ABSTRACT
Introduction
The recent approval in the USA (Food and Drug Administration), Canada (Health Canada), UK (Medicines and Healthcare products Regulatory Agency), and EU (European Medicines Agency) of once-weekly injectable semaglutide 2.4 mg, as an adjunct to a calorie-controlled diet and increased physical activity, for chronic weight management provides health-care practitioners with an additional option when prescribing weight-loss medication.
Areas covered
We describe the chemistry, mechanism of action, and pharmacological properties of semaglutide (a glucagon-like peptide 1 receptor agonist [GLP-1 RA]) and discuss clinical data and considerations for using once-weekly subcutaneous semaglutide 2.4 mg as treatment for overweight and obesity among patients with and without type 2 diabetes (T2D).
Expert opinion
Once-weekly subcutaneous semaglutide 2.4 mg is the most efficacious medication approved for chronic weight management among patients with overweight and obesity, with and without T2D, and is the first drug to induce sustained double-digit reductions in percentage body weight over 1- to 2-year treatment periods. It demonstrates a similar safety and tolerability profile to other GLP-1 RAs. Semaglutide 2.4 mg treatment could dramatically improve clinical approaches to weight management, but the relatively high cost might prevent patients accessing treatment. Further research exploring the cost-effectiveness of subcutaneous semaglutide 2.4 mg is required.
1. Introduction
1.1. Impact of obesity
Obesity is a prevalent, complex, progressive, and relapsing chronic disease characterized by abnormal or excessive body fat (adiposity) that impairs health [Citation1,Citation2]. The etiology of obesity is diverse and multifactorial, and results from a complex interplay of genetic, epigenetic, biological, and environmental factors [Citation1,Citation2]. Obesity is classified by the World Health Organization as a body mass index (BMI) of ≥30 kg/m2 [Citation3]. The presence of obesity increases a person’s risk of developing health complications, such as insulin resistance, hypertension, dyslipidemia, type 2 diabetes (T2D), subfertility, nonalcoholic fatty liver disease and steatohepatitis, obstructive sleep apnea, cardiovascular disease, and several forms of cancer [Citation2,Citation4]. Obesity also reduces a person’s health-related quality of life [Citation5], the number of healthy life years, which is defined as years free of obesity-associated cardiovascular disease and diabetes [Citation6], and total life expectancy. A modeling study demonstrated that, in people with a BMI between 30 and 35 kg/m2, up to 8.4 years of life were lost owing to obesity, and the number of healthy life years lost was two to four times higher than the total years of life lost [Citation6]. In 2017, obesity was responsible for an estimated 4.7 million deaths and 148 million disability-adjusted life-years in the USA [Citation7]. Furthermore, among patients with COVID-19, obesity has been linked to increased numbers of hospitalizations, increased need for mechanical ventilation, and increased risk of death compared with patients without obesity [Citation8,Citation9]. As would be expected, given the associated morbidity, obesity is directly related to increased health-care utilization costs (i.e. healthcare expenditure) and increased indirect costs (i.e. gross domestic product [GDP]) through loss of productivity and participation at work [Citation10]. Between 2020 and 2050, overweight- and obesity-associated healthcare expenditure in the USA is estimated to account for almost 14% of the total healthcare expenditure [Citation10]; the wider economic cost of overweight and obesity is estimated to range from 0.45% to 1.62% of GDP [Citation10].
1.2. Prevalence of obesity
The global prevalence of obesity almost tripled between 1975 and 2016 and, without effective interventions, is predicted to continue rising [Citation3,Citation11]. In 2015, it was estimated that more than 600 million adults globally (approximately 12% of the global adult population) were living with obesity [Citation11]. In the USA, the prevalence of obesity among adults has increased from approximately 30% in 1999–2000 to more than 40% in 2017–2018 [Citation12].
While obesity is widely recognized as a chronic disease, it remains underdiagnosed and undertreated in clinical practice partly owing to weight bias and stigma among health-care practitioners (HCPs) and people living with obesity [Citation2]. In a survey of more than 3,000 adults living with obesity in the USA, 71% of patients discussed their weight with their HCPs. Still, only 55% of patients received a formal diagnosis of obesity, highlighting the divergence in perceptions and attitudes around obesity among patients and HCPs [Citation13]. Given the rising prevalence of obesity and considering the impact of weight-associated comorbidities, obesity presents a serious and growing public health challenge, and there remains an urgent need for effective therapies that are accessible to HCPs and patients.
1.3. Treatment options for obesity
To improve patient outcomes, the primary goal of obesity management is weight loss. The extent of the required weight loss depends on the patient’s initial body weight and obesity-related comorbidities [Citation2]. Current obesity clinical practice guidelines continue to recommend lifestyle interventions as the cornerstone therapy for obesity, supported by the three pillars of behavioral and psychological therapy, pharmacotherapy, and bariatric surgery [Citation2,Citation14,Citation15]. Lifestyle interventions, such as increased physical activity and eating a reduced-calorie diet, lead to a modest weight loss of approximately 3–5%, which is often inadequate to achieve weight-related health goals [Citation2,Citation16–18]. In addition, patients who are successful with losing weight often cannot maintain the weight loss long-term because of compensatory physiological and metabolic adaptations following weight loss [Citation19–21]. Nonetheless, a modest weight loss of 5–10% of the initial body weight is clinically meaningful in reducing cardiometabolic risks and improving obesity-related comorbidities [Citation2,Citation22–24]. Recent evidence suggests that, for patients with a BMI ≥ 35 kg/m2, greater weight loss of ≥10% of initial body weight is beneficial and should be the primary treatment target when modest weight loss has insufficient effects [Citation25]. Bariatric surgery is highly effective at reducing and sustaining body weight loss of 20–30%, depending on the procedure. Unfortunately, <1% of eligible patients undergo bariatric surgery because of cost, accessibility, and other barriers [Citation26–28]. Pharmacotherapy is more readily available than surgery and is more efficacious than lifestyle interventions alone, thereby providing an attractive option to bridge this treatment gap.
Five weight-loss medications have been approved by the US Food and Drug Administration (FDA) for long-term chronic weight management, including orlistat (a lipase inhibitor), phentermine–topiramate, naltrexone–bupropion (neurotransmitter agonists and reuptake inhibitors), liliraglutide,nd, most recently, semaglutide; the latter two are glucagon-like peptide-1 receptor agonists [GLP-1 RAs]) [Citation29,Citation30]. These medications, except for phentermine–topiramate, are also approved for use in Europe by the European Medicines Agency (EMA) and in Canada [Citation15,Citation31].
Semaglutide is a GLP-1 RA that the FDA initially approved in 2017, and the EMA in 2018, as a once-weekly injectable treatment, at doses up to 1.0 mg, for the management of T2D. Once-weekly injectable semaglutide 2.4 mg was approved by the FDA (US), Health Canada (Canada) and the Medicines and Healthcare products Regulatory Agency (UK) in 2021, and by the EMA (EU) in 2022, as an adjunct treatment for weight management, in conjunction with diet and exercise [33].
We describe here the chemistry, mechanism of action, and pharmacological properties of semaglutide and discuss clinical trial data and clinical considerations for using injectable once-weekly subcutaneous semaglutide 2.4 mg as a treatment for overweight and obesity among individuals with and those without T2D.
2. Once-weekly injectable semaglutide 2.4 mg
2.1. Therapeutic indications, administration, and dose-escalation
Once-weekly injectable semaglutide 2.4 mg (marketed as ‘Wegovy’) is indicated as an adjunct to a calorie-reduced diet and increased physical activity for chronic weight management in adult patients with a BMI ≥30 kg/m2, or a BMI ≥27 kg/m2 with the presence of least one obesity-related comorbidity, such as hypertension, T2D, or dyslipidemia [33]. Treatment is administered as a once-weekly subcutaneous injection and should be administered on the same day each week with or without meals [33]. The injection time and injection site can be changed without any dose adjustment. In the USA, subcutaneous semaglutide for weight management is delivered as 0.25, 0.5 , 1.0, 1.7, or 2.4 mg doses [33]. Treatment should be initiated at 0.25 mg once weekly for 4 weeks, and the dose should be escalated at 4-week intervals until the maintenance dose of 2.4 mg is reached [33]. If patients do not tolerate a dose during the dose titration, further escalation can be delayed for 4 weeks [33].
2.2. GLP-1, semaglutide, and mechanism of action
GLP-1 is a 31-amino acid incretin peptide hormone that stimulates glucose-mediated insulin secretion, slows gastric motility, and reduces appetite and food intake [Citation32,Citation33]. GLP-1 is primarily secreted from L-cells in the gut but is also secreted from the nucleus tractus solitarius in the hindbrain [Citation32,Citation33]. After food ingestion, GLP-1 is rapidly released from the intestinal L-cells into the circulation and mediates its effects through activation of the GLP-1 receptor, which is expressed throughout the body, including the gastrointestinal tract, pancreas, and brain [Citation32,Citation33]. Endogenous GLP-1 released from the intestinal cells is enzymatically degraded by dipeptidyl peptidase 4 (DPP-4) and resulting in a short biological half-life of approximately 3 minutes [Citation32,Citation33]. In the pancreas, GLP-1 receptors act in a glucose-dependent manner to stimulate insulin secretion and inhibit glucagon release [Citation32,Citation33].
Semaglutide is a long-acting GLP-1 analog with 94% sequence homology to human GLP-1 that selectively binds to the GLP-1 receptors. Semaglutide consists of amino acids 7–37 of native GLP-1, with two amino acid substitutions at positions 8 (Aib8) and 34 (Arg34) and a C18 fatty diacid attached to lysine 26 via a long hydrophilic spacer [Citation32]. These structural modifications enable semaglutide to reversibly bind to albumin and prevent degradation by DPP-4, thereby reducing renal clearance [Citation32,Citation33]. This formulation provides semaglutide with a longer half-life (range, 155–184 h, ~7 days) than both liraglutide (range, 11–15 h) and native GLP-1 (range, 1–2 min) [Citation34], allowing for once-weekly subcutaneous dosing without compromising weight-loss efficacy [Citation32,Citation33].
In addition to promoting insulin secretion and decreasing glucagon secretion [Citation35,Citation36], semaglutide results in weight loss via reduced energy intake, with minimal effects on energy expenditure [Citation37]. Semaglutide activates GLP-1 receptors in the hindbrain and hypothalamus and exerts direct and indirect effects on neural pathways involved in homeostatic and hedonic appetite control [Citation38]. In adults with obesity, once-weekly semaglutide 2.4 mg reduced energy intake, huhunger,nd cravings; increased satiety and fullness; and improved executive control of eating (described further in Section 2.4.1) [Citation39].
2.3. Semaglutide pharmacokinetics
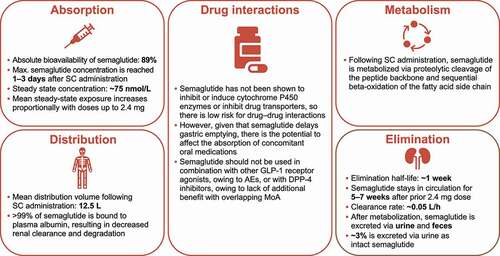
The pharmacokinetic profile relating to the absorption, distribution, metabolism, and elimination of subcutaneously administered once-weekly semaglutide is summarized in . Additional details can be found in the prescribing information [33].
2.4. Key phase 1 and 2 clinical data: subcutaneous semaglutide in people with overweight or obesity
2.4.1. Effects of once-weekly semaglutide on gastric emptying, appetite, and energy intake
A phase 1, randomized, double-blind, parallel-group trial (Clinicaltrials.gov; NCT03842202) assessed the effects of once-weekly subcutaneous semaglutide (dose-escalated to 2.4 mg) versus placebo on gastric emptying, appetite, control of eating, and energy intake in 72 adults (>18 years of age) with obesity (BMI, 30–45 kg/m2) over a 20-week treatment period [Citation39]. Gastric emptying was assessed by measuring paracetamol (acetaminophen) absorption following consumption of a standardized breakfast; patient-reported appetite ratings and Control of Eating Questionnaire responses were evaluated, and energy intake was measured during ad libitum lunch [Citation39]. From baseline to week 20, semaglutide 2.4 mg treatment suppressed appetite, reduced food cravings, and lowered ad libitum energy intake (semaglutide, 1736 kJ; placebo, 2676 kJ [a 35% reduction]) and mean body weight (semaglutide, −10.4 kg; placebo, −0.4 kg) [Citation39]. At week 20, there was no evidence of delayed gastric emptying with semaglutide versus placebo, and the incidence and nature of adverse events (AEs) were consistent with the known safety and tolerability profile of semaglutide, with no new identified safety signals [Citation39].
2.4.2. Efficacy and safety of semaglutide compared with liraglutide for weight loss in people with obesity in a phase 2 dose-finding study
A phase 2, randomized, double-blind, placebo- and active-controlled, dose-ranging trial (ClinicalTrials.gov; NCT02453711) investigated the efficacy and safety of semaglutide compared with liraglutide for weight loss in people with obesity [Citation40]. In this trial, once-daily semaglutide, at 0.05, 0.1, 0.2, 0.3, and 0.4 mg doses, induced greater reductions in body weight over 52 weeks versus placebo (at all doses) and versus once-daily liraglutide 3.0 mg (at 0.2, 0.3, and 0.4 mg doses of semaglutide), and was well tolerated at all doses [Citation40]. Semaglutide 0.4 mg daily was the most effective dose for weight loss with an acceptable tolerability profile [Citation40]. Semaglutide once-weekly 2.4 mg dose was selected for the phase 3 clinical trial program as pharmacokinetic modeling demonstrated that this dose did not exceed the Cmax at steady state seen for the daily 0.4 mg dose.
3. Semaglutide Treatment Effect in People (STEP) with obesity clinical trial program: phase 3 clinical data
The STEP program includes 15 phase 3 clinical trials investigating the efficacy and safety of high-dose 2.4 mg once-weekly injectable semaglutide among people with overweight and obesity, with or without diabetes. The trial status, study design, and interventions for these trials are summarized in . Of these 15 trials, results from 6 clinical trials (STEP 1–4, STEP 6, and STEP 8) have been published [Citation41–46]. Results from STEP 5 are currently published in abstract form [Citation47]; it is anticipated that the full STEP 5 results will become available in 2022. Overall, across these six published clinical trials, data were collected from more than 5000 subjects with overweight or obesity (including 1210 subjects with T2D [STEP 2]) [Citation41–46]. STEP 1–3, 6, and STEP 8 included a 68-week treatment period, STEP 5 included a 104-week treatment period [Citation47], and STEP 4 included a 20-week open-label run-in period followed by a 48-week randomized treatment period [Citation41–46]. All trials were placebo-controlled [Citation41–47]; STEP 8 also investigated the efficacy and safety of once-weekly semaglutide 2.4 mg versus once-daily liraglutide 3.0 mg [Citation46].
Table 1. Study design overviews for the phase 3 STEP trials
In all trials, semaglutide was administered using a prefilled pen injector [Citation41–47]. Treatment was initiated at a dose of 0.25 mg once weekly for the first 4 weeks and was increased every 4 weeks to reach the 2.4 mg maintenance dose by week 16. Lower maintenance doses were permitted if participants had unacceptable side-effects with the 2.4-mg dose. In STEP 8, liraglutide was administered once daily, initiated at 0.6 mg, and escalated to 3.0 mg over 4 weeks [Citation46].
Throughout the trial duration, all participants were provided with exercise equipment to encourage physical activity and received counseling from an interdisciplinary team, including a dietitian [Citation41–47]. In STEP 1, 2, 4, 5, 6, and STEP 8, patients were advised to perform 150 min of physical activity per week and to consume a hypocaloric diet (a reduction of 500 daily kilocalories) [Citation41,Citation42,Citation44–47]. In STEP 3, all participants received intensive behavioral therapy (IBT) and were advised to perform 100–200 min of physical activity per week [Citation43]. All participants also consumed a low-calorie diet (1000–1200 calories per day), which was provided as meal replacements (e.g. liquid shakes, meal bars, or portion-controlled meals) for the initial 8 weeks of the trial [Citation42]. They were then transitioned to a hypocaloric diet (1200–1800 calories per day) of conventional food for the remainder of the 68-week treatment period [Citation42].
3.1. Study endpoints and clinical assessments
For STEP 1–6 and STEP 8, the primary endpoint was the mean percentage change from baseline (at randomization) to end of treatment (EOT) in body weight [Citation41–47]. The co-primary endpoint for STEP 1–3, STEP 5, and STEP 6 was the proportion of patients achieving ≥5% weight loss from baseline to EOT. Additional endpoints included the proportion of participants achieving a body weight reduction of ≥10% or ≥15% from baseline to EOT; change from baseline to EOT in waist circumference, vital signs (systolic/diastolic blood pressure), and clinical outcome assessments (anthropometry and cardiometabolic parameters) [Citation41–47].
For all randomized patients, serial assessments were conducted, including height, body weight, BMI, waist circumference, fasting plasma glucose, glycated hemoglobin (HbA1c), fasting serum insulin, lipids, and vital signs [Citation41–47].
Clinical outcome assessments for physical functioning were also conducted in STEP 1–4 and STEP 6; these included the 36-Item Short Form Health Survey (SF-36), version 2, acute version; the Impact of Weight on Quality of Life, Lite (IWQOL-Lite), clinical trials version; and the Stanford Presenteeism Scale, version 2001. Additional measures included the Patient Global Impression of Change; the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form; the Work Productivity Activity Impairment-Specific Health Problem, version 2.0, and the Weight-Related Sign and Symptom Measure [Citation41–45]. However, it should be noted that not all assessments were used in each trial, and no clinical outcome assessments for physical functioning were included in STEP 5 and STEP 8 [Citation46,Citation47].
Safety assessments included physical examinations, electrocardiograms, biochemistry and hematology assessments, detection of antibodies against semaglutide, and vital signs. AE data were collected throughout the trial duration and during follow-up when patients were no longer receiving treatment [Citation41–47].
3.2. Study population
Patients’ baseline characteristics were generally comparable between trials () and were well-matched between randomized treatments [Citation41–47]. In STEP 1–5 and STEP 8, the racial composition of each trial population was predominantly White, with variations in race and ethnicities among different trials. In STEP 6, all patients were of Asian descent. Because subjects were recruited from different countries, these differences were to be expected; for example, STEP 1 and STEP 2 were designed to have Asian participants constitute at least 10% of the study populations, and STEP 6 was conducted in a population of East Asian adults. Additionally, because STEP 2 included patients with T2D, the mean HbA1c was expectedly higher among this trial population compared with the other STEP trials.
Table 2. Summary of patients’ baseline characteristics for STEP 1–6 and STEP 8 [Citation41–47]
3.3. Efficacy summary
The primary and secondary efficacy endpoints for STEP 1–6 and STEP 8 trials are summarized in and [Citation41–47]. Once-weekly semaglutide 2.4 mg treatment induced a greater reduction from baseline to EOT in body weight compared with placebo across all STEP trials. A recent meta-analysis of STEP 1, STEP 3, and STEP 4 reported that semaglutide reduced body weight by 12.6% over placebo; the absolute body weight loss was 11.9 kg [Citation48]. With respect to categorical weight loss, more participants achieved ≥5%, ≥10%, ≥15%, and ≥20% compared with placebo (risk ratio [RR], 2.11, 3.98, 6.73, and 11.36, respectively) [Citation48]. In STEP 2, the reduction in body weight was larger with semaglutide 2.4 mg (6.2% placebo-subtracted) than with semaglutide 1.0 mg (3.5% placebo-subtracted) [Citation42]. It should be noted that people with T2D experience more difficulty with weight loss compared with those without T2D for a variety of reasons [Citation49]. Indeed, compared with patients without diabetes, less weight loss was noted in the active treatment arms in the STEP 2 trial, and the Satiety and Clinical Adiposity-Liraglutide Evidence in nondiabetic and diabetic individuals (SCALE) Obesity and Prediabetes trial using liraglutide 3.0 mg [Citation42,Citation50]. In STEP 3, the mean weight change from baseline to EOT was −16.0% with semaglutide versus −5.7% with placebo, both combined with IBT and meal replacements [Citation43]. STEP 5 reported sustained weight loss of −15.2% over 104 weeks with semaglutide from baseline to EOT, compared with −2.6% with placebo [Citation47]. In STEP 8, the reduction in body weight from baseline to EOT was greater with once-weekly semaglutide 2.4 mg versus once-daily liraglutide 3.0 mg [Citation46]. The weight loss observed in STEP 8 with liraglutide was similar to the weight loss reported in the SCALE Obesity and Prediabetes trial [Citation51], therefore making it easier to make indirect comparisons between the STEP and SCALE programs. Across the STEP trials, the proportions of patients achieving weight reductions of ≥5%, ≥10%, ≥15%, and ≥20% of their initial body weight were greater with once-weekly semaglutide 2.4 mg versus placebo and once-daily liraglutide 3.0 mg treatments.
Figure 2. Effects of once-weekly subcutaneous semaglutide 2.4 mg on body weight from STEP 1–6 and STEP 8 [Citation41–47].
![Figure 2. Effects of once-weekly subcutaneous semaglutide 2.4 mg on body weight from STEP 1–6 and STEP 8 [Citation41–47].](/cms/asset/aefcf86f-7893-478d-b19b-4e244af3458b/ierj_a_2070473_f0002_oc.jpg)
Table 3. Summary of primary and secondary efficacy endpoints for STEP 1–6 and STEP 8 [Citation41–47]
In addition to body weight reductions, compared with placebo and liraglutide, semaglutide improved anthropometric and cardiometabolic parameters (including BMI, waist circumference, HbA1c, fasting plasma glucose, lipid profiles, and C-reactive protein levels) (). In a meta-analysis of STEP 1, STEP 3, and STEP 4, compared with placebo, semaglutide 2.4 mg improved cardiometabolic risk factors, including a reduction in waist circumference (−9.34 cm), BMI (−4.48 kg/m2), systolic and diastolic blood pressure (−4.62 mm Hg and −1.82 mm Hg, respectively), glycemic parameters in participants without T2D (HbA1c −0.25%, fasting plasma glucose −7.4 mg/dL), lipid profiles (total cholesterol −5.9%, triglycerides −18.3%, low-density lipoprotein cholesterol −6.6%, free fatty acids −11.8%), C-reactive protein levels, and physical function [Citation48]. Importantly, in STEP 1–4 and STEP 6 (), semaglutide 2.4 mg treatment improved health-related quality of life (SF-36 scores and IWQOL-Lite scores).
3.4. Safety summary
The AEs and tolerability profiles for STEP 1–6 and STEP 8 are summarized in [Citation41–47]. The safety profile of semaglutide 2.4 mg was similar to those reported for semaglutide 1.0 mg and liraglutide 3.0 mg, with no new signals reported. As with other GLP-1 RAs, gastrointestinal disorders (typically nausea, constipation, diarrhea and/or vomiting) were the most frequently reported events. Most of these AEs were transient and mild to moderate in severity and resolved without permanent discontinuation. Gastrointestinal events were the main AE-related cause for discontinuation (7% with semaglutide versus 3.1% with placebo in STEP 1). Notably, the weight-loss benefits were independent of and unrelated to the gastrointestinal side-effects of nausea.
Table 4. Adverse events and tolerability profiles for STEP 1–6 and STEP 8 [Citation41–47]
Semaglutide was associated with an increase in gallbladder-related disorders, mainly cholelithiasis, 2.6% versus 1.2% in placebo in STEP 1, and 2.6% versus 1.3% in placebo in STEP 5. Some of these events could be attributed to weight loss rather than being drug related [Citation52,Citation53]. There was no increase in benign and malignant neoplasms reported in STEP 1–6 and STEP 8.
GLP-1 RAs, including semaglutide, are known to increase the heart rate by 1–4 beats per minute. Heart rate should be routinely monitored with semaglutide treatment, and the drug should be discontinued with sustained increases. Notably, the small increase in heart rate did not appear to affect beneficial cardiovascular outcomes, as revealed by results of the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6). Long-term cardiovascular outcomes are currently being investigated in people with obesity (but without diabetes) and established cardiovascular disease in the Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (SELECT) trial. This event-driven trial is the largest cardiovascular disease outcome trial conducted in people with obesity and is estimated to complete in 2023.
3.5. Study limitations
Several study limitations should be considered when interpreting these STEP trial results [Citation41–47]. All the phase 3 trials could have been better balanced regarding race and sex [Citation41–47]. The efficacy and safety data from STEP 2 cannot be extended to individuals with T2D receiving insulin therapy, as they were excluded from the study [Citation42]. All trials included lifestyle modifications as part of the study methodology (i.e. a hypocaloric diet and increased physical activity) [Citation41–47], and patients in STEP 3 received IBT [Citation43]. However, given that subcutaneous semaglutide 2.4 mg should be used as an adjunct to a calorie-controlled diet and increased physical activity, these findings are likely representative of patients seen in clinical practice. It should be noted that increased exercise intensity may enhance cardiorespiratory fitness, thereby reducing fat mass and further augmenting weight loss with semaglutide. It has also been reported that, in adults with overweight or obesity, combining exercise with daily subcutaneous GLP-1 RA therapy (liraglutide 3.0 mg per day) improved healthy weight loss maintenance than either treatment alone [Citation54]. The synergistic effects of exercise in conjunction with lifestyle interventions were not explored in the STEP trials.
The strict run-in period of STEP 4 did not allow for any deviation away from the standard semaglutide dosing titration schedule [Citation44]. Because HCPs in clinical practice can tailor the dosing titration on a case-by-case basis, the STEP 4 methodology may not be representative of real-world experience. Additionally, in STEP 8, owing to the different semaglutide and liraglutide dose-escalation protocols, more patients could have permanently discontinued liraglutide after an AE than semaglutide [Citation46]. Furthermore, if patients discontinued liraglutide sooner than semaglutide, the achievable weight loss with liraglutide could have been reduced [Citation46].
Overall, in addition to the ongoing and planned STEP trials (), future real-world studies representing routine clinical practice that monitor the long-term efficacy and safety of once-weekly injectable semaglutide 2.4 mg use among people with overweight or obesity, with and without T2D, would be beneficial.
3.6. Ongoing and future STEP trials
In addition to STEP 1–6 and STEP 8, there are currently eight ongoing or planned trials from the phase 3 STEP clinical trial program (). STEP 7 and STEP 11 are regional placebo-controlled phase 3 trials investigating the efficacy and safety of once-weekly semaglutide in a Chinese population and adults from Thailand and South Korea, respectively, living with overweight or obesity; STEP TEENS is a multinational placebo-controlled trial evaluating the efficacy and safety of once-weekly semaglutide among adolescents (12–17 years of age) with overweight or obesity. STEP 9 and STEP 10 are placebo-controlled trials investigating the efficacy and safety of once-weekly semaglutide among patients with osteoarthritis and prediabetes, respectively, with overweight or obesity. In patients with T2D, the effects of once-weekly subcutaneous semaglutide (0.5 mg and 1.0 mg) on cardiovascular outcomes were previously assessed in SUSTAIN 6; in this trial, it was demonstrated that the rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was significantly lower among patients with T2D receiving semaglutide versus placebo [Citation55]. Because both diabetes and obesity are risk factors for cardiovascular disease, the SELECT trial will determine the effects of semaglutide 2.4 mg on cardiovascular outcomes among patients with overweight and obesity, without diabetes. Furthermore, there are two randomized, placebo-controlled phase 3 trials investigating the effects of once-weekly injectable semaglutide 2.4 mg on the body weight and heart failure symptoms among individuals with heart failure with preserved ejection fraction with T2D (STEP HFpEF DM) and without T2D (STEP HFpEF); these trials are expected to complete in 2023 ().
4. Semaglutide 2.4 mg versus other weight-management medications
In patients with overweight or obesity, with and without T2D, once-weekly injectable semaglutide 2.4 mg induced weight loss from baseline to EOT, ranging between 7.9% and 16.0% () [Citation41–47]. This is a greater reduction in body weight compared with other approved weight-management medications (range, 5.0–9.9%), which include once-daily liraglutide 3.0 mg [Citation50], orlistat (three times daily oral administration) [Citation56], phentermine–topiramate (once-daily oral administration) [Citation57], and naltrexone–bupropion (twice-daily oral administration) [Citation58]. Additionally, in the head-to-head STEP 8 trial, semaglutide 2.4 mg treatment induced greater weight loss from baseline than liraglutide 3.0 g, with no additional safety signals, among patients with overweight and obesity () [Citation46]. In a meta-analysis of STEP 1–3, it was reported that semaglutide 2.4 mg reduced body weight (–11.9 kg) and its percentage (–12.6%), respectively, relative to placebo [Citation48], which is greater than the reductions in body weight (–5.3 kg) [Citation59] and percentages (–4.16%) [Citation60] reported with liraglutide 3.0 mg, relative to placebo, in other meta-analyses. In a recent systematic review and network meta-analysis of weight-management drugs, phentermine–topiramate and GLP-1 RAs (liraglutide, semaglutide, exenatide) were more effective than lifestyle interventions alone in body weight reductions of ≥5% (odds ratio [OR], 8.02 and 6.33, respectively) [Citation61]. The authors conducted a post-hoc analysis of semaglutide 2.4 mg, since it showed much greater weight-loss benefits than other GLP-1 RAS as well as all other drug classes, and the OR for likelihood of weight loss of ≥5% was 9.82 [Citation61]. With respect to safety, phentermine–topiramate and naltrexone–bupropion were associated more adverse side-effects and higher rates of discontinuation than semaglutide [Citation61].
5. Emerging weight-management medications
The success of GLP-1 RAs, initially with liraglutide and more recently with semaglutide, in obesity treatment with double-digit percentage of body weight loss, sparks the development of drugs with even greater weight-loss efficacy and better safety and tolerability profiles.
Several novel weight-management drugs are under active development and investigations, including monotherapy or combination therapy with drugs that have different mechanisms of action in the hope of achieving greater weight loss than monotherapy.
Cagrilintide, a long-acting analog of amylin (a pancreatic hormone that promotes satiety), was recently investigated in a phase 2, multicenter, randomized, double-blind, placebo-controlled, and active-controlled, dose-finding 26-week trial (ClinicalTrials.gov; NCT03856047) [Citation62]. In total, 706 participants with obesity or overweight with one or more obesity-related comorbidities were randomized to once-weekly cagrilintide (0.3–4.5 mg), once-daily liraglutide 3.0 mg, or volume-matched placebo. Cagrilintide resulted in dose-dependent weight reductions that had not plateaued by week 26, and greater weight loss at all doses than placebo. Mean weight reductions with all doses of cagrilintide (0.3–4.5 mg) were 6.0–10.8% versus 3.0% in the placebo group after 26 weeks of treatment, including a 6-week dose-escalation period [Citation62]. Weight loss was greater with cagrilintide 4.5 mg than liraglutide 3.0 mg, and more participants lost >10% and >15% body weight with cagrilintide than with liraglutide.
Combination therapy of semaglutide and cagrilintide has been studied in a phase 1b randomized trial (Clinicaltrials.gov; NCT03600480) [Citation63]. In this trial, 96 adults (>18 years of age) with overweight or obesity (BMI 27.0–39.9 kg/m2) were randomized to once-weekly semaglutide (dose-escalated to 2.4 mg), co-administered with cagrilintide (0.16, 0.30, 0.60, 1.2, 2.4 or 4.5 mg) or matched placebo, all without lifestyle interventions [Citation63]. Cagrilintide and semaglutide doses were co-escalated in 4-week intervals to the desired dose over 16 weeks and were treated at the target dose for a further 4 weeks [Citation63]. At week 20, mean percentage body weight reductions from baseline were greater with semaglutide 2.4 mg plus cagrilintide 1.2 mg (–15.7% from baseline) and 2.4 mg (–17.1% from baseline) versus semaglutide 2.4 mg with placebo (–9.8% from baseline) [Citation63]. Glycemic parameters improved across all treatment groups independently of cagrilintide dose [Citation63]. Most AEs were mild to moderate in severity, and the proportions of participants reporting an AE were similar across treatments; the most reported AEs across treatments were gastrointestinal disorders [Citation63]. Overall, among adults with overweight or obesity, concomitant treatment with cagrilintide and semaglutide 2.4 mg was well tolerated and demonstrated an acceptable safety profile [Citation63]. A multicenter, randomized, double-blind, placebo-controlled clinical trial of cagrilintide–semaglutide combination therapy is currently being planned for obesity treatment.
A dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptor co-agonist, tirzepatide, which was initially developed for the treatment of T2D, is currently being investigated in individuals with overweight or obesity (SURMOUNT trial). In a head-to-head phase 3 trial (SURPASS-2), the efficacy and safety of tirzepatide (at 5, 10, or 15 mg) was compared with once-weekly semaglutide 1.0 mg among 1879 individuals with T2D [Citation64]. At all doses, tirzepatide was noninferior and superior to semaglutide with respect to mean change from baseline to week 40 in HbA1c, and the reductions in body weight were greater with tirzepatide versus semaglutide 1.0 mg (least-squares mean estimated treatment difference, – 1.9 kg [at 5 mg], – 3.6 kg [10 mg], and – 5.5 kg [15 mg]); for both tirzepatide and semaglutide treatments, the most common AEs were gastrointestinal-related events [Citation64].
Another class of co-agonists, the dual GLP-1 and glucagon receptor co-agonist, is currently in phase 1 and 2 clinical trials [Citation65,Citation66]. The rationale for pursuing the development of a dual GLP-1 and glucagon receptor co-agonist is to exploit the stimulatory effect of glucagon on energy expenditure, which might lead to greater weight loss than can be achieved through central appetite suppression with a GLP-1 RA alone. Oxyntomodulin is a naturally occurring gut hormone that activates both GLP-1 and glucagon receptors and has a potent effect on energy balance and body weight regulation. Oxyntomodulin analogs are unimolecular dual co-agonists that promote weight loss through distinct mechanisms of action [Citation66]. Unimolecular triple incretins, combining GLP-1, GIP, and glucagon receptor tri-agonist, are also under active investigation for obesity and diabetes treatment [Citation67].
6. Clinical implications and considerations
Semaglutide, like all GLP-1 RAs, is associated with gastrointestinal adverse side-effects (nausea, constipation, diarrhea, and/or vomiting) that are mild to moderate in severity [33]. These symptoms are usually dose-dependent and can be minimized with more gradual dose up-titration. Healthy diets with reduced refined sugar and fat content also help to reduce the symptoms. HCPs should discuss, assist, and advise their patients on how to manage and mitigate these symptoms before prescribing the medication [Citation68]. Semaglutide is contraindicated in pregnancy and in patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2, or a known hypersensitivity to semaglutide [33]. Semaglutide treatment is not recommended for patients with end-stage renal disease [33]. Rare cases of acute pancreatitis, hypoglycemia, acute kidney injury, diabetic retinopathy in patients with T2D, angioedema, and anaphylaxis have been reported with semaglutide treatment [33].
Patients with overweight or obesity are at increased risk of having obesity-related complications or comorbidities; therefore, these patients may require additional concomitant treatments. Because once-weekly semaglutide can cause delay in gastric emptying, its potential to affect the absorption of concomitantly administered oral medications should be considered [33]. Furthermore, to reduce the risk of hypoglycemia, HCPs can consider reducing the dose of concomitantly administered insulin secretagogues or insulin when initiating once-weekly injectable semaglutide treatment [33].
In terms of drug administration, some patients are reluctant to use injectable medications, and, for these patients, oral medications might improve treatment adherence. Currently, the oral formulation of semaglutide (marketed as Rybelsus), which is administered once daily, is approved for the treatment of T2D at doses up to 14 mg [Citation69]. Oral semaglutide is currently being investigated for weight management (clinicaltrials.gov; NCT05035095). If approved for weight management, this oral formulation might be a more appropriate treatment choice for those patients who are reluctant to self-inject.
In addition to the efficacy, safety, and dosing/administration methods, the medication price, and medication cost-effectiveness, should also be considered by HCPs. In the USA, the reported monthly prescription cost of once-weekly injectable semaglutide 2.4 mg ($1619) is higher than for other anti-obesity medications (orlistat: $823 [120 mg; dosed three times daily]; phentermine–topiramate: $223–239 [7.5 mg/46 mg; dosed once daily]; naltrexone–bupropion: $365 [16 mg/180 mg; dosed twice daily]) except for once-daily liraglutide 3.0 mg ($1619) [Citation70]. Furthermore, because anti-obesity medications are often labeled for cosmetic use rather than disease management, it is often difficult to gain insurance coverage for these agents, providing a treatment barrier for patients who could benefit from receiving semaglutide treatment. Several studies in patients with T2D across the UK and USA have shown cost savings with semaglutide 1.0 mg and have demonstrated the cost-effectiveness of semaglutide compared with dulaglutide, which is another diabetes medication [Citation71,Citation72]. However, data regarding the cost-effectiveness of semaglutide for weight management among patients with overweight or obesity without T2D are limited. One study to date reported that, because of the high prescription cost, semaglutide might not be a cost-effective treatment option for weight management. However, it should be noted that this study was conducted before the results of the phase 3 STEP clinical trials were published and does not consider the demonstrated weight-loss efficacy of once-weekly injectable semaglutide 2.4 mg [Citation73]. Future cost-effectiveness studies could investigate the potential short- and long-term cost savings with semaglutide 2.4 mg and other anti-obesity medications, including the benefits generated by improvements in weight and prevention and treatment of obesity-related comorbidities.
It should be noted that, across the STEP trials, a small proportion of patients with overweight or obesity did not respond to once-weekly injectable semaglutide 2.4 mg treatment (i.e. those patients who achieved a reduction of <5% of their initial body weight). The percentage of ‘non-responders’ in these trials was approximately 30% of patients with T2D (i.e. in STEP 2) and ranged between 13% and 23% of patients without diabetes (), highlighting that some individuals with overweight or obesity may need further interventions, or different treatment options, to help manage their body weight and obesity-related co-morbidities.
Finally, it is important to emphasize that, in addition to subcutaneous semaglutide 2.4 mg (or any other medication for chronic weight management), a successful weight-loss program also includes lifestyle modifications, such as a calorie-controlled diet, increased physical activity, and behavioral modifications.
7. Conclusions
Obesity is a progressive, relapsing, chronic disease that severely impairs health. People with obesity are at risk of developing concomitant diseases and many obesity-related comorbidities. Current obesity clinical practice guidelines recommend lifestyle interventions, such as a calorie-controlled diet and increased physical activity, as the cornerstone therapy for obesity, supported by behavioral and psychological therapy, pharmacotherapy, and bariatric surgery. The recent FDA approval of once-weekly injectable semaglutide 2.4 mg, as an adjunct to a calorie-controlled diet and increased physical activity, for chronic weight management provides HCPs with an additional option to consider when prescribing weight-loss drugs. The phase 3 STEP clinical development program, to date, has demonstrated that, among patients with overweight and obesity, with and without T2D, once-weekly injectable semaglutide 2.4 mg treatment induces greater weight loss from baseline, compared with placebo and once-daily liraglutide 3.0 mg. Semaglutide 2.4 mg treatment also demonstrates a tolerability profile that is similar to that for other GLP-1 RAs, with the most common AEs being gastrointestinal-related events. In addition to the efficacy and safety profile, HCPs should also consider the route and frequency of administration as well as the drug cost when choosing an anti-obesity medication. The cost of once-weekly injectable semaglutide 2.4 may constitute barriers for broad insurance coverage and limit patient access to this treatment option.
8. Expert opinion
Once-weekly subcutaneous semaglutide 2.4 mg is currently the most efficacious medication approved for chronic weight management and is the first drug that reduced the percentage of body weight in the double digits. In addition to being indicated as a treatment for T2D and chronic weight management (including patients with obesity with a monogenetic etiology) [Citation74], subcutaneous semaglutide is currently being investigated as a treatment option for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH). A phase 2 trial of patients with NASH demonstrated that, compared with placebo, subcutaneous semaglutide treatment resulted in a significantly (P < 0.001) higher proportion of patients with an improvement in fibrosis [Citation75]; these results were corroborated in a meta-analysis [Citation76].
In terms of chronic weight management, in STEP 1, semaglutide 2.4 mg reduced ≥5% body weight in close to 90%, ≥10% in about 70%, ≥15% in 50%, and ≥20% in about 32% of participants [Citation41]. Semaglutide 2.4 mg treatment not only leads to effective weight loss, but also sustained double-digit weight loss, as demonstrated in the 2-year STEP 5 trial [Citation47]. In addition, compared with placebo and liraglutide, semaglutide 2.4 mg reduced waist circumference and improved cardiometabolic parameters, including systolic and diastolic blood pressure, glycemic and lipid profiles, reduced C-reactive protein levels, physical function, and quality of life. Semaglutide 2.4 mg also improved body composition and increased the proportion of lean body mass relative to total body mass as quantified by a dual-energy X-ray absorptiometry scan [Citation41].
Semaglutide 2.4 mg will drastically change our current practice and heralds a new era of obesity treatment. For far too long, HCPs have emphasized lifestyle interventions as the cornerstone of obesity treatment, yet these interventions only lead to modest weight loss. Moreover, the weight loss is not sustained for most people living with obesity because of weight regain due to compensatory metabolic and hormonal adaptation that occur following weight loss. The lack of successful weight loss with lifestyle interventions fuels pervasive weight bias and stigma among HCPs of personal failure and casts blame and shame among people living with obesity. Weight bias and stigma are barriers that can hinder patients from accessing care and treatment [Citation13]. Semaglutide treatment will shift the focus from weight loss to improved health and well-being as well as reducing weight bias and clinical inertia.
Semaglutide 2.4 mg paves the way for ongoing STEP trials to demonstrate the clinical benefits of >15% weight loss in the amelioration of many obesity-related comorbidities. For example, STEP 9 is designed to determine whether double-digit weight loss will improve knee osteoarthritis among people with obesity, and STEP 10 will investigate whether double-digit weight loss will delay the progression from prediabetes to T2D. Additionally, two STEP trials have been planned to examine whether double-digit weight loss will improve heart failure with preserved ejection fraction (HFpEF), STEP HFpEF in people with obesity, and STEP HFpEF DM in people with obesity and T2D. The results from these ongoing STEP trials will provide direct evidence to support the clinical benefits of obesity treatment in improving obesity-related comorbidities, and not just the surrogate endpoints of body weight loss and, importantly, dispel the misperceived narrative that obesity is merely a body image rather than a health issue.
Pharmacotherapy for obesity, unlike T2D, is rarely prescribed by HCPs, and less than 1% of people with obesity in the USA who are eligible to receive treatment [Citation77]. The high cost of treatment and lack of insurance coverage are major reasons. Still, HCPs are also concerned with the low efficacy, as well as safety, of weight-loss medications, especially with a long tainted history of weight-management medications, such as fenfluramine, sibutramine, and lorcaserin, which were the latest medications to be withdrawn from the US market in 2020 [Citation78,Citation79]. Semaglutide 2.4 mg, which has demonstrated a good safety profile and acceptable tolerability profile in the STEP 1–5 trials, will hopefully change the perception and reduce therapeutic inertia in prescribing medical therapy for a chronic relapsing disease like obesity. While we await the results of SELECT, the largest ongoing cardiovascular outcome trial with semaglutide 2.4 mg expected to be complete in 2023, we can rely on the cardiovascular safety data from the SUSTAIN-6 cardiovascular outcomes trial with once-weekly semaglutide 1.0 mg in people with T2D [Citation55].
Semaglutide 2.4 mg and future weight-management medications will also dramatically change our approach to diabetes management. The alarming increase in the global prevalence of T2D is attributable to the increased prevalence of obesity. While lifestyle interventions remain the first-line treatment, the modest weight loss that results from using these interventions alone is inadequate to achieve the glycemic targets for most people with T2D. The Look-AHEAD trial in people with T2D demonstrated the benefits of lifestyle interventions on glycemic and metabolic control, but the modest weight loss achieved during the 8-year follow-up study failed to reduce cardiovascular disease outcomes [Citation21]. Current clinical practice guidelines recommend adjunctive glucose-lowering therapies at or within the first 3 months of diagnosis [Citation80,Citation81], and HCPs should preferably choose drugs that have been proven to confer cardiovascular benefits through cardiovascular outcome trials mandated by the FDA and EMA as requirements for new drug approval [Citation80,Citation81]. The treatment goals remain glucocentric and focused on preventing downstream metabolic and vascular complications. Over the past several years, bariatric surgery has been advocated as an effective method, not only for diabetes treatment but also to induce diabetes remission through sustained weight loss of 20% or more [Citation82]. About 25–30% of patients remained in remission 5 years following bariatric surgery, and the remission rates are related to the magnitude of weight loss [Citation82]. Recent data from the DiRECT trial provide compelling evidence that >15% body weight loss is necessary to induce diabetes remission and potentially reverse the underlying pathophysiological defects of insulin resistance and secondary β-cell failure [Citation83]. With the availability of semaglutide 2.4 mg and promising weight-loss medications on the horizon, we can now achieve double-digit percentage body weight loss with pharmacotherapies, approaching the weight loss reported with bariatric surgery, providing a treatment option to bridge the gap between lifestyle interventions and bariatric surgery. Importantly, we now have the opportunity of focusing on weight management as the primary treatment goal for diabetes and potentially modifying the natural progression of T2D.
HCPs should, however, be aware of several challenges associated with semaglutide treatment. Gastrointestinal side-effects are common, and HCPs should become familiar with strategies to mitigate these AEs by providing proper counseling to patients, especially during the dose up-titration period, as well as providing ongoing support for the full duration of semaglutide treatment. Semaglutide should be discontinued when non-responders are identified earlier on with treatment. Semaglutide is an injectable medication, and some patients may require counseling to overcome their fear of needles. High medication costs can be a barrier for some patients, which is especially true for the vulnerable population of patients without health insurance plans and access to medications. The pharmacoeconomics and cost-effectiveness of semaglutide 2.4 mg treatment has not been established and raises the question of whether obesity pharmacotherapy can be justified.
Semaglutide 2.4 mg is the first weight-loss medication that bridges the treatment gap in obesity and can lead to improvement in several obesity-related comorbidities. However, such a breakthrough in pharmacological advancement must also be accompanied by increased awareness and education of both HCPs and people living with obesity, easier access to care, government and institutional support, and reduced cost barriers. Coverage through government and third-party insurance plans will go a long way to reduce the devastating impact of obesity and related complications on our society.
Article highlights
Current obesity clinical practice guidelines recommend lifestyle interventions, such as dietary changes, increased physical activity, and behavioral modifications, as the cornerstone therapy for obesity, supported by behavioral and psychological therapy, pharmacotherapy, and bariatric surgery.
The recent approval in the USA (Food and Drug Administration), Canada (Health Canada), UK (Medicines and Healthcare products Regulatory Agency), and EU (European Medicines Agency) of once-weekly injectable semaglutide 2.4 mg (a glucagon-like peptide-1 receptor agonist [GLP-1 RA]), as an adjunct to a calorie-controlled diet and increased physical activity, for chronic weight management provides health-care practitioners with an additional option to consider when prescribing drugs for weight loss.
This article describes the chemistry, mechanism of action, and pharmacological properties of semaglutide, a GLP-1 RA, and discusses the clinical data and considerations for using once-weekly subcutaneous semaglutide 2.4 mg as a treatment for overweight and obesity among patients with and without type 2 diabetes (T2D).
The phase 3 STEP clinical development program to date has demonstrated that, among patients with overweight and obesity, with and without T2D, once-weekly injectable semaglutide 2.4 mg treatment induces greater weight loss from baseline than placebo (STEP 1–6) and once-daily liraglutide 3.0 mg (STEP 8).
In STEP 1–6 and STEP 8, semaglutide 2.4 mg treatment demonstrated a tolerability profile similar to that of other GLP-1 RAs, with the most common adverse events being gastrointestinal-related events.
Declaration of interest
DCW Lau reports clinical research grant funding from Novo Nordisk. He serves on advisory boards for Amgen, Bayer, Boehringer Ingelheim, HLS Therapeutics, Eli Lilly, Novartis, Novo Nordisk, and Pfizer. He serves on speaker bureaus for Amgen, Bayer, Boehringer Ingelheim, CME at Sea, HLS Therapeutics, Eli Lilly, Novartis, Novo Nordisk, and Obesity Canada. RL Batterham reports clinical research grant funding from Novo Nordisk. She serves on advisory boards for Eli Lilly, Gila, Novo Nordisk, Pfizer, and ViiV. She serves on speaker bureaus for Eli Lilly, International Diabetes Canada, Medical Press, Novo Nordisk, and ViiV. CW le Roux reports grants from Anabio, the Health Research Board, the Irish Research Council, and Science Foundation Ireland. He serves on advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GI Dynamics, Glia, Herbalife, Janssen, Johnson & Johnson, Novo Nordisk, and Sanofi-Aventis. CWlR is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He has been Chief Medical Officer and Director of the Medical Device Division of Keyron since January 2011. Both of these are unremunerated positions. CWlR was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Information resources
For further information regarding the published phase 3 randomized STEP trials, please refer to the cited references (see reference list). These articles can also be accessed via the following links.
STEP 2: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00213-0/fulltext
STEP 3: https://jamanetwork.com/journals/jama/fullarticle/2777025
STEP 4: https://jamanetwork.com/journals/jama/article-abstract/2777886
STEP 6: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00008-0/fulltext
STEP 8: https://jamanetwork.com/journals/jama/article-abstract/2787907
Reviewer disclosures
A reviewer on this manuscript has disclosed receiving a research grant from Novo Nordisk. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Novo Nordisk A/S conducted the initial literature searches and had a role in the review of the manuscript for scientific accuracy.
Additional information
Funding
References
- Frühbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131–136.
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. Can Med Assoc J. 192(31): E875–E891. 2020.
- World Health Organization. Obesity and Overweight 2021. Last accessed 2022 Mar 24. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- Obes Res. National Institutes of Health. 1998.
- Stephenson J, Smith CM, Kearns B, et al. The association between obesity and quality of life: a retrospective analysis of a large-scale population-based cohort study. BMC Public Health. 2021;21(1):1990.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modeling study. Lancet Diabetes Endocrinol. 2015;3(2):114–122.
- de Cosio Fg, Diaz-Apodaca B, Baker A, et al. US obesity mortality trends and associated noncommunicable diseases contributing conditions among white, black, and hispanic individuals by age from 1999 to 2017. SN Compreh Clin Med. 2021;3(6):1334–1343.
- Yang J, Hu J, Zhu C. Obesity aggravates COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2021;93(1):257–261.
- Sanchis-Gomar F, Lavie CJ, Mehra MR, et al. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clinic Proc. 2020;95(7):1445–1453.
- OECD. The heavy burden of obesity. 2019. Available at: https://www.oecd.org/health/the-heavy-burden-of-obesity-67450d67-en.htm. Last accessed 2022 Mar 24.
- Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020(360):1–8.
- Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the National ACTION Study. Obesity. 2018;26(1):61–69.
- Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424.
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(3):1–203.
- Peirson L, Douketis J, Ciliska D, et al. Treatment for overweight and obesity in adult populations: a systematic review and meta-analysis. CMAJ Open. 2014;2(4):E306–E317.
- Barte JCM, Veldwijk J, Teixeira PJ, et al. Differences in weight loss across different BMI classes: a meta-analysis of the effects of interventions with diet and exercise. Int J Behav Med. 2014;21(5):784–793.
- Singh N, Stewart RAH, Benatar JR. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: a meta-analysis of randomised trials. BMJ Open. 2019;9(8):e029966.
- Varkevisser RDM, van Stralen MM, Kroeze W, et al. Determinants of weight loss maintenance: a systematic review. Obes Rev. 2019;20(2):171–211.
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604.
- Research Group LAHEAD. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity. 2014;22(1):5–13.
- Jensen MD, Ryan DH, Apovian CM, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American heart association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–38. 2013.
- World Health Organization. World Health Organization obesity: preventing and managing the global epidemic. Report of a WHO consultation WHO Technical Report Series. 2000;894.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- Tahrani A, Morton J. Benefits of weight loss of 10% or more in patients with overweight or obesity: a review. Obesity. 2022;30(4):802–840. (In Press).
- Gasoyan H, Tajeu G, Halpern MT, et al. Reasons for underutilization of bariatric surgery: the role of insurance benefit design. Surg Obes Relat Dis. 2019;15(1):146–151.
- Love KM, Mehaffey JH, Safavian D, et al. Bariatric surgery insurance requirements independently predict surgery dropout. Surg Obes Relat Dis. 2017;13(5):871–876.
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using Andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404–412.
- González-Muniesa P, Mártinez-González M-A, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3(1):17034.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64(11):1376–1385.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362.
- Lau J, Bloch P, Schäffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analog semaglutide. J Med Chem. 2015;58(18):7370–7380.
- Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155.
- Vilsbøll T, Agersø H, Krarup T, et al. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88(1):220–224.
- Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266.
- Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354.
- Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251.
- Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6). DOI:10.1172/jci.insight.133429.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23(3):754–762.
- O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 384(11): 989–1002. 2021.
- Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 397(10278): 971–984. 2021.
- Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 325(14): 1403–1413. 2021.
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 325(14): 1414–1425. 2021.
- Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 10(3): 193–206. 2022.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 327(2): 138–150. 2022.
- W Tea G. 2021. Two-year effect of semaglutide 2.4 mg vs. placebo in adults with overweight or obesity (STEP 5). Presented at the 39th annual meeting of The Obesity Society (TOS) held at ObesityWeek, Dallas, TX (Texas), Nov 1-5.
- Zhong P, Zeng H, Huang M, et al. Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis. Endocrine. 2022;75(3):718–724.
- Lau DCW, Teoh H. Current and emerging pharmacotherapies for weight management in prediabetes and diabetes. Can J Diabetes. 2015;39:S134–S141.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
- Nreu B, Dicembrini I, Tinti F, et al. Cholelithiasis in patients treated with glucagon-like peptide-1 receptor: an updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2020;161:108087.
- Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12(12):1347–1352.
- Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719–1730.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated co-morbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained- release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029.
- Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315(22):2424–2434.
- Moon S, Lee J, Chung HS, et al. Efficacy and safety of the new appetite suppressant, liraglutide: a meta-analysis of randomized controlled trials. Endocrinol Metab. 2021;36(3):647–660.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet. 2022;399(10321):259–269.
- Lau DCW, Erichsen L, Francisco AM, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398(10317):2160–2172.
- Enebo LB, Berthelsen KK, Kankam M, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397(10286):1736–1748.
- Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515.
- Sánchez-Garrido MA, Brandt SJ, Clemmensen C, et al. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60(10):1851–1861.
- Hope DCD, Vincent ML, Tan TMM. Striking the balance: GLP-1/glucagon co-agonism as a treatment strategy for obesity. Front Endocrinol. 2021;12:735019.
- Bossart M, Wagner M, Elvert R, et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab. 2022;34(1):59–74.e10.
- Wharton S, Davies M, Dicker D, et al. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. 2021;134(1):14–19.
- Rybelsus (semaglutide) prescribing information. Novo Nordisk A/S. 2021.
- American Diabetes Association Professional Practice Committee. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Suppl 1):S113–S124. 8.
- Viljoen A, Hoxer CS, Johansen P, et al. Evaluation of the long‐term cost‐effectiveness of once‐weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611.
- Wilkinson L, Hunt B, Johansen P, et al. Cost of achieving HbA1c treatment targets and weight loss responses with once-weekly semaglutide versus dulaglutide in the United States. Diabetes Therapy. 2018;9(3):951–961.
- Lee M, Lauren BN, Zhan T, et al. The cost‐effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obes Sci Pract. 2020;6(2):162–170.
- Lepsen EW, Zhang J, Thomsen HS, et al. Patients with obesity caused by melanocortin-4 receptor mutations can be treated with a glucagon-like peptide-1 receptor agonist. Cell Metab. 2018;28(1):23–32.e3.
- Newsome MB, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonlcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124.
- Mantovani A, Petracca G, Beatrice G, et al. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites. 2021;11(2):73.
- Zhang S, Manne S, Lin J, et al. Characteristics of patients potentially eligible for pharmacotherapy for weight loss in primary care practice in the United States. Obes Sci Pract. 2016;2(2):104–114.
- Curfman GD, Morrissey S, Drazen JM. Sibutramine — another Flawed Diet Pill. N Engl J Med. 2010;363(10):972–974.
- Mahase E. Weight loss pill praised as “holy grail” is withdrawn from US market over cancer link. BMJ. 2020;368:m705.
- Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic Glycemic Management of Type 2 Diabetes in Adults: 2020 Update. Can J Diabetes. 2020;44(7):575–591.
- American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Suppl 1):S125–S143. 9.
- Chumakova-Orin M, Vanetta C, Moris DP, et al. Diabetes remission after bariatric surgery. World J Diabetes. 2021;12(7):1093–1101.
- Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diab Endocrinol. 2019;7(5):344–355.