1. Introduction
Neuroblastoma (NB) is the most frequent solid tumor in children after cerebral tumors. The prognosis of patients over 18 months of age with stage 4 NB remains poor, despite increasingly aggressive treatments using no-cross resistant chemotherapy combinations. Several chemotherapeutic agents are active against NB, but the emergence of drug-resistant clones during induction treatment and the persistence of minimal residual disease are real problems, because resistant tumors are often fatal. At the present time, the percentage of long-term event-free survivors in advanced NB is still around 30% [Citation1]. Myeloablative consolidation chemotherapy has provided some improvement, but only in a minority of patients. Encouraging results in event-free survival were recently reported by Park et al. [Citation2] with tandem autologous transplant.
Thus, there is an urgent need for new effective therapeutic agents and innovative strategies.
2. 131-I-metaiodobenzylguanidine: a ‘new’ drug
It is well known that NB cells are sensitive to radiation and external beam irradiation is an important part of treatment for primary tumor of disseminated NB. However, toxicity in normal tissue has limited the maximum deliverable irradiation dose to the tumor. By delivering cytotoxic radionuclides conjugated to tumor-seeking agents, targeted radiotherapy can determine a specific irradiation at primary tumor and metastatic sites while avoiding toxicity in normal tissue. Being a radiosensitive tumor, NB is eligible for targeted radiotherapy.
Metaiodobenzylguanidine (MIBG) is a structural analogue of noradrenaline. Part of noradrenaline is normally recaptured by noradrenergic tissues including NB cells and the same process permits eventually to incorporate MIBG. NB is characterized by MIBG avidity in more than 90% of the cases, promoting the use of 131-I radiolabeled MIBG (131-I-MIBG) for treatment of this tumor.
For the diagnosis, staging and follow-up of NB, radio-iodinated MIBG has been shown to be the most specific and powerful agent. Moreover, owing to its capacity to concentrate in NB cells, when 131-I-MIBG is administered at a higher dose, a significant amount of radiation will be delivered to primary and metastatic sites [Citation3]. In a number of studies, when administered alone in patients with relapsed or resistant NB, 131-I-MIBG determined up to 64% of objective response rate, mostly partial responses [Citation1], while the only meaningful toxicity reported was hematologic. Hence, 131-I-MIBG appears to be one of the most effective single drugs against NB.
Notably, the Goldie-Coldman hypothesis predicts that the use of non-cross resistant treatment modalities, such as chemotherapy and radiation as close together as possible appears to be the optimal strategy for very aggressive malignant tumors [Citation4]. Of course, the ‘optimal strategy’ is referring to a theoretical therapeutic approach, since the best treatment for high-risk NB is still unknown.
On the basis of the above considerations, our group at the Catholic University of Rome has made many attempts, in the course of several years, to closely combine 131-I-MIBG with chemotherapy in resistant patients [Citation5–9]. It was first established to administer 131-I-MIBG 10 days after the initial chemotherapy, even if at that time the nadir of neutrophils and platelets should not allow the use of an additional myelotoxic agent [Citation10]. Some experiments in murine model have suggested the rationale for the chemotherapy-131-I-MIBG sequence [Citation11]. If myelosuppressive drugs are administered a few days before total body irradiation, normal tissue including bone marrow appears to be spared from radiation toxicity. However, tumor cells are not protected if myelosuppressive agents are administered before irradiation.
Thus, by gradually intensifying chemotherapy and 131-I-MIBG dosage, we have designed a regimen based on a close combination of both modalities and planned to be administered within a month period. Because of the very encouraging therapeutic results and an acceptable toxicity, we then decided to include that model in the treatment induction of patients with NB at diagnosis [Citation12].
3. An innovative strategy
In recent years, the treatment of NB has been based on an intensive induction regimen, primary tumor resection, consolidation with myeloablative therapy followed by radiotherapy on tumor bed and retinoic acid with immunotherapy [Citation13]. Each component has its own effect on the long-term outcome. However, the role of the induction regimen is the most crucial because, during this step, resistant clones may emerge. Moreover, although a complete remission (CR) after this phase does not ensure long-term survival, the majority of patients who do not obtain a CR will die [Citation1].
Therefore, several attempts have been made to include 131-I-MIBG in advanced NB at diagnosis during remission induction. However, even at diagnosis, 131-I-MIBG has been generally used alone, because of the possible hematologic toxicity due to the close integration of the two therapeutic modalities within an intensive regimen [Citation14]. A combination of 131-I-MIBG, followed weeks later by high-dose chemotherapy and autologous stem cell rescue, has also been tried on residual tumors but with minor therapeutic benefit [Citation15].
Topotecan, which is also a radiosensitizer, has been administered at diagnosis in close combination with 131-I-MIBG [Citation16]. It is unlikely, with current regimens, to expect any improvement in treatment results. It is hoped that better results will be obtained with the recent ongoing COG ANBL1531 trial (ClinicalTrials.gov identifier NCT03126916), designed to evaluate the role of 131-I-MIBG during induction in children with high-risk NB.
Indeed, there is a need for a novel strategy in the treatment of advanced NB. We feel that the main objective of any treatment should be the killing of a maximum number of tumor cells within a few weeks after diagnosis. Notably, it is been clearly shown that a better and early response after induction is correlated with better long-term survival [Citation17]. In the current protocols, the interval between the several courses of induction chemotherapy is of 3–4 weeks, but tumor cells are likely to recover during the interval between pulses of chemotherapy and resistant clones may develop. Pearson et al. [Citation18] have attempted a rapid and intensive schedule of chemotherapy at diagnosis based on a 10-day interval between chemotherapy courses and concentrated in a 70-day period. However, the percentage of long-term survivors did not significantly improve.
Furthermore, in childhood leukemia and lymphoma, where very intensive and rapid chemotherapy regimens were used in the first few weeks of treatment, excellent long-term survival has been obtained.
Based on our long previous experience with the combination chemotherapy-131-I-MBG in resistant NB we have proposed a trial never attempted before at diagnosis, with the critical contribution of 131-I-MIBG at a very high dosage (). This schedule allows for almost continuous exposure of tumor cells to both chemotherapy and radiation during the entire first month of induction [Citation12]. Following the preliminary results, altogether 24 patients were investigated, with excellent therapeutic results. A high percentage of major responses (complete and good partial response) was observed and within a very short time, e.g. 40 days after the start of treatment. Moreover, there was a clear correlation between the amount of 131-I-MIBG (around 16 mCi/kg) and the major responses. A moderate hematologic toxicity was observed, the only toxicity noted. Hematologic toxicity appears to be mainly related to the chemotherapeutic agents rather than to 131-I-MIBG. No serious infection or bleeding problems were observed. Last but not least, the general condition of these children has been excellent.
Figure 1. Combined therapy schedule. * 131-I-metadiodobenzylguanidine irradiation protracted for a few weeks
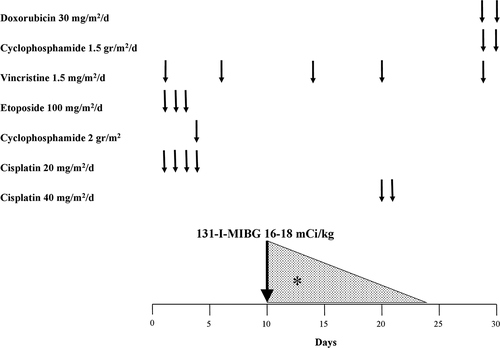
The administration of further cycles of chemotherapy is not hampered by the initial intensive chemotherapy-131-I-MIBG schedule. Furthermore, the mobilization and collection of peripheral blood stem cells was possible with an adequate number of harvested CD34 + . Hematologic recovery time after busulfan and melphalan high-dose chemotherapy and autologous stem cell transplantation was acceptable. Hence, moderately intensive multidrug chemotherapy closely combined with a single substantial dose of 131-I-MIBG does not seem to determine any damage to bone marrow and hematopoietic stem cells [Citation12].
The long-term results of our pilot study appear very encouraging. The regimen lends itself to a randomized multicentric trial, and the value of this novel approach to the treatment of high-risk NB will only be defined by its impact on the final percentage of cure rate. Finally, the use of high-dose 131-I-MIBG in these patients poses challenges resulting from radiation safety requirements associated with the administration of this therapy. Treatment of these patients should be carried out in highly specialized centers because it requires close collaboration between pediatric oncologist, nuclear medicine doctors and technologists, nursing staff and radiation safety officers.
In conclusion, we have proposed a unique therapeutic approach for advanced NB, never attempted before at diagnosis. We avoided adhering to the widely accepted convention of chemotherapy ‘pulses’ with the risky interval between courses, waiting for the hematologic recovery. Conversely, we succeeded in obtaining a persistent exposure of the tumor cells to both chemotherapy and low dose-rate radiation () during the entire first month of induction.
While the main significance of the foregoing findings lies in the rapid tumor cell destruction, the moderate hematologic toxicity observed allows for a new field of investigation in patients with such a devastating tumor.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers in this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Wilson JS, Gains JE, Moroz V, et al. A systematic review of 131I-metaiodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50(4):801–815.
- Park JR, Kreissman SG, London WB, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA. 2019;322(8):746–755.
- Mastrangelo R, Tornesello A, Mastrangelo S. Role of 131I-metaiodobenzylguanidine in the treatment of neuroblastoma. Med Pediatr Oncol. 1998;31(1):22–26.
- Goldie JH, Coldman AJ. Quantitative model for multiple levels of drug resistance in clinical tumors. Cancer Treat Rep. 1983;67:923–931.
- Mastrangelo S, Tornesello A, Diociaiuti L. et al. Treatment with meta-[131I]iodobenzylguanidine and cisplatin in stage IV neuroblastoma. Q J Nucl Med. 1995;39(4 Suppl 1):69–71.
- Mastrangelo R, Tornesello A, Riccardi R, et al. A new approach in the treatment of stage IV neuroblastoma using a combination of [131I]meta-iodobenzylguanidine (MIBG) and cisplatin. Eur J Cancer. 1995;31A(4):606–611.
- Mastrangelo R, Tornesello A, Lasorella A, et al. Optimal use of the 131-I-metaiodobenzylguanidine and cisplatin combination in advanced neuroblastoma. J Neurooncol. 1997;31:153–158.
- Mastrangelo S, Tornesello A, Bembo V, et al. Radioresistant sensitization of neuroblastoma by cisplatin? Med Pediatr Oncol. 2000;35(1):77–79.
- Mastrangelo S, Servidei T, Iavarone A, et al. Role of 131I-metaiodobenzylguanidine (MIBG) in the treatment of neuroblastoma: a review. Inter J Pediatr Hematol Oncol. 1996;3:287–296.
- Mastrangelo S, Tornesello A, Diociaiuti L, et al., Treatment of advanced neuroblastoma: feasibility and therapeutic potential of a novel approach combining 131-I-MIBG and multiple drug chemotherapy. Br J Cancer. 2001;84(4):460–464.
- Millar JL, Hudspith BN. Sparing effect of cyclophosphamide (NSC-26271) pretreatment on animals lethally treated with gamma-irradiation. Cancer Treat Rep. 1976;60:409–414.
- Mastrangelo S, Rufini V, Ruggiero A, et al., Treatment of advanced neuroblastoma in children over 1 year of age: the critical role of 131I-metaiodobenzylguanidine combined with chemotherapy in a rapid induction regimen. Pediatr Blood Cancer. 2011;56(7):1032–1040.
- Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1617–1629.
- De Kraker J, Hoefnagel KA, Verschuur AC, et al. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer. 2008;44:551–556.
- Lee JW, Lee S, Cho HW, et al. Incorporation of high-dose 131 I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: results of the SMC NB-2009 study. J Hematol Oncol. 2017;10(1):108.
- Kraal KC, Tytgat GA, van Eck-Smit BL, et al. Upfront treatment of high-risk neuroblastoma with a combination of 131I-MIBG and topotecan. Pediatr Blood Cancer. 2015;62(11):1886–1891.
- Schmidt M, Simon T, Hero B, et al. The prognostic impact of functional imaging with (123) I-mIBGin patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German neuroblastoma trial NB97. Eur J Cancer. 2008;44(11):1552–1558.
- Pearson AD, Pinkerton CR, Lewis IJ, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9(3):247–256.
