?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
Despite therapeutic vancomycin is regularly monitored, its dose requirements vary considerably between individuals. Various innovative vancomycin dosing strategies have been developed for dose optimization; however, the utilization of individual factors and extensibility is insufficient. We aimed to develop an optimal dosing algorithm for vancomycin based on the high-dimensional data using the proposed variable engineering and machine-learning methods.
Methods
This study proposed a variable engineering process that automatically generates second-order variable interactions. We performed an initial examination of independent variables and interactive variables using eXtreme Gradient Boosting. The vancomycin dose prediction model was established based on the derived variables.
Results
Based on the evaluation of the model performance in the validation cohort, our algorithm accounted for 67.5% of variations in the vancomycin doses. Subgroup analysis showed better performance in patients with medium and high body weight (with the ideal predictive percentage of 72.7% and 73.7%), and low and medium levels of serum creatinine (with the ideal predictive percentage of 77.8% and 73.1%) than in other groups.
Conclusion
The new vancomycin dose prediction model is potentially useful for patients whose population profiles are similar to those of our patients and yielded desired reference of clinical indicators with specific breakpoints.
1. Introduction
Vancomycin has remained a therapeutic mainstay in treating serious infections caused by gram-positive bacteria, especially the methicillin-resistant Staphylococcus aureus (MRSA) [Citation1-4]. As one of the most frequent pathogens, MRSA infections increased by 1.28 times between 2007 and 2015 [Citation5]. This microorganism leads to a diversity of human diseases, ranging from skin and soft tissue infections to invasive and life-threatening infections, such as pneumonia, bacteremia, osteomyelitis, meningitis, endocarditis, and sepsis [Citation6]. The optimal serum trough concentrations for vancomycin should be maintained over 10 mg/L and 15–20 mg/L for serious infections [Citation1,Citation2]. Unfortunately, recent evidence proposes that vancomycin dosing in clinic fails to reach target troughs in more than 50% of patients [Citation7].
Due to the complex pharmacokinetic and pharmacodynamic (PK/PD) situations, the optimal dosing strategy for vancomycin remains undefined. In most contemporary vancomycin dosing schemes, simple formulas with limited parameters [i.e. patients’ weight, age, serum creatinine (SCr), and gender] were used in estimations of area under the concentration–time curve (AUC) value [Citation8–10]. However, in clinical practice, there are considerable inter-individual variability in vancomycin responses, and it is difficult to calculate the exposure in a consolidated formula alone. Evidence from clinical trials suggests biochemical markers, such as white blood cells (WBC) and serum C-Reactive protein (CRP) in serum, changing dramatically when patients are exposed to infections [Citation11–14]. Besides, co-administrated medications are commonly delivered in invasive infections caused by MRSA strains, and some regimens are known to have potential toxicities [Citation1,Citation15]. These differences in vancomycin dose requirements caused by these available clinical factors have not been well analyzed and utilized. In this study, we tracked those factors in electronic health records which contain an individual’s integrated and comprehensive clinical history and evaluated their contribution to the appropriate routine therapeutic dose.
To deal with the generated high-dimensional and multivariate datasets, our study adopted a powerful machine learning algorithm – eXtreme Gradient Boosting (XGBoost) for feature selection and model construction [Citation16]. Given that the versatility of datasets provides more generalized prediction results with high levels of confidence, and unique advantages and greater analytic flexibility of machine learning, the clinical construction of dose–response prediction models based on has become more effective and is in higher demand [Citation17,Citation18]. Therefore, our study aimed to construct and validate a new vancomycin routine therapeutic dose prediction model that is able to utilize an individual’s clinical information to capture previously ignored but potentially powerful predictors. It is hoped that this new approach could ultimately predict the optimal vancomycin dose with improved accuracy.
2. Methods
2.1. Study Population
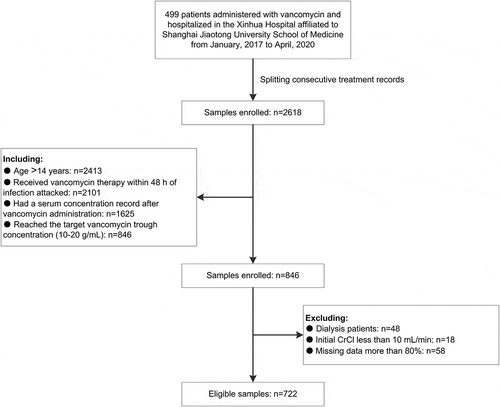
This study was conducted at Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine. The protocol of this study has been approved by the Ethics Committee of Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine, and in the ethical approval documents, the informed consent has been exempted. A total of 499 patients (January 2017 to April 2020) with MRSA and other gram-positive bacterial infections were routinely administered with vancomycin using the hospital-approved dosage strategy, the indications of which included but were not limited to meningitis, bacteremia, pneumonia, endocarditis, and sepsis. The electronical medication records were reviewed by clinical pharmacists to determine the dose, infusion frequency, and any other clinical information relating to vancomycin therapy. All the samples for the study were compliant with the criteria below, and a flow diagram is shown in :
The inclusion criteria: (1) Patients older than 14 years; (2) received vancomycin therapy within 48 h of infection attacked; (3) monitored the therapeutic vancomycin serum concentration after vancomycin administration; and (4) vancomycin serum trough concentrations in the range of 10–20 g/mL were recorded.
The exclusion criteria: (1) Patients undergoing dialysis; (2) with initial creatinine clearance (CrCl) less than 10 mL/min (as estimated by the Cockcroft-Gault equation); and (3) data were missing more than 80%.
2.2. Study Design
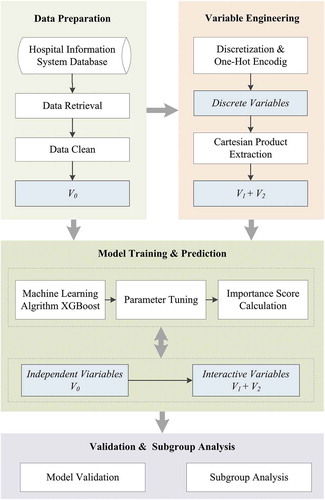
This work was a single-center and retrospective cohort study. Our experiment followed the pipeline illustrated in , which consisted of four major steps: data preparation, variable engineering, model training and prediction, and validation and subgroup analysis. The model was designed to predict vancomycin routine therapeutic dose by estimating the available indicators in clinical practice. In our patient cohort, pediatric patients (age<14 years) were excluded because of their diverse PK parameter values [Citation2]. The predictive outcome of the applied models was defined as the actual dose of vancomycin which was monitored serum trough level in the range of 10 to 20 g/mL in patients. Each patient may have multiple trough concentration records fell in target level, the observation window for clinical indicators was defined at the recent time of determining vancomycin trough concentration. This serum trough value in this study was defined as the therapeutic monitoring result of vancomycin serum trough concentration between the two consecutive vancomycin administrations.
2.3. Candidate Predictors
As the predictors for the initial machine learning models, we included available information at hospitalization and critical care settings– for instance, patient demographic information, vancomycin medical record, combination, and laboratory result. A total of 29 variables were included for candidate predictors (). Among the variables, the overall rate of missing data was 3.18% in the entire cohort. To deal with missing values, we discretized the continuous variables to low (L), medium (M), and high (H) levels, based on the normal reference interval, tri-sectional quantiles of interquartile range (IQR), or prior knowledge, which are demonstrated in the Supplementary . The discrete variables will be assembled by the variable engineering and used as predictors to participate in the model training process.
Table 1. The description of the study samples
2.4. Prediction Model Using XGBoost
Assembling algorithms combine multiple individually weak prediction models to produce a robust predictive estimator. This combination could be achieved in tree boosting. The adopted XGBoost was designed to be a scalable machine learning framework for tree boosting, that makes a prediction based on a tree-like structure [Citation16]. The XGBoost learn the values of input features by a series of split points and nodes between the trees and generates new decision tree in a gradient boosting manner to fit the residual predicted by the previous one and better predict the objective. More importantly, XGBoost algorithm can learn from incomplete data automatically by adding a default direction for the missing values in each tree node, that missing values are viewed as a situation when generating the decision rules. In present experiment, the XGBoost model was trained on the derivation cohort using all candidate predictors to predict vancomycin dose and tuned using hyperparameters (Supplementary ). The objective function is as follows:
Table 2. Variables selected via XGBoost and the corresponding variable importance score
where denotes the kth tree,
and
represent the predicted and true values, respectively. The regularization
describes the tree complexity, in which
and
are two parameters. In terms of different choices of the base function
, XGBoost can not only achieve the classification tasks, but also complete the regression tasks. We performed the XGBoost algorithm using Python [https://www.python.org/] and the scikit-learn framework [https://www.scikit-learn.org/stable/]. To achieve their best performance, AutoML [https://github.com/ClimbsRocks/auto_ml] method was adopted to select the algorithm parameters automatically.
XGBoost provides the importance score of each variable, which was computed by the Gini coefficient among the corresponding splits within all trees. To interpret individual variable impacts on the model better, we used the Shapley Additive exPlanations (SHAP) method, which is a visualized approach to explain the output of a machine learning model [Citation19]. In brief, SHAP is an additive variable attribution method that provides a transformation of the ensemble of decision trees’ summing impact in the form of particular feature attributions and is relatively easily interpreted.
2.5. Variable Engineering
The proposed variable engineering process was based on variable discretization to explore the second-order variable interactions, such as patient with lower hematokrit (HCT), and higher SCr, to introduce the new variables for prediction modeling, that represent the complex variable relations and further enhance the model performance. We only included the positive responses and missing value was imputed by zero, representing patients without these variables. Variabletools [https://www.variabletools.com/], an open source Python [https://www.python.org/] framework for automated variable engineering, was adopted after to discover the potential variable interactions automatically.
2.6. Statistical analysis
Baseline data were described as frequencies (percentages) for categorical variables and as median (IQR) based on the test of normality for continuous variables. Kolmogorov-Smirnov test was applied to test the normality. It is important to note that the vancomycin order events containing missing values were excluded in the process of the statistical test of some variables.
2.7. Model validation
In order to evaluate the model predictive performance, we use the metrics of R–squared (R2), Mean Square Error (MSE), Root Mean Square Error (RMSE), and Mean Absolute Error (MAE) in the validation cohort to evaluate the models. R2 indicates the explanation degree of the independent variable to the dependent variable. When R2 is closer to 1, the reference value of the prediction model is higher; conversely, the closer to 0, the lower the reference value. In order to test the clinical utility of our method, we computed the under, ideal, and over prediction percentages. Ideal was defined as a predicted dose falling within ± 20% of the actual dose. Under and over were, respectively, defined as a predicted dose being more than 20% lower and higher than the actual dose [Citation20].
3. Results
3.1. Study Population
A total of 184 consecutive samples of eligible patients treated with vancomycin were evaluated. The baseline characteristics description and the included variables can be found in as counts or medians and IQR. As demonstrated, overall, the median of routine therapeutic vancomycin dose was2000 mg/d. Loop diuretics (47.8%) and aminoglycosides (44.0%) were the most commonly used with vancomycin. Notably, we also collected laboratory results in period of treatment for analysis and modeling, which were obtained from laboratory tests conducted the day before the vancomycin trough concentration dropped by 10–20 mg/L. Moreover, the missing percentages of each variable in the data set are also shown. There was relatively low collection of weight from the results in this study, with the missing rate of 16.3%, which came from the critically patients with limited mobility. For categorical variables, only the positive responses were extracted to the data set, and the others were imputed by zero, that is no missing values existing.
3.2. Variable Importance and Prediction Model Construction
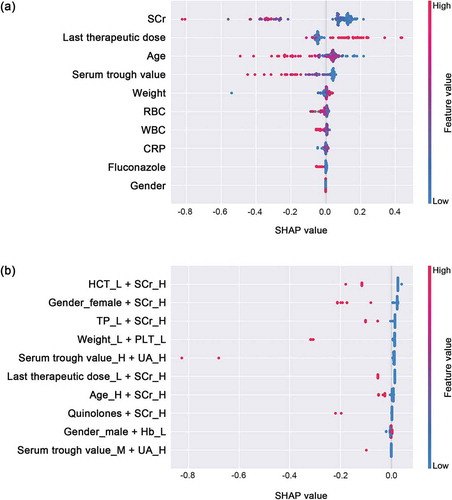
In order to determine the included variables, we first proposed a variable engineering process to randomly combined variables with specific levels to introduce the variable interactions for vancomycin dose prediction. We then performed XGBoost models on the dataset of the overall 29 variables and the generated interacting variables. To gain insights into the importance of each predictor, summarized the 10 most important independent and interacting predictors of models for the optimal vancomycin dose. SCr, latest vancomycin therapeutic dose and age are the uppermost predictors, and when the SCr level is high (>97 µmol/L for female; >106 µmol/L for male), the combined effect with other discretized variables is strongly correlated with the dose of vancomycin.
The final vancomycin dose prediction model was constructed on these derived variables. In the process of model building, all samples were randomly assigned to a derivation cohort for training the model and a validation cohort for evaluating the model according to a 7:3 ratio. Then, we tested the model on a variety of different variable combinations. The performance of models approximately tended to be stabilized after the size of variables combined with 10 single variables and 10 interactive variables, the increasing and decreasing number of variables did not improve the model performance.
To provide clinicians with a directly perceived understanding of feature contributions, the SHAP approach was used to interpret the XGBoost predicting results, and the nonlinear impact of the variables on the vancomycin therapeutic routine dose is illustrated in . Our results indicate that in independent variables, patients with higher previous therapeutic dose were associated with a higher therapeutic dose of vancomycin. On the contrary, our results suggest that smaller doses were appropriate when patients had higher SCr, older age, and higher last vancomycin trough concentration, that is, their values were negatively correlated with the therapeutic dose. In terms of the interactive variables, most of them that had greater influence on the model output were negatively correlated with vancomycin doses.
Figure 3. XGBoost model feature importance explained by SHAP values
3.3. Evaluation of the predictive model
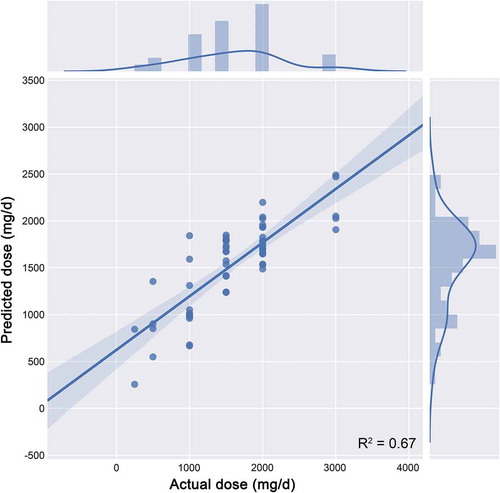
In terms of the overall predictive performance achieved as measured in the validation cohort, shown in , we found that the R2 of the model was 67.5%, which suggests that our methodology can well explain the inter-individual dose variation of vancomycin. Considering the prediction error, the precision is measured by the MAE, MSE, and the RMSE, respectively, 0.212, 0.097, and 0.310. shows the comparison of the predicted vancomycin dose with the observed dose in the validation cohort. The solid line indicates the ideal predictions, and the additional curve represents the distribution of values. As the results, the proposed XGBoost model for the dose prediction of vancomycin had the preferable performance.
Table 3. The prediction performance of the vancomycin dose prediction model
3.4. Clinical relevance
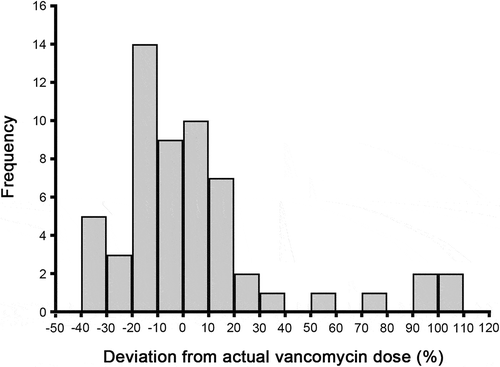
To analyze the clinical relevance, we also computed the percentage deviation of the XGBoost dosing model from actual vancomycin dose requirements (). Of 57 cases in the validation cohort, our performed algorithm showed 40 (70.2%) of ideal prediction. There were 5 (8.8%) overestimated cases by more than 50%. In the poorest predictions, there were four predicted doses that were out of range in 70% of actual dose, the corresponding actual vancomycin doses were quite low (mean = 375 mg/d). This limitation is expected, because the predicted results may have large deviations in the minimal samples.
We further tested the robustness of model results, the percentages of under, ideal, and over predictions for each subgroups in validation cohort are listed in . In view of derived results by XGBoost and their importance in the literatures, the selected variables were the target vancomycin through concentration, body weight, and SCr for comparison of accuracy in subgroups. For the patients with normal and higher weight, the ideal prediction proportion was 72.7% and 73.7%, respectively. As regards the SCr in the observation window, the ideal prediction were 77.8% and 73.1% in the lower group and medium group, respectively. Moreover, the vancomycin therapeutic trough was divided into two groups, and the predicted accuracy was basically stable within the range of 10–15 mg/L, 15–20 mg/L and the overall target trough (10–20 mg/L, ideal proportion = 70.2%).
Table 4. Characteristics of subgroups which predicted vancomycin doses were below, within, and above ideal range of actual doses
4. Discussion
Optimizing vancomycin dosing regimen with therapeutic drug monitoring is broadly recommended [Citation21,Citation22]. Using the mathematical methods enables integration of high-dimensional data and provides additional information for data mining [Citation23]. In this report, we developed a new variable selection method, combined discrete variables in electronic medical records randomly using a variable engineering tool to fit feature interactions in individuals. Based on a powerful machine learning algorithm-XGBoost, the independent and interactional variables were screened and illustrated to predict connections of appropriate vancomycin therapeutic dose. Furthermore, we validated an accurate model for vancomycin dose prediction, showing promising performance.
Consistent with other studies on vancomycin therapy, the major predictors in our model were supported by these results. It was proven that about 85% of the variability in vancomycin clearance can be explained by CrCl in adult patients with various degrees of impaired renal function [Citation24,Citation25, **Citation26]. The most widely used index of renal function was the Cockcroft–Gault equation, which used age, weight, SCr, and gender to estimate CrCl [Citation25]. Regarding this equation, multiple evidences have shown the correlation between the used parameters and vancomycin serum concentration [Citation8–10,Citation27,Citation28]. Furthermore, close monitoring of vancomycin serum concentrations is recommended to ensure that targets are met and maintained [Citation1]. In this study, all the above predictors were included in our proposed prediction model ( and ). Specifically, the interactive variables consisting of higher levels of SCr (>92/110 μmol/L for female/male, equal to>1.04/1.24 mg/dL for female/male) were mostly correlated with lower vancomycin therapeutic doses. It is comparable with the study of Pritchard et al., in which researchers suggested that the baseline SCr ≥1.7 mg/dL as independent predictors of vancomycin-related nephrotoxicity [Citation29]. With increasing reports of vancomycin-induced nephrotoxicity, evidences demonstrated the correlation between vancomycin dose, trough concentrations, and incidence of nephrotoxicity [Citation30–32].
Interestingly, as seen in this study, certain biochemical markers were inferred to be important in the established vancomycin dose prediction model, including UA, PLT, TP, CRP, WBC, RBC, HCT, and Hb. Among them, UA was considered as a marker of kidney disease [Citation33], and hyperuricemia was revealed as an independent risk factor for vancomycin-induced acute kidney injury [Citation34]. Thrombocytopenia can be caused by vancomycin-dependent antibodies produced by immune mechanisms [Citation35]. In terms of serum TP, Butterfield et al. found that TP ≥6.7 g/dL was a predictor to estimate unbound vancomycin that was active and available for clearance [Citation36]. It is comparable with the correlation between lower level of TP (≤6.0 g/dL) with high SCr and higher dose of vancomycin in our report. Leukocyte and CRP, as the markers of infection and inflammation, recent researches have reported the immunization pathway that CRP leads to enhanced leukocytes recruitment [Citation11,Citation13]. Periodic monitoring of the WBC during long-term vancomycin treatment was recommended by Mackett et al. [Citation12], whereas, vancomycin-related neutrophil changes were not derived as a vancomycin dose-dependent factor in our model. Moreover, the indicators relating to erythrocytes were necessary in our estimation of vancomycin dose, but rarely relevant studies have demonstrated the association. This can be attributed to the learning pattern of machine learning. The variables selection of XGBoost were based on the better prediction performance, which may not totally fit the clinical cognition with the limited data source.
Quinolones and fluconazole are widely used antibacterial agents in treatment against infection clinically [Citation37,Citation38]. Credito et al. has reported there was vitro synergy in combination of a new des-F(6)-quinolone DX–619 and vancomycin. In renal dysfunction, dose adjustment for fluconazole was recommended in fungal infections [Citation39–41]. Wang et al. demonstrated fluconazole might be potential risk factors for vancomycin nephrotoxicity [Citation42]. Therefore, it can be speculated that quinolones and fluconazole may have potential effects on the dose–response of vancomycin. Although concern has been expressed about the raised nephrotoxicity risk when vancomycin and aminoglycoside are used concurrently, it is not shown the influence with vancomycin dose in our results [Citation43]. However, the effect of these combinations on the dose of vancomycin has not been reported and may be worth further study. Loop diuretics, immunosuppressors, and glycopeptides, as the result, they have little influence overestimation of vancomycin doses.
Currently, various innovative vancomycin dosing strategies based on PK/PD have been developed for dose optimization and individualization. Two major methods of dose individualization used to target vancomycin-specific pharmacokinetic parameters are nomograms methods and Bayesian estimation procedures [Citation44–50]. Available data show that both approaches provide considerable improvements over the conventional dosing methods [Citation9,Citation10]. However, there exist limits that all nomograms assume the PK parameters are stable, such as the rate of renal clearance of vancomycin, which may not occur in a critically ill patient [Citation9]. The Bayesian programs offer the advantage that they adopt more priori and posteriori information in the population model to provide AUC–guided dosing recommendations at the bedside [Citation10]. In fact, the Bayesian forecasting approaches for dosage individualization relied on the prior general population PK information and the limited clinical covariates [Citation51]. In this work, we used the available clinical data to search for potential predictors associated with vancomycin dose through a powerful machine learning method, XGBoost, and proposed a vancomycin dosing model. It has been shown in previous studies that machine learning can improve the predictive ability of various drug delivery models (e.g. warfarin, tacrolimus) [Citation52–54].
The superiorities for the powerful predictive abilities in the machine learning models are multiple. The machine-learning methods are adept at dealing with high-dimensional coactions and non-linear relationships between the predictors with the objective [Citation55,Citation56]. In the age of health information technology, machine learning-based prediction has the advantage of scalability, for example, the prediction model is updated by automatically extracting electronic health records and continuously monitoring physiological data [Citation57,Citation58]. Taken together, our results suggest that the XGBoost can illustrate the variables importance and establish a dose prediction model with high accuracy. This method can be combined with the existing computerized medical and healthcare information system, and it is expected to be applied in practical applications. While external validations are necessary, the current study lends support to the application of machine learning-based predication to vancomycin dosing as a decision support technology.
5. Conclusion
Based on the analysis of adult vancomycin therapy visit data, we developed the dose prediction model. The model yielded a promising performance in predicting routine therapeutic dose of vancomycin. Furthermore, the model increased the reference of clinical indicators with specific breakpoints that yielded a greater net benefit across wide ranges of threshold probabilities. Machine learning methods-as assistive technologies offered new avenues for improving the vancomycin therapy decision-making, which will optimize resource utilization in clinical practice.
Article highlights
• We developed a dose prediction model for vancomycin based on the high-dimensional data using the proposed variable engineering and machine learning methods.
• The application of machine learning methods offered new avenues for improving the vancomycin therapy decision-making, which will optimize resource utilization in clinical practice.
• The model yielded a promising performance in predicting routine therapeutic dose of vancomycin.
• The model increased the reference of clinical indicators with specific breakpoints that yielded a greater net benefit across wide ranges of threshold probabilities.
• XGBoost was used to screen the independent and interactional variables and predict connections of appropriate vancomycin therapeutic dose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability
The data and code are available on https://github.com/yp-Leo/Vancomycin.
Additional information
Funding
References
- Rybak M, Lomaestro B, Rotschafer JC, et al., Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 66(1): 82–98. 2009. .
- Rybak MJ, Le J, Lodise TP, et al., Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 77(11): 835–864. 2020. .
- Gyssens IC. Kucers’ the Use of Antibiotics. London: Hodder Arnold; 2010.
- Health Communication Network. Australian Medicines Handbook. Adelaide, SA: Australian Medicines Handbook; 2012.
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. .
- Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. .
- Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–629.
- Álvarez R, López Cortés LE, Molina J, et al. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother. 2016;60(5):2601–2609.
- Elyasi S, Khalili H. Vancomycin dosing nomograms targeting high serum trough levels in different populations: pros and cons. Eur J Clin Pharmacol. 2016;72(7):777–788.
- Pai MP, Neely M, Rodvold KA, et al. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–57.
- Braig D, Nero TL, Koch HG, et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. 2017;8(1):14188. .
- Mackett RL, Guay DR. Vancomycin-induced neutropenia. Can Med Assoc J. 1985;132(1):39–40.
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
- Chantziara V, Georgiou S, Tsimogianni A, et al. The predictive role of C-reactive protein and procalcitonin biomarkers in central nervous system infections with extensively drug resistant bacteria. Crit Care. 2016;20(Suppl 2): P013.
- Deresinski S. Vancomycin in Combination with Other Antibiotics for the Treatment of Serious Methicillin-Resistant Staphylococcus aureus Infections. Clin Infect Dis. 2009;49(7):1072–1079.
- Chen T, Guestrin C XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, CA, ACM, 2016:785–794.
- Denny JC, Van Driest SL, Wei WQ, et al. The influence of big (clinical) data and genomics on precision medicine and drug development. Clin Pharmacol Ther. 2018;103(3):409–418.
- Linden A, Yarnold PR, Nallamothu BK. Using machine learning to model dose-response relationships. J Eval Clin Pract. 2016;22(6):856–863.
- Lundberg SM, Lee SI. A unified approach to interpreting model predictions. In: Advances in Neural Information Processing Systems. Long Beach, CA: Neural Information Processing Systems; 2017. p. 4765–4774.
- Shin J, Cao D. Comparison of warfarin pharmacogenetic dosing algorithms in a racially diverse large cohort. Pharmacogenomics. 2011;12(1):125–134.
- Colin PJ, Allegaert K, Thomson AH, et al. Vancomycin pharmacokinetics throughout life: results from a pooled population analysis and evaluation of current dosing recommendations. Clin Pharmacokinet. 2019;58(6):767–780.
- De Velde F, Mouton JW, De Winter BCM, et al. Clinical applications of population pharmacokinetic models of antibiotics: challenges and perspectives. Pharmacol Res. 2018;134:280–288.
- Batzel JJ, Hinghofer-Szalkay H, Kappel F, et al. Bridging different perspectives of the physiological and mathematical disciplines. Adv Physiol Educ. 2012;36(4):265–274.
- Moellering RC Jr, Krogstad DJ, Greenblatt DJ. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med. 1981;94(3):343–346.
- Roberts JA, Taccone FS, Udy AA, et al. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother. 2011;55(6):2704–2709.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
- Hall RG 2nd, Payne KD, Bain AM, et al. Multicenter evaluation of vancomycin dosing: emphasis on obesity. Am J Med. 2008;121(6):515–518.
- Young T, Daniel M, Baumhover S, et al. Methodological study of vancomycin dosing in elderly patients using actual serum creatinine versus rounded serum creatinine. Drugs R D. 2017;17(3):435–440.
- Pritchard L, Baker C, Leggett J, et al. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med. 2010;123(12):1143–1149.
- Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144.
- Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–1336.
- Jeffres MN, Isakow W, Doherty JA, et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. ClinTher. 2007;29(6):1107–1115.
- Giordano C, Karasik O, King-Morris K, et al. Uric acid as a marker of kidney disease: review of the current literature. Dis Markers. 2015;2015:382918.
- Pan KM, Wu Y, Chen C, et al. Vancomycin-induced acute kidney injury in elderly Chinese patients: a single-centre cross-sectional study. Br J Clin Pharmacol. 2018;84(8):1706–1718.
- Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356(9):904–910.
- Butterfield JM, Patel N, Pai MP, et al. Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob Agents Chemother. 2011;55(9):4277–4282.
- Bhagwat SS, Nandanwar M, Kansagara A, et al. <p>Levonadifloxacin, a Novel Broad-Spectrum Anti-MRSA Benzoquinolizine Quinolone Agent: review of Current Evidence. Drug Des Devel Ther. 2019;13:4351–4365.
- Goa KL, Barradell LB, Fluconazole. An update of its pharmacodynamic and pharmacokinetic properties and therapeutic use in major superficial and systemic mycoses in immunocompromised patients. Drugs. 1995;50(4):658–690.
- Credito K, Lin G, Appelbaum PC. Antistaphylococcal activity of DX-619 alone and in combination with vancomycin, teicoplanin, and linezolid assessed by time-kill synergy testing. Antimicrob Agents Chemother. 2007;51(4):1508–1511.
- Debruyne D, Ryckelynck JP. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993;24(1):10–27.
- Garey KW, Pai MP, Suda KJ, et al. Inadequacy of fluconazole dosing in patients with candidemia based on Infectious Diseases Society of America (IDSA) guidelines. Pharmacoepidemiol Drug Saf. 2007;16(8):919–927.
- Li L, Li X, Xia Y, et al. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol. 2020;11:786.
- Timpe EM. Nephrotoxicity with combination vancomycin-aminoglycoside therapy. J Pediatr Pharmacol Ther. 2005;10(3):174–182.
- Avent ML, Vaska VL, Rogers BA, et al. Vancomycin therapeutics and monitoring: a contemporary approach. Intern Med J. 2013;43(2):110‐9.
- Baptista JP, Roberts JA, Sousa E, et al. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care. 2014;18(6):654.
- Imai S, Yamada T, Ishiguro N, et al. Validating the effectiveness of switching the vancomycin TDM analysis software based on the predictive accuracy. Yakugaku Zasshi. 2017;137(9):1185‐92.
- Kullar R, Leonard SN, Davis SL, et al. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15-20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy. 2011;31(5):441–448. .
- Bayard DS, Jelliffe RW. A Bayesian approach to tracking patients having changing pharmacokinetic parameters. J Pharmacokinet Pharmacodyn. 2004;31(1):75‐107.
- Macdonald I, Staatz CE, Jelliffe RW, et al. Evaluation and comparison of simple multiple model, richer data multiple model, and sequential interacting multiple model (IMM) Bayesian analyses of gentamicin and vancomycin data collected from patients undergoing cardiothoracic surgery. Ther Drug Monit. 2008;30(1):67‐74.
- Aubron C, Corallo CE, Nunn MO, et al. Evaluation of the accuracy of a pharmacokinetic dosing program in predicting serum vancomycin concentrations in critically ill patients. Ann Pharmacother. 2011;45(10):1193‐8.
- McClellan SD, Farringer JA. Bayesian forecasting of aminoglycoside dosing requirements in obese patients: influence of subpopulation versus general population pharmacokinetic parameters as the internal estimates. Ther Drug Monit. 1989;11(4):431–436.
- Sharabiani A, Bress A, Douzali E, et al. Revisiting warfarin dosing using machine learning techniques. Comput Math Methods Med. 2015;2015:560108.
- Cosgun E, Limdi NA, Duarte CW. High-dimensional pharmacogenetic prediction of a continuous trait using machine learning techniques with application to warfarin dose prediction in African Americans. Bioinformatics. 2011;27(10):1384–1389.
- Tang J, Liu R, Zhang YL, et al. Application of machine-learning models to predict tacrolimus stable dose in renal transplant recipients. Sci Rep. 2017;7(1):42192.
- Kuhn M, Johnson K. Applied predictive modeling. New York: Springer-Verlag; 2013.
- James G, Witten D, Hastie T, et al. An introduction to statistical learning: with applications in R. New York: Springer-Verlag; 2013.
- Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–2223.
- Kolachalama VB, Singh P, Lin CQ, et al. Association of pathological fibrosis with renal survival using deep neural networks. Kidney Int Rep. 2018;3(2):464–475.