-020-00944-5.
- de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55(5):491–493.
- Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178–184.
- Liao YT, Yang SY, Liu HC, et al. Cardiac complications associated with short-term mortality in schizophrenia patients hospitalized for pneumonia: a nationwide case-control study. PLoS One. 2013;8(7):e70142.
- Rohde C, Polcwiartek C, Kragholm K, et al. Adverse cardiac events in out-patients initiating clozapine treatment: a nationwide register-based study. Acta Psychiatr Scand. 2018;137(1):47–53.
- Siskind D, Sidhu A, Cross J, et al. Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy. Aust N Z J Psychiatry. 2020;54:467–481.
- Verdoux H, Quiles C, de Leon J. Clinical determinants of fever in clozapine users and implications for treatment management: A narrative review. Schizophr Res. 2019;211:1–9. .
