ABSTRACT
Introduction
Norgestimate (NGM) is a testosterone derivative with peculiar receptor activities.
Areas covered
This is a narrative review of the available data on the pharmacotherapy of NGM in combined hormonal contraceptives (CHCs) in terms of contraceptive efficacy, venous thromboembolism (VTE) risk, safety, tolerability and bleeding patterns. A comprehensive literature review was conducted in August 2020 using PubMed with the keyword ‘norgestimate’.
Expert Opinion
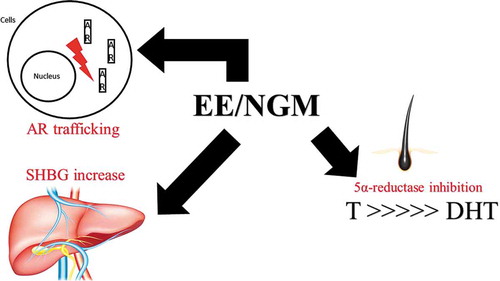
NGM shows a mild estrogenic activity associated with anti-mineralocorticoid and anti-androgenic properties, largely responsible for the cardiovascular safety profile. The anti-androgenic property depends on the androgen receptor (AR) nuclear translocation (AR trafficking and its subnuclear distribution), the inhibition of 5α-reductase activity (it possesses higher activity compared to other available progestins), and the increase on sexual hormone binding globulin (SHBG) levels if combined with an estrogenic counterpart. NGM is one of the molecules that best modulates the power of ethinyl-estradiol on the thromboembolic risk, being associated with the lowest VTE risk between different CHCs. NGM has the advantage of retaining peripheral anti-androgenic activity, demonstrated by the impact on lipid and glucose metabolism, and it should be preferred if compared with other similar progestins of the same class of risk which are much more androgenic, such as levonorgestrel.
1. Introduction
The choice of the most suitable progestin for a woman’s needs plays a crucial role in assuring good compliance toward hormonal contraception. The synthetic progestins available are generally divided into progesterone and testosterone derivatives, and an analog of spironolactone [Citation1,Citation2]. The common ability of the different ‘progestational’ steroids is to bind to the progesterone receptor (PR), with high or low affinity, guaranteeing the secretory maturation of a previously estrogen-modified proliferative endometrium. On the other hand, the main differences between different available progestins are due to their pharmacological, pharmacokinetic and pharmacodynamic properties, and to their different abilities to work on other intracellular steroid receptors. The various progestins are characterized by different affinity to the estrogen (ER), androgen (AR), glucocorticoid (GR) and mineralocorticoid (MR) receptors, expressing their possible agonist or antagonist actions [Citation3]. Such interactions may either induce transactivation or prevent activation of the different steroid receptors and may be responsible for some positive or for some of the undesirable side effects of progestins. In other words, even if they belong to the same ‘progestin family’, they can show a completely different clinical profile [Citation4]. Different generations of progestins have followed one another, and new molecules are and will be put on the market; nevertheless, some older molecules continue to maintain peculiar advantages in this continuous evolutionary process [Citation5]. In this wide panorama, norgestimate (NGM) represents a particular progestin, as it is a testosterone derivative with a peculiar receptor activity. The aim of this narrative review is to focus on the main advantages of NGM, related in particular to its unique peripheral anti-androgenic activity, the biological explanation of its low thrombotic risk and its reassuring tolerability and safety, nearly 30 years after its first release on the hormonal contraception market.
2. Materials and methods
This narrative review paper includes selected pharmacotherapy data of interest about the progestin NGM, in particular contained in combined hormonal contraceptive (CHC) oral formulations in combination with ethinyl-estradiol (EE), in monophasic or multiphasic association, published in English up to July 2020. The research was carried out in August 2020 and the databases were searched from 1 January 1977 to 31 July 2020. Relevant documents were identified through a literature search in PubMed using the keyword ‘norgestimate’.
We recovered and evaluated all potentially relevant articles and checked their reference lists to identify any additional relevant publications. Only papers in the English language in press or just published were considered. We did not consider abstracts and/or case reports. All identified references were reviewed by a second author (MC.DS).
The initial search retrieved 407 documents. Studies that were not relevant to the outcomes of interest of the review or duplicates were not considered. The final reference list consisted of 74 papers. The data are presented in a narrative structure, divided into paragraphs for each subtopic.
3. Chemistry
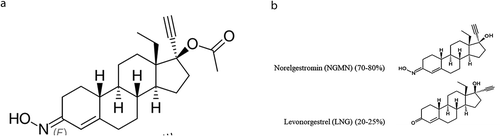
NGM () is a 19-nortestosterone derivative of the gonane family [Citation1]. NGM is 17-ethylinated, 18-methyl steroid with a 17-position acetate and an oxime at position 3 (). The C-17 acetate group as well as a unique C-3 oxime group inhibit its ability to bind to ARs. Because the C-3-keto group is typical of androgenic compounds, its replacement by the oxime group can contribute to reduce the androgenicity of NGM as compared with norgestrel (NGL) and levonorgestrel (LNG) [Citation6], in addition to other peripheral mechanisms that will be reported later.
4. Pharmacokinetics and metabolism
NGM is considered a prohormone or a prodrug. Through a rapid hydrolysis of the acetate at position 17, it forms 17-deacetyl-norgestimate (norelgestromin, NGMN) (), which carries most of the progestational activities of NGM, being its major metabolite [Citation7]. A low concentration of NGMN can be detected in the blood for about 5 hours after 250 µg NGM oral assumption, with a mean peak of 100 pg/mL, compared with a mean peak concentration of NGM of 3,500 pg/mL after 1.5 hours [Citation6]. On the other hand, the half-life of NGMN exceeds 24 hours, due to the protection of oxime group from further rapid metabolism [Citation6] (). Other metabolic transformations include cleavage at the oxime, ketone reduction at position 3, A + D ring hydroxylation and reduction of the C4-C5 double bond. By final conjugation, it goes to glucuronide or sulfate metabolites. The last step includes that a small amount of NGM, about 20–25%, is then metabolized to LNG [Citation8] ().
Table 1. Pharmacokinetic parameters of norgestimate (NGM) and its metabolite norelgestromin (NGMN)
5. Pharmacodynamics
5.1. Progestational activity
5.1.1. Effect on progesterone receptor (PR)
NGM and its metabolites have a similar progestational activity to that of progesterone (P) molecule itself on the endometrium (as shown by its strong binding affinity for PRs in the uterus of the rabbit [Citation9]) (). Highly progestational responses to NGM have been demonstrated in many experimental models. Another parameter used to describe a typical progestational activity is the suppression of ovulation. In rabbits, oral doses of NGM have been shown to inhibit ovulation by blocking the preovulatory surge of luteinizing hormone (LH) [Citation10]. In addition, NGM replacement permits pregnancy continuation despite the absence of endogenous P in ovariectomized rats. In estrogen primed immature rabbits, endometrial stimulation follows both oral and subcutaneous administration of NGM: the maintained potency of NGM after subcutaneous administration can indicate that its progestational activity does not depend on first-pass hepatic metabolism [Citation9].NGM and NGMN are PR partial agonists, with half-maximal effective concentration (EC50) values of 13 and 11.1 nM, respectively; these concentrations fall within the total serum concentration of NGM and its metabolite after oral administration (10−9-10−7 M) [Citation11].
Figure 2. A) Relative binding affinities of common used progestins to progesterone receptor (PR). natural progesterone is the reference (1). b) Relative binding affinities of common used progestins to androgen receptor (AR). dihydrotestosterone is the reference (1). the assay measures displacement of 3H R5020 from the PR isolated from the uterus of the rabbit. data from reference [Citation9]
![Figure 2. A) Relative binding affinities of common used progestins to progesterone receptor (PR). natural progesterone is the reference (1). b) Relative binding affinities of common used progestins to androgen receptor (AR). dihydrotestosterone is the reference (1). the assay measures displacement of 3H R5020 from the PR isolated from the uterus of the rabbit. data from reference [Citation9]](/cms/asset/5d5ea559-2f92-462b-bb12-7cacfa494ae0/ierj_a_1878876_f0002_oc.jpg)
In humans, the ovulation inhibition dose in quantity per day per os for NGM is 200 µg, while its transformation dose per cycle is 7 mg, resulting in a uterotrophic index of 2.9% [Citation2].
5.2. Estrogenic activity
5.2.1. Effect on estrogen receptor (ER)
Inhibition of estrogenic activity by NGM has also been demonstrated experimentally. In ovariectomized rats, cornification of the vagina induced by estrone is inhibited by oral and subcutaneous administration of NGM [Citation10]. However, in contrast to its anti-estrogenic properties, NGM has been found to be virtually inactive in a series of estrogenic test systems. Thus, the anti-estrogenic properties are rather a progestin-mediated action, as opposed to direct interference with the interaction between estrogen and its receptor. Indeed, ER activity has been demonstrated, in particular selective agonism at ERα (EC50 30.4 and 43.4 nM for NGM and NGMN), while NGM and its metabolite are not active on ERβ [Citation11].
5.3. Mineralocorticoid activity
5.3.1. Effect on mineralocorticoid receptors (MR)
Though the relative binding affinities of NGM to MR has been initially described as null [Citation2], in subsequent experiments, its affinity for MR was described as moderate, i.e. a half maximal inhibitory concentration (IC50) of 81.2 and 83.7 nM [Citation11]. The half-maximal inhibition (Ki) of 1 nM aldosterone binding to MR occurred at 232 ± 53.3 nM of NGM, 140 ± 32.1 nM of NGMN and 4 ± 0.49 nM of spironolactone (). This NGM anti-mineralocorticoid activity may be clinically important, i.e. by reducing water and sodium retention and decreasing the risk of hypertension and cardiovascular disease during its use.
Figure 3. Antagonistic mineralocorticoid activity of norgestimate (NGM) and norelgestromin (NGMN), in comparison to spironolactone. data adjusted from reference [Citation11]
![Figure 3. Antagonistic mineralocorticoid activity of norgestimate (NGM) and norelgestromin (NGMN), in comparison to spironolactone. data adjusted from reference [Citation11]](/cms/asset/64e0c72c-12c4-4f2b-952a-ad05a37dbb0d/ierj_a_1878876_f0003_oc.jpg)
5.4. Glucocorticoid activity
5.4.1. Effect on glucocorticoid receptor (GR)
The relative binding affinity of NGM to GR is negligible, as is the potential agonist/antagonist activity on this type of steroid receptor [Citation2]. Similarly, NGM has been found to be clinically inactive on GR. Its affinity for GR is very low (IC50 of 325 and 255 nM) [Citation11].
5.5. Androgenic activity
The androgenic or anti-androgenic activity of NGM is not only based on AR direct bond but depends on other receptor and peripheral effects, such as AR nuclear translocation (AR trafficking and its subnuclear distribution), effect on 5α-reductase activity and on sex hormone-binding globulin (SHBG) levels if combined with an estrogenic counterpart. These different activities confer NGM with some clinical anti-androgenic effects, despite its direct derivation from testosterone and possible partial transformation into a mild androgenic molecule such as LNG ().
Figure 4. Antiandrogenic activities of norgestimate (NGM) in combination with EE. T: testosterone. DHT: dihydrotestosterone. SHBG: sex hormone-binding globulin. AR: androgen receptor. EE: ethinylestradiol. NGM: norgestimate
5.5.1. Effect on androgen receptors (AR)
5.5.1.1. Receptor affinity
The knowledge about NGM binding to the AR and thereby inducing androgenic or anti-androgenic effects is based mainly on animal experiments. NGM has essentially minimal activity on AR. This has been proven by its very weak affinity for AR in rats [around 1.3% times that of dihydrotestosterone (DHT)] () [Citation12].In these same assays its two principal metabolites, NGMN and LNG, were proven to be more androgenic than NGM, which makes it unlikely that they made any significant contribution to the biologic activity of NGM [Citation13].
An ideal progestin should achieve a progestational response when given at a low concentration and it has to elicit an androgenic response only at a high concentration. In AR binding assays, only very high concentrations of NGM (IC50 of 764 nM) displaced radiolabeled DHT, the androgen standard. In PR binding assays, the NGM’s IC50 was only 3.5 nM, with a relative binding affinity of 1.24. NGM androgen-to-progestin receptor binding ratio was 219, indicating a highly selective progestational response, higher than P itself. The descending order of selectivity of different progestin is indeed NGM, P and LNG [Citation14]. The partial AR agonist activities of NGM and NGMN seem even higher than that of cyproterone acetate (CPA). However, NGM and NGMN (10−6 M) induced luciferase activity of up to 70% of that observed with a synthetic androgen agonist (10−9 M), in comparison to 50% for CPA (10−6 M) [Citation15].
5.5.1.2. AR trafficking and its subnuclear distribution
After androgen binding to the ligand-binding domain of AR, the AR-androgen complex moves into the nucleus where it can interact with target DNA sequences. The complex can then induce the recruitment of downstream transcription factors and the subsequent transcription of target genes [Citation16].
Anti-androgens can affect each step of the molecular action of androgens. It was demonstrated that NGM permits green fluorescent protein AR transport into the nucleus, but less efficiently than with natural ligands. In a cell line well-known as a useful tool for studying androgen and anti-androgen actions, it was observed in competitive studies that the half-maximal inhibition (Ki) of NGM (4.2 + 0.561078 M) was close to that of cyproterone acetate (CPA) (6.6 + 0.861078 M), which is the gold standard for hyperandrogenism treatment. If we consider the dosage of NGM and its intracellular concentration in target tissue, this Ki is compatible with a biological effect of NGM on AR. Moreover, these data show that NGM and NGMN decreased AR transcriptional activity (24%), although less efficiently than that observed with CPA at the same concentration (47%) () [Citation15].
5.5.1.3. Effect on 5α-reductase activity
Increased 5α-dihydrotestosterone (DHT) concentration due to elevated peripheral androgen metabolism is very important in the pathogenesis of acne, hirsutism and androgenic alopecia. The clinical manifestations of hyperandrogenism, such as hirsutism and acne, are disturbing to most patients and it can be associated with other conditions, including polycystic ovary syndrome (PCOS), insulin resistance and cardiovascular diseases risk [Citation17]. In many androgen-responsive organs, such as skin, androgenic activity is elicited mainly by DHT [Citation18]. The reduction of testosterone to DHT is catalyzed by the 5α reductase enzyme, that can exist in two isoforms, which differ pH requirement and in Michaelis-Menten constants [Citation19]. Both isoenzymes are simultaneously present in many tissues including the central nervous system, the respiratory, urogenital and gastrointestinal tract, the skin and the endocrine system [Citation20]. Finasteride, a potent 5α-reductase type 2 inhibitor, suppressed circulating DHT beyond 70% and led to improvements in women suffering from hirsutism and acne. The effect of other progestins on the inhibition of 5α-reductase is variable. In an in vitro model using breast skin, NGM showed 50% inhibition of 5α-reductase at a concentration of 10 pM, in the range of finasteride activity (IC50 of 1 pM) () [Citation21]. These results provide evidence for the usefulness of NGM for the treatment of hirsutism and male-pattern baldness in women. In the same study, LNG, dienogest (DNG), CPA and gestodene (GSD) had IC50 values between 52 and 98 pM. Out of all progestins tested, only CPA and NGM have been reported to inhibit skin 5α-reductase activity. On the other hand, the EE component does not influence 5α-reductase activity (). In genital skin tissue, estradiol (E2) has been shown to inhibit 5α-reductase only at high concentrations, suggesting that estrogens appear not to be a major factor influencing 5α-reductase activity.
Figure 5. Progestins inhibit skin 5α-reductase activity in vitro. The most potent inhibitor in comparison to finasteride was norgestimate, followed by levonorgestrel, dienogest, cyproterone acetate, gestodene, norgestrel, norethisterone and 3-keto-desogestrel. No effect was seen with ethinyl estradiol. Data from reference [Citation21]
![Figure 5. Progestins inhibit skin 5α-reductase activity in vitro. The most potent inhibitor in comparison to finasteride was norgestimate, followed by levonorgestrel, dienogest, cyproterone acetate, gestodene, norgestrel, norethisterone and 3-keto-desogestrel. No effect was seen with ethinyl estradiol. Data from reference [Citation21]](/cms/asset/04c8efbe-5820-4eb4-9b26-630369dcfb33/ierj_a_1878876_f0005_oc.jpg)
5.5.1.4. Effect on sex hormone-binding globulin (SHBG) levels
SHBG is a carrier protein produced by the liver. Its production has been demonstrated to be highly estrogen sensitive. Oral intake of EE alone results in an important dose-dependent increase in SHBG levels. Progestins administration results in varying degrees of SHBG decrease, depending on both the dose and type of molecule used, which could be interpreted as an expression of differences in the anti-estrogenic potency of the progestin. Therefore, the combined effect on SHBG could be used as a measure of the total estrogenic effect of EE and the anti-estrogenic effect of the progestin, or the total estrogenicity of the combination. This marker changes have been even proposed to predict the risk of venous thromboembolism (VTE) during CHC use [Citation22].
Moreover, the elevations in SHBG can effectively reduce the amount of bioactive T and decreasing its potential androgenic activity. A strongly androgenic progestin can inhibit this action by competitively displacing T from SHBG and allowing freer active T to produce its effects.
The increase in SHBG in users of CHCs containing NGM is particularly evident in monophasic associations (+167% and +161%), +163%, +69% and +126% in triphasic formulations, significantly lower than with other anti-androgenic progestins such as drospirenone (DRSP) (+270%), DNG (+269%) and CPA (+325% and +402%), but higher than with second-generation androgenic progestins such as LNG (+50-60%) () [Citation23]. In in vitro studies of human SHBG, both NGM and NGMN, showed little affinity for SHBG, as they were unable to displace T from SHBG even at concentrations >10,000 nM ()[Citation24].
Figure 6. Relative binding affinity of different progestins for human sex hormone-binding globulin (SHBG) measured as displacement of ‘H-testosterone. data from reference [Citation24]
![Figure 6. Relative binding affinity of different progestins for human sex hormone-binding globulin (SHBG) measured as displacement of ‘H-testosterone. data from reference [Citation24]](/cms/asset/c9538cae-e225-4d86-9186-4fda42fda8e4/ierj_a_1878876_f0006_oc.jpg)
6. Coagulatory effect of CHCs containing NGM
6.1. Effect of the estrogenic component
EE-containing CHCs can normally increase the activity of some coagulation factors by about 30 to 50% [factors I (fibrinogen), II, VII, VIII, IX, X and XI], with a contemporary decrease of the activity of naturally occurring anticoagulants, like protein S by approximately 30 to 40% [Citation25]. The acquired hypercoagulability seems to be independent of the route of administration of EE [Citation26], but directly dependent on its dose [Citation27,Citation28]. Substitution of EE by oral E2, as performed in the last 10 years, may reduce but not abolish the coagulation stimulus of CHCs [Citation29], though the transdermal/vaginal route of administration would be more neutral in comparison to the oral one [Citation30].
6.2. Effect of the progestin component
As reported above, different progestins may differently interact with PR, ER, GR, MR and AR, exerting either agonistic or antagonistic effects. Three effects may be relevant to the effect of a progestin molecule on coagulation: glucocorticoid, anti-mineralocorticoid and androgenic activity, but in the end, the latter are the most relevant in clinical practice.
6.2.1. Due to glucocorticoid activity
Activation of GR increases the effect of thrombin and, to a lesser extent, enhances procoagulation factors [Citation31]. Several progestins may bind and activate GR [Citation32], but at the doses used in vivo only medroxyprogesterone acetate (MPA) can exert some glucocorticoid-mediated procoagulant effects [Citation33]. As reported, the activity of NGM on GR is not clinically relevant.
6.2.2. Due to mineralocorticoid activity
Activation of the renin-angiotensin-aldosterone system increases blood coagulation though several mechanisms, including elevation of plasminogen activator inhibitor-1, which inhibits fibrinolysis, and platelet adhesiveness [Citation34], which is antagonized by aldosterone [Citation35]. Traditionally, besides P, the progestins norethisterone acetate (NETA), GSD and mostly DRSP can have some anti-mineralocorticoid properties [Citation2]. However, recent evidences also suggest a mild anti-mineralocorticoid activity for NGM in vitro [Citation11] (), which could be clinically relevant regarding the specific VTE risk of this progestin.
6.2.3. Due to androgenic activity
The androgenic action of progestins has different coagulatory functions. It counteracts, in a dose- and potency-dependent way, the estrogen-induced reduction of activated protein C (APC) and of tissue factor pathway inhibitor and the induced increase in factor VIIa. It does not counteract the estrogen-induced increase in fibrinogen and the decrease in antithrombin III [Citation36–38]. Accordingly, androgenic progestins, to a greater extent than other progestins, antagonize the estrogen-induced activation of the coagulation system. For this reason, the activation of coagulation is less pronounced with more androgenic CHCs, at a similar EE dose. The most potent androgenic progestins are first and second-generation progestins, such as NETA, NGL and LNG [Citation39,Citation40]. Following the administration of 250 µg NGM, systemic exposure to LNG should be theoretically about a third that observed in a pill containing 150 µg LNG (20–25% of 250 µg = 50–62.5 µg), provided it is clinically relevant [Citation41]. Indeed, 250 µg dose of NGM counteracts the 30 µg EE-induced increase in SHBG less than a 150 µg LNG dose (see above). Accordingly, in theory the combination of EE with NMG is less androgenic than that with LNG, and its use should be associated with a slightly higher risk of VTE: however, this is not confirmed by epidemiological data.
6.3. VTE risk during CHCs containing NGM
As we will see, epidemiological data are largely supported by the reported biological data. The risk of VTE is lower with pills containing more androgenic progestins such as LNG in combination with EE. The data show that LNG does not relevantly decrease the risk of VTE induced by 50 µg EE, but it counteracts, in part, the effect of the 30 µg EE doses. The same counteraction can be observed with 100 µg LNG added to 20 µg EE [Citation42]. NGM is partially metabolized to LNG (20–25%), and, at the doses administered, it exposes the user to levels of LNG like those achieved with the administration of 50 µg LNG. It seems that this exposure is sufficient to reduce the risk of VTE induced by EE, at levels similar to those observed with a CHC containing LNG [Citation42].
According to a recent Danish historical registry-based cohort study, in the group of progestins in combination with 30–40 μg EE, NGM shows a similar VTE risk of LNG and NETA (1.18 95% CI 0.86–1.62), while other progestins in combination with the same EE dose show a significantly doubled risk between 2.09 and 2.24, i.e. desogestrel (DSG), GSD, DRSP and CPA. In comparison with non-users, this risk with ≥30 µg EE + NGM is still lower than with products containing 20 μg EE in combination with GSD, DSG or DRSP (2.56 vs. 3.26 to 4.84) [Citation43]. NGM is the progestin that, associated with EE, has shown the lowest risk of VTE (5–7 cases for 10.000 women users/year) according to the European Medicines Agency, together with first- and second-generation progestins such as NETA and LNG [Citation44] (). Interestingly, the risk of its principal metabolite, NGMN, is considered slightly higher (6–12 cases for 10,000 women users/year), probably for the most used and studied route of administration (transdermal) that is associated with higher cumulative serum doses of EE () [Citation45].
Table 2. Risk of developing a blood clot (VTE) in a year according to European medicines agency [Citation44]. 1: further studies are ongoing or planned to collect sufficient data to estimate the risk for these products. CHC: combined hormonal contraceptive
7. Contraceptive efficacy
NGM are available combined to EE in two different formulations: a monophasic (days 1 to 21, 35 μg of EE/250 μg of NGM) and a triphasic preparation (days 1 to 7, 35 μg of EE/180 μg of NGM; days 8 to 14, 35 μg of EE/215 μg of NGM; days 15 to 21, 35 μg of EE/250 μg of NGM). The triphasic combination has the rationale to permit a lower total dose of NGM in comparison with the monophasic one without an increase of breakthrough bleeding (BTB) occurrence. In general, no significant difference in contraceptive efficacy has been demonstrated among all the various mono and triphasic formulations ().
Table 3. Experimental process of ethinylestradiol/norgestimate (EE/NGM) in a monophasic or triphasic formulation in relation to its contraceptive efficacy. X: study without a comparator. PI: Pearl index. GSD: gestodene. NGL: norgestrel, LNG: levonorgestrel
An open-label, noncomparative clinical trial conducted in Germany with 59,701 women enrolled showed an overall Pearl Index (PI) of 0.25 (95% CI: 0.19 to 0.31) [Citation46]. The contraceptive efficacy was demonstrated also by two smaller multicenter trials conducted in Austria and in Germany, respectively on 97 and 147 women, with no pregnancies occurring during the entire follow-up period [Citation47,Citation48]. An excellent contraceptive efficacy (overall and theoretical PI of 0.55 and 0.37, respectively) was achieved also with the triphasic EE/NGM combination during a 12-month open, non-comparative study on 661 women [Citation49].
These formulations have proved to be equally effective as the other contraceptive combinations commonly used. Two multicenter, double-blinded, randomized clinical trials conducted in Unites States (US) on 1,473 women comparing the monophasic EE/NGM combination with monophasic EE/NGL showed no statistically significant differences between the two formulations with respect to pregnancy rates [Citation50]. The same results have been achieved in an open-label, comparative clinical trial of triphasic EE/NGM versus triphasic EE/LNG conducted on 4,234 women [Citation51] ().
8. Impact on lipids and glucose metabolism
Lipid levels are biomarkers of cardiovascular risk, even if it is not clear whether CHC-induced changes of lipid metabolism can be really translated into clinically significant effects on this risk. Past studies have shown that users of high-dose CHCs experienced a marked increase in total cholesterol and triglycerides levels. With the evolution of CHCs, the gradual reduction of the EE doses has reduced their impact on lipid metabolism.
The impact of CHC on lipoprotein levels changes depending on the potency of the estrogen and its counteraction by the androgenic potency of the progestin [Citation52]. In general, the EE stimulus causes a positive effect, with a decrease of LDL and an increase of HDL and triglycerides [Citation53]. Progestins with androgenic properties antagonized these EE effects, in a dose-related fashion, with an increase in LDL and a decrease of HDL and triglycerides levels. Accordingly, at similar EE doses, CHCs with less androgenic/anti-androgenic molecules, such third-generation progestins as NGM, have shown greater increases in HDL than other formulation containing more androgenic progestins, such as LNG-based CHCs.
This effect was confirmed for NGM in clinical trials, both for the comparisons with second-generation (LNG and NGL) and other third-generation progestins: Chapdelaine et al. reported that serum levels of HDL were significantly increased from baseline values in the EE/NGM group but were significantly decreased in the EE/NGL group. Increases in LDL levels were moderate in the EE/NGM group but were pronounced in the EE/NGL group, resulting in a significant difference between formulations in the impact on LDL/HDL ratio, a common marker of arterial risk [Citation54]. A German study showed essentially no change in cholesterol from baseline after six cycles of use during EE/NGM treatment, also in subjects with higher basal cholesterol levels [Citation46]. Another US randomized controlled trial comparing monophasic NGM and NGL and showed that HDL levels were constantly elevated in the NGM group and depressed in the NGL group, and that EE/NGL significantly increased levels of LDL. For this reason, the LDL/HDL ratio decreased by 7.7% in the NGM group while it increased by 18.5% in the NGL group [Citation50] ().
Figure 7. Percentage change from baseline in serum lipids and lipoproteins: all users of monophasic EE/NGM and EE/NG. EE: ethinyl-estradiol. NGM: norgestimate. NGL: norgestrel *: Two-sided t-test significant at the 5% level. modified from reference [Citation50]
![Figure 7. Percentage change from baseline in serum lipids and lipoproteins: all users of monophasic EE/NGM and EE/NG. EE: ethinyl-estradiol. NGM: norgestimate. NGL: norgestrel *: Two-sided t-test significant at the 5% level. modified from reference [Citation50]](/cms/asset/55276f1a-84dc-4406-88a6-d8f45b7afa14/ierj_a_1878876_f0007_b.gif)
In the comparison between EE/NGM and EE/GSD, there was no significant difference between formulations with regard to the influence on any measured cholesterol parameter. A similar significant increase was observed in the plasma levels of HDL-cholesterol levels. In contrast, the LDL-cholesterol levels were significantly decreased. These changes in lipid metabolism appear to reflect a predominance of the effect of the estrogen component in combination with an anti-androgenic progestin, in particular for NGM [Citation55].
CHCs have also been associated with subclinical disturbances in carbohydrate metabolism. They can include impaired glucose tolerance and increased insulin resistance, which are known risk factors for type 2 diabetes mellitus. Both the estrogen and progestin components of CHC may be responsible for inducing insulin resistance [Citation56].
Traditionally, it was proposed that high-dose (50 μg EE) and high androgenic progestin containing CHCs have a more pronounced negative effect on glucose metabolism when compared to lower doses preparations. In general, a progestin with high androgenic activity has been shown to cause a greater decline in insulin sensitivity (SI) compared to a progestin with an anti-androgenic activity. Indeed, pills containing second-generation progestins, such as LNG, can normally decrease SI [Citation57].
The EE/NGM combination caused an increase of mean fasting glucose of only 0.1% at cycle 12 and of 2.3% at cycle 24 in comparison to a significant increase of 1% at cycle 12 and 4.6% at cycle 24 for EE/NGL group [Citation50]. This evidence to date would suggest that NGM formulations have little or no effects on carbohydrate metabolism in studies conducted in up to 24 cycles of use.
9. Safety and tolerability
9.1. Body weight
Body weight did not change significantly during the use of EE/NGM in clinical trials and withdrawal rates for weight gain are very low, constantly below 2% of users (). In a study conducted by Runnenbaum et al. [Citation46], the body weight of the women enrolled increased by only 0.6% at cycle 6. The same trend was achieved by Huber [Citation47] in a dual center trial on an EE/NGM monophasic formulation. Discontinuation because of weight gain during monophasic EE/NGM versus EE/NGL was observed in two different clinical trials: both samples demonstrated a slightly higher rate of discontinuation with NGL (1.4% with EE/NGL versus 1% with EE/NGM, by Corson [Citation50]; 1.54% versus 0.84%, by Andolsek [Citation58]). There were no significant changes in body weight at the end of 6 cycles in a comparative study by Tantbirojn et al. (EE/LNG vs. EE/NGM) [Citation59] and of a comparative study by Affinito et al. between EE/GSD and EE/NGM formulations [Citation60]. The triphasic formulation EE/NGM showed the same moderate effects on body weight [Citation49], with a similar effect in comparison with EE/LNG [Citation61].
Table 4. Experimental process of ethinylestradiol/norgestimate (EE/NGM) in a monophasic or triphasic formulation in relation to its safety and tolerability. X: study without a comparator. GSD: gestodene. NGL: norgestrel, LNG: levonorgestrel
9.2. Blood pressure
None the studies analyzed found any clinically significant changes in ambulatory blood pressure in the subjects during monophasic EE/NGM treatment [Citation47,Citation50,Citation59–61]. The same trend was observed with the triphasic formulation [Citation49,Citation61]. All these data seem to support the clinical anti-mineralocorticoid activity of progestin in decreasing the risk of hypertension, despite the concomitant presence of ≥ 30 µg of EE than can lead to a mild increase in blood pressure [Citation62]. However, no included study was based on more accurate Holter 24-h ambulatory blood pressure measurements, as more recent trials have performed for other contraceptive formulations [Citation63].
9.3. Acne
The treatment with EE/NGM was shown to be effective on mild to moderate acne vulgaris, reducing the lesions count from the beginning of the treatment cycle to the end [Citation13,Citation64]. Jaisamrarn et al. [Citation65] conducted an investigator-blinded, randomized, parallel-group trial at three centers in Thailand to compare the effectiveness and the safety of triphasic EE/NGM and biphasic EE/DSG. The efficacy and safety parameters including body weight, body mass index, vital signs, acne lesions count, facial sebum output, adverse events and concomitant medication were recorded. The total lesions count continuously decreased throughout the 6 months of treatment in both groups compared to baseline: the relative decrease from baseline to cycle 6 in the mean percentage of total lesions count in EE/NGM and EE/DSG was 74% and 65%, respectively, with a non-statistically significant mean difference of 9%. Regarding facial seborrhea, treatment with EE/NGM showed greater improvement compared to baseline than EE/DSG after 6 months of treatment (p = 0.005). The response to treatment was evaluated by the investigators as ‘excellent’ or ‘good’ for 87% of subjects in the EE/NGM group and for 74% in the EE/DSG group. In the subjects’ self-assessment, those treated with EE/DSG (96%) showed a similar improvement to EE/NGM (93%). In conclusion, this study demonstrated that the effects of EE/NGM and EE/DSG on total acne lesions count are similar, but EE/NGM has a more beneficial effect on facial seborrhea.
Efficacy on facial acne was also demonstrated in a non-comparative study by Runnenbaum et al. [Citation46] investigating the monophasic EE/NGM association: the incidence of acne was reduced from 12% during the pretherapy cycle to 9% after six cycles of EE/NGM treatment. In another trial, acne was cited as the reason for withdrawal by 0.8% of subjects using EE/NGM compared with 1.2% of those using EE/NGL [Citation54].
9.4. Adverse effects
The occurrence of adverse effects during EE/NGM is similar to that reported with other CHCs. The percentage of women who experienced nausea or headache at the end of six cycles of treatment was lower than the percentage with these symptoms before treatment with EE/NGM, decreasing, respectively, from 6% to 4% and from 13% to 5% [Citation46]. Affinito et al. [Citation60] compared the adverse reaction during EE/NGM in comparison to EE/GSD in the monophasic formulation: the safety profile was good for both contraceptives, but patients in the EE/NGM group reported a higher incidence of breast pain, whereas patients in the EE/GSD group experienced a higher incidence of headache (with no statistically significant differences). The primary reason for discontinuation were adverse reactions in 3% of women in the EE/GSD group (persistent abdominal and gastric pain, insomnia, vasomotor syndrome, tachycardia) and in 2% in the EE/NGM group (varicose veins, mastodynia, vertigo, headache). The most common adverse events during the study by Tantbirojn et al. [Citation59] were dizziness, nausea, and headache: the only statistically significant differences obtained were for headache and dizziness, finding more prevalent in the EE/LNG group in comparison of the EE/NGM group.
10. Bleeding patterns
In CHCs users, cycle control is crucial for compliance, which in turn is the major determinant of use-effectiveness. Runnenbaum et al. [Citation46] demonstrated the excellent cycle control exercised by the monophasic formulation of EE/NGM. The incidence of breakthrough (BTB) and intermenstrual spotting decreased after the therapy, from 4.5% to 3% and from 9% to 4% after six cycles of EE/NGM treatment, respectively. The occurrence of amenorrhea was only 1.4% of the women in cycle 6. Almost all (97%) women reported an absence of irregular bleeding by the end of cycle 6. Reductions in cycle length and duration of bleeding by 4% and 16%, respectively, was noted and 32% of the women experienced a reduction in intensity of bleeding. In the comparative study by Corson [Citation50] on monophasic formulation EE/NGM versus EE/NGL, the daily incidence of BTB and spotting was similar with the two treatment regimens. Amenorrhea was reported more frequently by subjects treated with EE/NGL than by those treated with EE/NGM. The difference between the two groups in the reporting of failure to have withdrawal bleeding was statistically significant (0.7% for EE/NGM versus 1.5% for EE/NGL, p < 0.001). Tantbirojn et al. [Citation59] compared the cycle control of monophasic formulation of EE/LNG with the EE/NGM one: the formulation with LNG appeared to provide better cycle control than NGM one, but without statistical significance. No differences in the mean cycle length of both groups were observed, as well no significant differences in amount of withdrawal bleeding. The mean duration of the bleeding in the EE/NGM group was longer than in the EE/LNG group with a significant difference. Affinito et al. [Citation60] compared the monophasic EE/NGM formulation with the EE/GSD one: the majority of cycles, 94.4% in the EE/GSD group and 92.8% in the EE/NGM group, were normal. BTB occurred in 0.2% of cycles in the EE/GSD group and 1.6% of cycles in the NGM group; the frequency of spotting was 5.4% in the EE/GSD group and 5.6% in the EE/NGM group. Similar results were achieved with the triphasic formulation of EE/NGM. Gauthier et al. [Citation49] demonstrated a decrease in the occurrence of severe flow from 14.8% before the study to 4% after cycle 12. Also, BTB and amenorrheic cycles decreased from 12.1% during cycle 1 to 2.6% at the end of the evaluation and from 4.1% to 3.7%, respectively. The same trend for a positive impact on cycle control was observed by Andolsek [Citation58] in two multicenter non-comparative studies on the EE/NGM triphasic formulation conducted in the US. The incidence of scheduled withdrawal bleeding in EE/NGM was constantly higher than 95%, as demonstrated in .
Table 5. Experimental process of ethinylestradiol/norgestimate (EE/NGM) in a monophasic or triphasic formulation in relation to its bleeding pattern. X: study without a comparator. %*: percentage of cycles. %+: percentage of patients. BTB: breakthrough bleeding. GSD: gestodene. NGL: norgestrel, LNG: levonorgestrel, DSG: desogestrel
11. Conclusions
The results from this narrative review try to biologically explain many of the advantages found with the use of NGM in the clinical practice as a hormonal contraceptive. The combination of EE with NGM, both in monophasic and in triphasic regimens, represents a valid therapeutic ally in many situations.
The receptor activity of this progestin is particularly effective and well-balanced. The highly selective progestational response in relation to the androgenic one, higher than that of natural P itself, confers to this molecule an intrinsic progestational activity demonstrated in many experimental models and by the extremely low dose of ovulation inhibition (200 µg), among the lowest between the progestins we commonly use. Through rapid hydrolysis, NGM forms NGMN, which carries most of the progestational activities of the parent compound, while only about 20–25% is finally metabolized to LNG.
The mild estrogenic activity of the molecule mainly expresses itself on type α ER; ERα and ERβ have different downstream transcriptional actions, resulting in specific tissue-specific biological activities. ERα is highly expressed in the uterus, ovarian theca cells, mammary gland and liver. Indeed, ERβ is highly present in ovarian granulosa cells, bone marrow and brain. There are some common physiological roles for the two ERs, in particular in the development and function of the ovaries, and in the protection of the cardiovascular system. The α subtype exerts its more prominent role in the uterus and the breast, as well as in the preservation of skeletal homeostasis [Citation66].
NGM is also devoid of any relevant GR activity, which is harmful to the skeletal and cardiovascular system, like other modern progestins. The most interesting receptor activities of NGM, however, are the anti-mineralocorticoid and the anti-androgenic ones. As shown, these properties are also largely responsible for the cardiovascular safety profile of the molecule, both on the arterial and on the venous side. The first property is clinically important, reducing the classical side effects of CHCs such as bloating and water retention and decreasing the risk of hypertension during their use. Moreover, it can show a positive effect in women suffering from premenstrual syndrome up to premenstrual dysphoric disorder [Citation67]. The second property is more complex for NGM: it depends on other receptor and peripheral effects, such as the AR nuclear translocation (AR trafficking and its subnuclear distribution), the inhibition of 5α-reductase activity (it possesses higher activity compared to other available progestins), and the increase on SHBG levels if combined with an estrogenic counterpart (). This anti-androgenic activity is invaluable when we have to manage particular diseases such as PCOS or signs of hyperandrogenism such as acne, hirsutism and androgenic alopecia, as demonstrated by the use in clinical practice for the treatment of such conditions [Citation68]. It is no coincidence that the excellent receptor activity of this hormonal contraceptive translates into high acceptability of patients in real-life use, among the highest among the various CHCs available [Citation69].
This receptor activity, in particular the anti-androgenic and the anti-mineralocorticoid one, is extremely important also for the safety profile of NGM. This progestin is one of the molecules that best modulate the power of EE on the thromboembolic risk and that are associated with the lowest VTE risk between different CHCs. However, NGM has the advantage of keeping retaining peripheral anti-androgenic activity, demonstrated by the impact on lipid and glucose metabolism, and it should be preferred if compared with other similar progestins of the same class which are much more androgenic, such as LNG [Citation61] and NETA.
On the contrary, the most important limitation of this molecule is that it is still used in oral contraceptives only with synthetic estrogens such as EE, even at medium-high dosages (up to 35 µg). In the last years, the market of CHCs has been evolving toward a reduction of EE dose and more metabolically neutral estrogens such as E2 [Citation29] and estetrol (E4) [Citation70] and toward products that will be able to administer these weaker estrogens by non-oral ways of administration [Citation30]. NGM would probably not be suitable for use in these formulations that require progestins with a more uterotropic effect (such as DNG, nomegestrol acetate or nestorone) [Citation29], as NETA and DSG were not, mainly for the not satisfactory cycle control [Citation71,Citation72]. However, NGM’s main metabolite (NGMN), has been and will be an excellent component of transdermal patch CHC, in combination with EE [Citation45].
Despite this, we believe that contraceptive formulations containing NGM occupy and will maintain an important role in combined hormonal contraception, despite the fact that 30 years have already passed since the first release of this progestin onto the market.
We have to report some limitations to the performance of this review. The most important of them is its narrative design: despite including a systematic review process of the literature, this study tends to be mainly descriptive and thereby often focuses on a subset of studies in an area chosen based on availability or author selection. Thus, this narrative review, while informative, can often include an element of selection bias and it is likely to overstate its health benefits and to underestimate its negative effects. For these reasons, these conclusions should be taken with caution and confirmed in future investigations.
12. Expert opinion
During the whole reproductive lifespan, women need safe hormonal contraceptives, with the sole purpose of avoiding unintended pregnancies. The most important and relevant adverse effect, fortunately rare, that could happen during CHC use is the occurrence of VTE. This effect can naturally occur without the use of any CHC (2 women per 10,000 women/year) and even more during pregnancy and especially the puerperium period (up to 350 women per 10,000 women/year) [Citation73]. During modern CHC use, this risk can vary between 5 and 12 women per 10,000 women/year. Statements from the EMA include that ‘products that contain LNG, NETA and NGM are associated with the lowest risk of VTE. The decision to use any other product than one known to have the lowest risk should be taken only after a discussion with the woman to ensure she understands the risk of VTE with CHCs, how her current risk factors influence this risk, and that her VTE risk is highest in the first ever year of use’. Although these recommendations are not always followed in clinical practice by physicians [Citation74] and may tend to limit the correct customization of hormonal contraceptives, they cannot be forgotten. For this reason, there are molecules such as LNG and NGM (NETA is no longer readily available), which will always play an important role in hormonal contraception technology. Furthermore, NGM has undeniable advantages over LNG, which we have summarized in this review, the first of them being of a generation beyond (third vs. second). LNG is a much more androgenic molecule, presenting no anti-mineralocorticoid activity, and these effects, from a therapeutic point of view, can only overshadow it. The pros of EE/NGM combinations (both mono and triphasic) include the satisfactory use in the woman with water retention and bloating or with premenstrual syndrome or in the woman with signs of hyperandrogenism, such as hirsutism and acne, or PCOS, for which they could be proposed as first choices between different CHCs. All these features are associated with very high contraceptive efficacy and excellent cycle control, which are fundamental requirements for a treatment that, besides being safe, must make the woman who uses it feel good.
There will never be a ‘pill’ for all women and neither is this the case with this combination; having many products allows us to customize the best treatment for a specific woman, always keeping in mind what other therapeutic effects we want to obtain from this wonderful tool that, almost 60 years after its introduction, has gone very far beyond family planning.
Article highlights
Norgestimate shows a mild estrogenic activity associated with anti-mineralocorticoid and anti-androgenic properties, largely responsible for the cardiovascular safety profile.
The anti-mineralocorticoid activity of norgestimate is clinically important, reducing the classical side effects of CHCs such as bloating and water retention and decreasing the risk of hypertension during their use.
The anti-androgenic activity of norgestimate is complex, depending on the androgen receptor trafficking and its subnuclear distribution, the peripheral inhibition of 5α-reductase activity and the important increase of SHBG levels.
Norgestimate is one of the progestins that best modulate the power of ethinyl-estradiol on the thromboembolic risk, being associated with the lowest venous thromboembolism risk between different CHCs.
Norgestimate, having the advantage of keeping retaining peripheral anti-androgenic activity, should be preferred if compared with other similar progestins of the same class of risk which are much more androgenic, such as levonorgestrel.
Declaration of interest
G. Grandi received honoraria for sponsored lectures and participation in advisory boards from Bayer AG, Teva/Theramex, Sandoz Novartis, Exeltis Italy, Merck Sharp & Dohme and Italfarmaco/Effik Italy. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Abbreviations
| APC: | = | Activated Protein C |
| AR: | = | Androgen Receptor |
| BTB: | = | Breakthrough Bleeding |
| CHC: | = | Combined Hormonal Contraceptive |
| COC: | = | Combined Oral Contraceptive |
| CPA: | = | Cyproterone Acetate |
| DHT: | = | 5α-Dihydrotestosterone |
| DNG: | = | Dienogest |
| DRSP: | = | Drospirenone |
| DSG: | = | Desogestrel |
| E2: | = | Estradiol |
| E4: | = | Estetrol |
| EE: | = | Ethinyl-Estradiol |
| ER: | = | Estrogen Receptor |
| GR: | = | Glucocorticoid Receptor |
| GSD: | = | Gestodene |
| LNG: | = | Levonorgestrel |
| MR: | = | Mineralocorticoid Receptor |
| NETA: | = | Norethisterone Acetate |
| NGL: | = | Norgestrel |
| NGM: | = | Norgestimate |
| NGMN: | = | Norelgestromin |
| P: | = | Progesterone |
| PCOS: | = | Polycystic Ovary Syndrome |
| PI: | = | Pearl Index |
| PR: | = | Progesterone Receptor |
| SHBG: | = | Sex-Hormone-Binding Globulin |
| VTE: | = | Venous Throboembolism |
Additional information
Funding
References
- Del Savio MC, De Fata R, Facchinetti F, et al. Drospirenone 4 mg-only pill (DOP) in 24+4 regimen: a new option for oral contraception. Expert Rev Clin Pharmacol. 2020;13(7): 685–69. DOI:10.1080/17512433.2020.1783247.
- Schindler AE, Campagnoli C, Druckmann R, et al., Classification and pharmacology of progestins. Maturitas. 2003;46(1): S7–S16.
- Grandi G, Mueller MD, Papadia A, et al. Inflammation influences steroid hormone receptors targeted by progestins in endometrial stromal cells from women with endometriosis. J Reprod Immunol. 2016;117:30–38.
- Kuhl H. Comparative pharmacology of newer progestogens. Drugs. 1996;51(2):188–215.
- Grandi G, Cagnacci A, Volpe A. Pharmacokinetic evaluation of desogestrel as a female contraceptive. Expert Opin Drug Metab Toxicol. 2014;10(1):1–10.
- Henzl MR. Norgestimate. J Reprod Med. 2001;46:648–661.
- Back DJ, Madden S, Orme MLE. Gastrointestinal metabolism of contraceptive steroids. AL J Obstetric Gynecol. 1990;163(6):2138–2145.
- Corson SL. Norgestimate. Clin Obstet Gynecol. 1995;38(4):841–848.
- Phillips A. The selectivity of a new progestin. Acta Obstet Gynecol Scand Suppl. 1990;152(s152):21–24.
- Phillips A, Hahn DW, Klimek S, et al. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Paris F, Balaguer P, Rimbault F, et al., Molecular action of norgestimate: new developments. Gynecol Endocrinol. 2015;31(6): 487–490.
- Phillips A, Hahn DW, McGuire JL. Preclinical evaluation of norgestimate, a progestin with minimal androgenic activity. Am J Obstet Gynecol. 1992;167(4):1191–1196.
- Phillips A, Demarest K, Hahn DW, et al. Progestational and androgenic receptor binding affinities and in vivo activities of norgestimate and other progestins. Contraception. 1990;41(4):399–410.
- Anderson FD. Selectivity and minimal androgenicity of norgestimate in monophasic and triphasic oral contraceptives. Acta Obstet Gynecol Scand. 1992;71(156):15–21.
- Paris F, Rabeolina F, Bacquet A, et al., Antiandrogenic activity of norgestimate in a human androgen-dependent stable-trasfected cell line. Gynecological Endocrinol. 2007;23(4): 193–197.
- Gobinet J, Paujol N, Sultan C. Molecular action of androgens. Mol Cell Endocrinol. 2002;198(1–2):15–24.
- Rittmaster RS. Clinical relevance of testosterone and dihydrotestosterone metabolism in women. Am J Med. 1995;98(1):17–21.
- Vermorken AJ, Goos JM, Roelofs HM. A method for the evaluation of the local antiandrogenic action of 5 alpha-reductase inhibitors on human skin. Br J Dermatol. 1980;102(6):695–701.
- Anclersson S, Russell DW. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc Nut1 Acad Sci USA. 1990;87(10):3640–3644.
- Aumuller G, Eicheler W, Renneberg H, et al. Immunocytochemical evidence for differential subcellular localization of 5α-reductase isoenzymes in human tissues. Acta Anat. 1996;156(4):241–252.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5α-reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14(4):223–230.
- Lete I, Chabbert-Buffet N, Jamin C, et al. Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: a literature review. Eur J Contracept Reprod HealthCare. 2015;20(5):329–343.
- Odlind V, Milsom I, Persson I, et al. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet Gynecol Scand. 2002;81(6):482–490.
- Phillips A, Hahn DW, McGuire JL. Relative binding affinity of norgestimate and other progestins for human sex hormone-binding globulin. Steroid. 1990;55(8):373–375.
- Rott H. Contraception, venous thrombosis and biological plausibility. Minerva Med. 2013;104(2):161–167.
- Sitruk-Ware RL, Menard J, Rad M, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92(6):2074–2079.
- Wiegratz I, Lee JH, Kutschera E, et al. Effect of four oral contraceptives on hemostatic parameters. Contraception. 2004;70(2):97–106.
- Kluft C, Endrikat J, Mulder SM, et al. A prospective study on the effects on hemostasis of two oral contraceptives containing drospirenone in combination with either 30 or 20 microg ethinyl estradiol and a reference containing desogestrel and 30 microg ethinyl estradiol. Contraception. 2006;73(4):336–343.
- Grandi G, Facchinetti F, Bitzer J. Estradiol in hormonal contraception: real evolution or just same old wine in a new bottle? Eur J Contracept Reprod Health Care. 2017;22(4):245–246.
- Grandi G, Barra F, Ferrero S, et al., Estradiol in non-oral hormonal contraception: a “long and winding road”. Expert Rev Endocrinol Metab. 2019;14(3): 153–155.
- Isidori AM, Minnetti M, Sbardella E, et al. Mechanisms in endocrinology: the spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol. 2015;173(3):R101–3.ù.
- Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–682.
- Herkert O, Kuhl H, Sandow J, et al. Sex steroids used in hormonal treatment increase vascular procoagulant activity by inducing thrombin receptor (PAR-1) expression: role of the glucocorticoid receptor. Circulation. 2001;104(23):2826–2831.
- Gromotowicz-Poplawska A, Stankiewicz A, Kramkowski K, et al. The acute prothrombotic effect of aldosterone in rats is partially mediated via angiotensin II receptor type 1. Thromb Res. 2016;138:114–120.
- Sawathiparnich P, Kumar S, Vaughan DE, et al. Spironolactone abolishes the relationship between aldosterone and plasminogen activator inhibitor-1 in humans. J Clin Endocrinol Metab. 2002;87(2):448–452.
- Cagnacci A. Hormonal contraception: venous and arterial disease. Eur J Contracept Reprod Health Care. 2017;22(3):191–199.
- Norris LA, Bonnar J. Haemostatic changes and the oral contraceptive pill. Baillieres Clin Obstet Gynaecol. 1997;11(3):545–563.
- Oral Contraceptive and Hemostasis Study Group. The effects of seven monophasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomized multicentre study. Contraception. 2003;67(3):173–185.
- Darney PD. The androgenicity of progestins. Am J Med. 1995;98(1):104–110.
- Deckers GH, Schoonen WG, Kloosterboer HJ. Influence of the substitution of 11-methylene, delta(15), and/or 18-methyl groups in norethisterone on receptor binding, transactivation assays and biological activities in animals. J Steroid Biochem Mol Biol. 2000;74(3):83–92.
- Hugon-Rodin J, Gompel A, Plu-Bureau G. Epidemiology of hormonal contraceptives-related venous thromboembolism. Eur J Endocrinol. 2014;171(6):R221–30.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Lidegaard Ø, Nielsen LH, Wessel Skovlund C, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: danish cohort study, 2001–9. BMJ. 2011;343:d6423.
- European Medicines Agency, Benefits of combined hormonal contraceptives (CHCs) continue to outweigh risks, 16 January 2014, available at https://www.ema.europa.eu/en/documents/referral/benefits-combined-hormonal-contraceptives-chcs-continue-outweigh-risks_en.pdf. last accessed 2020 Aug 5th
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72(3):168–174.
- Runnebaum B, Grunwald K, Rabe T. The efficacy and tolerability of norgestimate/ethinyl estradiol (250 μg of norgestimate/35 μg of ethinyl estradiol): results of an open, multicenter study of 59,701 women. Am J Obstet Gynecol. 1992;166(6):1693–1698.
- Huber J. Clinical experience with a new norgestimate-containing oral contraceptive. Int J Fertil. 1992;37 Suppl 1(suppl):47–53.
- Becker H. Supportive European data on a new oral contraceptive containing norgestimate. Acta Obstet Gynecol Scand. 1990;152(s152):33–39.
- Gauthier A, Upmalis D, Dain M-P. Clinical evaluation of a new triphasic oral contraceptive: norgestimate and ethinyl estradiol. Acta Obstet Gynecol Scand. 1992;156(s156):27–32.
- Corson SL. Efficacy and clinical profile of a new oral contraceptive containing norgestimate. U S Clinial Trials Acta Obstet Gynecol Scand. 1990;152(s152):25–31.
- London RS, Chapdelaine A, Upmalis D, et al. Comparative contraceptive efficacy and mechanism of action of the norgestimate-containing triphasic oral contraceptive. Acta Obstet Gynecol Scand. 1992;156(s156):9–14.
- Fotherby K. Oral contraceptives and lipids. BMJ. 1989;298(6680):1049–1050.
- Grandi G, Piacenti I, Volpe A, et al. Modification of body composition and metabolism during oral contraceptives containing non-androgenic progestins in association with estradiol or ethinyl estradiol. Gynecol Endocrinol. 2014;30(9):676–680.
- Chapdelaine A, Desmarais J-L, Derman RJ. Clinical evidence of minimal androgenic activity of norgestimate. Int J Fertil. 1989;34(5):347–352.
- Wiegratz I, Jung-Hoffmann C, Gross W, et al. Effect of two oral contraceptives containing ethinyl estradiol and gestodene or norgestimate on different lipid and lipoprotein parameters. Contraception. 1998;58(2):83–91. Aug.
- Crook D, Godsland I. Safety evaluation of modern oral contraceptives. Effects on Lipoprotein and Carbohydrate Metabolism Contraception. 1998;57:189–201.
- Oelkers W, Foidart JM, Dombrovicz N, et al. Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clin Endocrinol Metab. 1995;80(6):1816–1821.
- Andolsek KM. Cycle control with triphasic norgestimate and ethinyl estradiol, new oral contraceptive agent. Acta Obstet Gynecol Scnd. 1992;156(s156):22–26.
- Tantbirojn P, Taneepanichskul S. Clinical comparative study of oral contraceptives containing 30 μg ethinylestradiol/150 μg levonorgestrel, and 35 μg ethinylestradiol/250 μg norgestimate in Thai women. Contraception. 2002;66(6):401–405.
- Affinito P, Monterubbianesi M, Primizia M, et al. Efficacy, cycle control and side-effects of two monophasic combination oral contraceptives: gestodene/ethinyl estradiol and norgestimate/ethynilestradiol. Gynecol Endocrinol. 1993;7(4):259–266.
- Janaud A, Rouffy J, Upmalis D, et al. A comparison study of lipid and androgen metabolism with triphasic oral contraceptive formulations containing norgestimate or levonorgestrel. Acta Obstet Gynecol Scand. 1992;156(s156):33–38.
- Grandi G, Napolitano A, Cagnacci A. Metabolic impact of combined hormonal contraceptives containing estradiol. Expert Opin Drug Metab Toxicol. 2016;12(7):779–787.
- Grandi G, Xholli A, Napolitano A, et al. Prospective measurement of blood pressure and heart rate over 24 h in women using combined oral contraceptives with estradiol. Contraception. 2014;90(5):529–534.
- Corson SL. Efficay and safety of a monophasic and triphasic oral contraceptive containing norgestimate. Am J Obstet Gynecol. 1994;170(5):1556–1561.
- Jaisamrarn U, Chaovisitraree S, Angsuwathana A, et al. A comparison of multiphasic oral contraceptives containing norgestimate or desogestrel in acne treatment: a randomized trial. Contraception. 2014;90(5):535–541.
- Paterni I, Granchi C, Katzenellenbogen JA, et al. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29.
- Nyberg S. Mood and physical symptoms improve in women with severe cyclical changes by taking an oral contraceptive containing 250-mcg norgestimate and 35-mcg ethinyl estradiol. Contraception. 2013;87(6):773–781.
- Cibula D, Sindelka G, Hill M, et al., Insulin sensitivity in non-obese women with polycystic ovary syndrome during treatment with oral contraceptives containing low-androgenic progestin. Hum Reprod. 2002;17(1): 76–82.
- Aikins Murphy P, Brixner D. Hormonal contraceptive discontinuation patterns according to formulation: investigation of associations in an administrative claims database. Contraception. 2008;77(4):257–263.
- Grandi G, Del Savio MC. Lopes da Silva-Filho A, et al. Estetrol (E4): the new estrogenic component of combined oral contraceptives. Expert Rev Clin Pharmacol. 2020;13(4):327–330.
- Wenzl R, Bennink HC, van Beek A, et al. Ovulation inhibition with a combined oral contraceptive containing 1 mg micronized 17 beta-estradiol. Fertil Steril. 1993;60(4):616–619.
- Astedt B, Jeppsson S, Liedholm P, et al. Clinical trial of a new oral contraceptive pill containing the natural oestrogen 17 beta-oestradiol. Br J Obstet Gynaecol. 1979;86(9):732–736.
- Jackson E. Controversies in postpartum contraception: when is it safe to start oral contraceptives after childbirth? Thromb Res. 2011;12(Suppl 3):S35–9.
- Lindh I, Skjeldestad FE, Gemzell-Danielsson K, et al. Contraceptive use in the Nordic countries. Acta Obstet Gynecol Scand. 2017;96(1):19–28.