ABSTRACT
Background
Several studies have reported an association between perinatal complications and the severity of the hypertensive disease. The increasing number of pregnancies complicated by hypertension and the small assurance about the perinatal effects of hypertensive drug use during pregnancy involves the need of studying the better management of hypertensive mothers.
Objective
To evaluate the association between maternal use of antihypertensive drugs and maternal and neonatal outcomes in women with chronic hypertension.
Study design
We conducted a population-based study including all deliveries of hypertensive women that occurred between 2007–2017 in the Lombardy region, Italy. We evaluated the risk of several maternal and neonatal outcomes among women who filled antihypertensive prescriptions within the 20th week of gestation. Propensity score stratification was used to account for key potential confounders.
Results
Out of 5,553 pregnancies, 2,138 were exposed to antihypertensive treatment. With respect to no-users, users of antihypertensive drugs showed an increased risk of preeclampsia (RR:1.68, 95%CI:1.42–1.99), low birth weight (1.30,1.14–1.48), and preterm birth (1.25,1.11–1.42). These results were consistent in a range of sensitivity and subgroup analyses.
Conclusion
Early exposure to antihypertensive drugs in the first 20 weeks of gestation was associated with an increased risk of preeclampsia, low birth weight, and preterm birth.
1. Introduction
Hypertensive disorders are the most common medical complication of pregnancy and represent one of the major causes of maternal and perinatal morbidity and mortality worldwide [Citation1–8]. Based on different diagnostic and therapeutic factors, hypertensive disorders of pregnancy (HDPs) should be classified as preexisting or gestational hypertension and pre-eclampsia [Citation3]. According to population-based studies, complications from gestational hypertension without proteinuria affect 10–16% of pregnancies, while pre-eclampsia regards 1–2% of them [Citation9]. Due to the increasingly postponing of maternity, HDPs are expected to increase in the following years [Citation3,Citation5].
During pregnancy, significant changes in the cardiovascular system occur due to increased maternal and fetal metabolic requirements. Blood pressure usually falls during the early stages of gestation, to then increases during the third trimester to a level equal to or even higher than those before pregnancy. High blood pressure alone does not much affect pregnancy outcome, but rises in blood pressure may be associated with other complications. Of these, the most common is pre-eclampsia [Citation10]. Previous studies have reported an association between maternal complications and severity of hypertensive disease (categorized as either low risk or high risk depending on the systolic and diastolic blood pressure readings and organ involvement), as well as an increased risk of preterm birth and low birth weight [Citation8,Citation11,Citation12]. Seely et al. reported an increased rate of preterm delivery ranging from 12% to 34% among women with chronic hypertension, these rates increase from 62% to 70% in women with severe hypertension [Citation11].
In this setting, antihypertensive drugs are used to treat chronic hypertension, to prolong pregnancy for as long as safely possible, thereby maximizing the gestational age, but also taking into account the need of minimizing fetal exposure to medications that may have adverse effects. Unfortunately, however, several antihypertensive drugs are contraindicated for use during pregnancy, while little evidence is still available of the effect on the fetus of almost all the other medicaments, so the choice of antihypertensive therapy during pregnancy is complex [Citation13].
The increasing number of pregnancies complicated by hypertension and the small assurance about the maternal and perinatal effects of hypertensive drugs involves the need of studying the better management of hypertensive mothers and relative babies. We conducted an observational population-based cohort study, aimed to investigate the association between the use of antihypertensive therapy during pregnancy and maternal and newborn outcomes in women with chronic hypertension.
2. Material and methods
2.1. Setting
The data used for the present study were retrieved from the healthcare utilization (HCU) database of Lombardy, a Region of Italy that accounts for about 16% (almost 10 million) of the total population, already described elsewhere [Citation14–16]. Briefly, the HCU data include a variety of information on residents, such as diagnosis at discharge from public or private hospitals, outpatient drug prescriptions, reasons for exemption from healthcare service co-payment, specialist visits and diagnostic exams provided fully or part free-of-charge by the National Health Service. In addition, a database reporting the certificates of delivery assistance (i.e. the so-called CeDAP) which provides detailed information on pregnancy, childbirth, and child presentation at delivery is consistently managed. These various types of data can be interconnected since a single individual identification code is used by all databases for each citizen enrolled. To preserve privacy, each identification code was automatically anonymized, the inverse process is only allowed to the Regional Authority upon request of judicial Authorities.
2.2. Cohort selection
Criteria for selecting the study cohort almost completely overlap with those previously reported by our group [Citation14–16]. Briefly, using the CeDAP database, we identified deliveries that occurred in Lombardy between 2007 and 2017 from women who (i) were beneficiaries of NHS and residents in Lombardy from at least two years before the last menstrual period date (LMP), (ii) were aged 12 to 55 years at delivery, (iii) had 28 to 46 weeks of gestation, based on LMP, and (iv) suffered from hypertension (defined as a systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg, or both [Citation17]), consistently with at least two distinct prescriptions of blood-pressure-lowering drugs, and/or a diagnosis for hypertension, and/or an exemption from healthcare co-payment for hypertension in the period ranging from one and two years before LMP, a time window we appointed as pre-pregnancy period (supplementary Table S1, Figure S1). We also excluded mothers who did not have a hospital admission ICD‐9 code for deliveries, and who had birth to two or more babies or stillbirths. Finally, pregnancies were excluded whether information on Apgar score and/or the weight at birth were missing (). The final study population, therefore, consisted of 5,553 deliveries of hypertensive women.
2.3. Antihypertensive medication during pregnancy
We identified all prescriptions of antihypertensive drugs dispensed to the included women during the first 20 weeks of gestation. Women were considered users whether at least an antihypertensive prescription was withdrawn within the twentieth week of gestation, no-users otherwise (Supplementary Figure S1).
2.4. Maternal and neonatal outcomes
Maternal outcomes of interest were inpatient diagnoses of exacerbation of hypertension and pre-eclampsia (i.e. gestational hypertension with proteinuria or end-organ manifestation consistent with pre-eclampsia), both diagnosed at equal to or greater than the 20th week of gestation [Citation18]. Diagnostic codes used for the above outcomes are given in supplementary material (Table S2).
Neonatal outcomes diagnosed at presentation were identified from the CeDAP database. The outcomes of interest were low 5-min Apgar score (less than 7 [Citation19]), low birth weight (less than 2,500 grams [Citation20]), and preterm birth (less than 37 gestation weeks [Citation21]).
2.5. Maternal covariates
Supplementary information was obtained from the CeDAP database, the inpatient hospital discharges registry, and the outpatient drug prescriptions registry. Maternal covariates and healthcare utilization were measured during the pre-pregnancy period, while concomitant medication was measured from the year before the LMP to the 20th gestational week. We collected information on (i) socio-demographic features, such as age at delivery, parity, nationality, marital status, employment, and educational attainment; (ii) clinical history of diabetes mellitus, hypothyroidism, renal dysfunction, dyslipidemia, obesity, migraine and/or headache, cardiovascular diseases (e.g. arrhythmia, and heart attack), neurologic and mental health disorders (i.e. depression, epilepsy, stress, anxiety and substance abuse like smoke, alcohol, and drugs); (iii) diagnosis of gestational hypertension, gestational diabetes and pre-eclampsia occurred in previous pregnancies; (iv) concomitant medication (i.e. antiplatelet, antipsychotics and NSAIDs), and (v) use of healthcare services (i.e. counting the number of distinct hospital admission and the number of different drugs prescription excluded the antihypertensive one).
2.6. Statistical analyses
Users and no-users of antihypertensive medications were compared according to the standardized mean difference of their maternal covariates.
Log-binomial regression models were fitted to estimate the relative risk (RR) and the 95% confidence interval (95% CI) of maternal and neonatal outcomes associated with exposure to antihypertensive drugs. Use of the robust variance estimator to account for correlations within women with more than one pregnancy in the observed period did not change the confidence intervals considerably in the unadjusted analyses, so correlation structures were omitted from all analyses. Other than the unadjusted (raw) estimate, adjusted RRs were obtained by the propensity score stratification approach [Citation15]. Propensity scores (PS), i.e. the individualized probabilities of receiving antihypertensive medication during pregnancy conditional on a set of maternal features, were generated using a logistic regression model including the above-reported covariates as predictors and antihypertensive use as the outcome. Fifteen equally sized strata were then created according to PS values of antihypertensive users so that each no-user should be assigned to the appropriate stratum according to its PS value, so making similar the distribution of observed characteristics between users and no users of antihypertensive [Citation22]. According to the commonly accepted rule, standardized differences lower than 10% were considered as suggestive of to be reached good between-group balance [Citation23,Citation24]. Finally, weighted regression models were used to derive the adjusted exposure effect [Citation3].
Because it has been reported that hypertensive disorders of pregnancy affect the considered neonatal outcomes [Citation11,Citation12,Citation25–27], and because indications for using antihypertensive drugs may be affected by these maternal complications, we conducted subgroup analyses restricted to women without such complications.
2.7. Sensitivity analyses
The robustness of estimates was addressed by the following five sensitivity analyses. First, an alternative criterion for classifying women as users and no-users was adopted. According to the new criterion, we redefined exposure as having at least one day of supply that overlaps with the exposure window of interest (i.e. the first twenty weeks of gestation). Second, the potential bias introduced by residual confounding was checked by including into the model socio-demographic characteristics, other than with medical conditions only as in the main analysis. As socio-demographic features were sometimes missing for some women, the analysis was restricted to the 5,239 women with complete information. Third, as continuous treatment with antihypertensive drugs might be not indicated for all the included women (we remember that in the main analysis only two sporadic prescriptions might be dispensed in the pre-pregnancy period for classifying a woman as hypertensive) we restricted the analysis to the 858 women who had a long history of antihypertensive treatment and high adherence with the considered medicaments. By considering a temporal horizon of two years before the LMP (i.e. a back time window with 730 days of length), we divided the cumulative number of days in which the antihypertensive medication was available on the days of observation, i.e. 730 days, a measure known as ‘proportion of days covered’ (PDC) [Citation28]. The duration of each prescription was calculated by dividing the total amount of the drug prescribed by the defined daily dose. For overlapping prescriptions, the woman was assumed to have used all the drugs included in the former prescription before starting the second one. Only women who experienced a PDC > 70% were included in this secondary analysis. Fourth, we assessed the pattern of utilization of the recommended (i.e. antiadrenergic agents, beta-blocking agents, and calcium channel blockers) and the unrecommended drugs before and during pregnancy; and we performed a subgroup analysis for specific classes of antihypertensive medications. The reference group was always composed of women not exposed to any antihypertensive during the first 20 weeks of gestation. Finally, the rule-out approach was applied to investigate the potential bias associated with unmeasured confounders, detecting the extension of the confounding required to fully account for the exposure-outcome association [Citation29]. We set the possible generic unmeasured confounder: (i) to have a 30% prevalence in the study population; (ii) to increase the risk of maternal and neonatal outcomes up to 10-fold more in women who were exposed than in those unexposed to the confounder, and (iii) to be up to 10-fold more common in users than no-users of antihypertensive drugs.
All analyses were performed using the Statistical Analysis System Software (version 9.4; SAS Institute, Cary, NC, USA). Statistical significance was set at the 0.05 level.
3. Results
Among the 5,553 deliveries that met the inclusion criteria, 2,138 women (38.5%) were classified as users of antihypertensive drugs during the first 20 weeks of gestation. Of those, 116 (5.4%) started antihypertensive medication during the first 20 weeks of gestation (supplementary Table S3).
3.1. Baseline features
compares maternal characteristics of users and no-users of antihypertensive drugs during the first 20 weeks of gestation. With respect to no-users, users were slightly younger and more often received drug prescriptions as a whole, specific medications such as antiplatelet and NSAIDs, but less often experienced hospital admissions. Additionally, users had more often foreign nationality and higher education (supplementary Table S4). A good between-group balance of the considered covariates was reached after accounting for the PS ( and Supplementary Table S4).
Table 1. Selected cohort characteristics of women among the exposure groups. Lombardy Region, Italy, 2007–2017.
3.2. Maternal and neonatal outcomes
Exacerbation of hypertension and preeclampsia respectively occurred in 7.8% against 7.6%, and 11.7% against 6.3% of women who used and those who did not use antihypertensive (supplementary Table S5). Low Apgar score, low birth weight, and preterm birth respectively occurred in 0.7% against 0.5%, 15.9% against 10.9%, and 17.6% against 11.9% of newborns from women who used and those who did not use antihypertensive (supplementary Table S6).
3.3. Association between antihypertensive use and maternal and neonatal outcomes
Unadjusted and adjusted estimates of relative risks are shown in . There was statistical evidence that, with respect to no-users, users of antihypertensive drugs had a higher risk of preeclampsia, low birth weight, and preterm birth with increased relative risks ranging from 25% (95% CI: 11% – 42%, preterm birth) to 68% (42% – 99%, preeclampsia). There was no evidence that antihypertensive use was associated with exacerbation of hypertension and low Apgar score. These neonatal findings were confirmed by restricting the analysis to women without evidence of hypertensive disorders of pregnancy ().
Figure 2. Forest plot of the risk ratio for the association between antihypertensive treatment and maternal and neonatal outcomes, unadjusted and propensity score stratified adjusted analyses. Lombardy Region, Italy, 2007–2017.
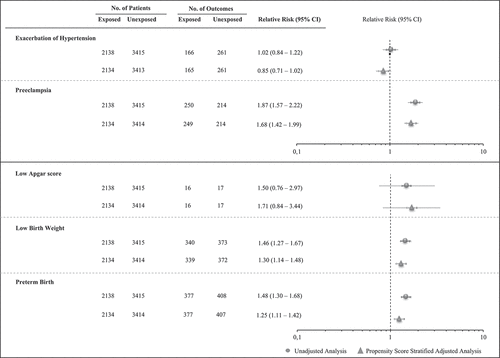
Table 2. Adjusted relative risk of neonatal outcome among women without pregnancy hypertensive disorders. Lombardy, Italy, 2007–2017 (N = 4689).
3.4. Sensitivity analyses
Results were substantially confirmed (i) by changing criteria for classifying women in users and no-users, and (ii) by adjusting estimates for socio-economic features in the portion of the sample with complete data (supplementary Figure S2).
The relationship between the use of antihypertensive drugs and the increased risk of preeclampsia was confirmed by restricting the analysis to women with a history of long-term adherence with the considered medicaments (supplementary Figure S3).
Most of the pregnancies (72%) were exposed to recommended drugs compared to the 14% exposed to not recommended, and 14% of pregnancies were exposed to both recommended and not recommended drugs during the first 20 weeks of gestation. The most common antihypertensive drugs used were calcium channel blockers, followed by beta-adrenergic blocking agents, ACE inhibitors, and ARBs (Supplementary material Table S7, Table S8, and Table S9). shows the adjusted relative risk of maternal and neonatal outcomes according to the recommended class of antihypertensive medication (i.e. antiadrenergic agents, beta-blocking agents, and calcium channel blockers) used during the first 20 weeks of gestation. It seems that the beta-blocking agents have a less negative impact on both maternal and neonatal outcomes (). Differently, among antiadrenergic agents and calcium channel blockers classes were confirmed the main results ().
Table 3. Adjusted relative risk of maternal and neonatal outcome among recommended drug classes. Lombardy, Italy, 2007–2017.
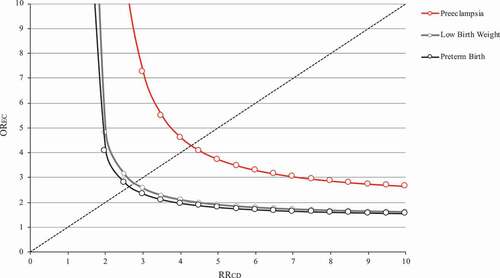
Finally, the results obtained by applying the rule-out approach are shown in . Assuming that the confounder prevalence in the study population was 30% (e.g. that less than one-third of the included women suffered from severe hypertension) and that women who used antihypertensive drugs had a three-fold higher odds of exposure to confounder than those who did not use antihypertensive drugs (i.e. women suffering severe hypertension kept antihypertensive drug therapy three-fold more than women with less severe hypertension), the analysis shows that exposure to the confounder (e.g. severe hypertension) should increase the outcome risk of 7-fold, 2.5-fold to nullify the observed harmful drug effect on preeclampsia, and low birth weight and preterm birth, respectively.
Figure 3. Influence of an unmeasured confounder on the relationship between use of antihypertensive treatment during the first 20 weeks of gestation, with respect to no-use, and the risk of preeclampsia, low birth weight, and preterm birth. The graph indicates the combinations of confounder‐outcome (RRCD) and exposure‐confounder (OREC) associations that would be required to move the observed effect of antihypertensive treatment toward the null. Lombardy Region, Italy, 2007–2017.
4. Discussion
Our population-based study offers evidence that among hypertensive women, those who kept antihypertensive drug therapy in the first 20 weeks of gestation had an excess risk of 68% (95% CI: 42% – 99%) of preeclampsia, and 30% (14% – 48%) and 25% (11% – 42%) of low birth weight and preterm birth, respectively. These results were confirmed in subgroup analyses restricted to women without hypertensive disorders of pregnancy, as well as in sensitivity analyses accounting for potential biases.
Previous studies that investigated the maternal and fetal morbidity following exposure to antihypertensive drugs during pregnancy in women with chronic hypertension reported inconsistent results. Our results confirm, with more exhaustive analyses, the findings of Sibai et al. which also concluded that treatment of maternal blood pressure in mild chronic hypertension during pregnancy did not improve perinatal outcome [Citation30]. In contrast, Rezk et al. has reported a significant positive association between cessation of antihypertensive drug use in patients with mild to moderate chronic hypertension and both maternal and fetal morbidity [Citation31].
There is currently a great deal of uncertainty about the advisability of continuing antihypertensive drug treatment in hypertensive women during pregnancy. A recent Cochrane systematic review did not clarify whether antihypertensive drug therapy for mild to moderate hypertension during pregnancy is worthwhile [Citation32]. On the other hand, the effect of less-tight versus tight control of hypertension on pregnancy complications was not entirely clarified from the so-called CHIPS (Control of Hypertension in Pregnancy Study) trial [Citation33]. Consequently, the American Society for Maternal-Fetal Medicine is awaiting the results of the Chronic Hypertension and Pregnancy (CHAP) Project due in 2022 at the earliest (ClinicalTrials.gov Identifier: NCT02299414) before advising on the treatment of chronic hypertension.
The main weakness of our study is that we do not know the clinical profile of women who kept and those who discontinued antihypertensive drug therapy during pregnancy, but we can only speculate on this issue. Because any effort for making comparable users and no-users was made in our study (e.g. by using a stratified PS design), we might be tried to affirm that users and no-users did not substantially differ for factors influencing the considered outcomes. If this was true, we must understand that the observed relationship between antihypertensive drug therapy and maternal/fetal outcomes should be directly due to drug action. This reading is consistent with the concern that antihypertensive treatment might decrease uteroplacental perfusion and fetal nutrition, leading to adverse fetal and newborn outcomes [Citation34]. On the other hand, we cannot exclude that residual confounder may be biased our findings. For example, women who kept antihypertensive drug therapy during pregnancy were likely affected by more severe hypertension than those who discontinued therapy, and despite our efforts, we did not reach a complete between-group balancing. As severe hypertension is a recognized risk factor for adverse maternal/fetal outcomes [Citation35,Citation36], if this was true, we must understand that the observed drug → outcome relationship should be due to the underlying severity of hypertension, and/or to other unmeasured confounders, rather than to a direct drug action. We must consider this hypothesis given that even a slight imbalance between groups in the severity of hypertension is sufficient to nullify the observed relationship for low birth weight and preterm birth (please see rule-out analysis, ). This reading would lead to infer that antihypertensive drug therapy is insufficient for annulling the effects of hypertension on maternal/fetal outcomes.
A recent bulletin published by the American Congress of Obstetricians and Gynecologists recommended that ‘women with chronic hypertension should be evaluated before conception to ascertain possible end-organ involvement’ [Citation37]. Indeed, a reduction in the risk of complications of pregnancy outcomes in women with chronic hypertension would be reached by optimizing the treatment based on an individual basis according to also the mother’s comorbidity and/or by reassurance on the continuation of drugs that are safe in pregnancy in a preconception consultation in severe hypertension (sBP of ≥160 mm Hg or dBP of ≥110 mm Hg). However, all guidelines agree on the definition and need of medical management, the need for more frequent antenatal care, and fetal surveillance, but no agreement has been still spotted regarding optimal treatment blood pressure target and need for treating mild-to-moderate hypertension [Citation17,Citation38].
The effectiveness of antihypertensive drug therapy during pregnancy has to be balanced with the risk of maternal and fetal adverse events, however. Accordingly, antihypertensive therapy during pregnancy should be managed with drugs including methyldopa, nifedipine, and labetalol [Citation1,Citation3,Citation5,Citation39,Citation40]. Conversely, angiotensin-converting enzyme (ACE) inhibitors and recalls of angiotensin II receptor blockers (ARBs) are strictly contraindicated during pregnancy and lactation due to adverse fetal and neonatal outcomes [Citation1,Citation3,Citation5]. Risk-benefit balance must be considered still uncertain for diuretics and some beta-blockers such as atenolol [Citation1,Citation5]. The pattern of antihypertensive utilization during the first 20 weeks of pregnancy appears to be in line with what is recommended [Citation39]. In our study, 72% of women were treated solely with recommended drugs, 14% with not recommended drugs, and 14% with both recommended and not recommended. The most common antihypertensive drugs used were calcium channel blockers, beta-adrenergic blocking agents, followed by ACE inhibitors and ARBs (Supplementary material Table S7, Table S8, and Table S9).
A strength of our study was the use of administrative data and CeDAP registry of Lombardy, which allowed us to provide a very large population-based cohort, with sociodemographic characteristics, clinical information, and drug utilization, and to test the robustness of our findings in several sensitivity and subgroup analyses. Since healthcare utilization databases are collected for administrative purposes instead of scientific investigations, certain biases, such as nonresponse bias, recall bias, and bias from losses to follow-up, are implicitly avoided.
However, our study had also some important limitations. First, the exclusion of mother-newborn pairs lacking identification codes could mainly affect less healthy women. Second, the implicit exclusion of spontaneous and elective pregnancy termination led to the selective exclusion of outcomes, potentially due to medicine fetal exposure. Third, exposure misclassification might affect our findings. For example, a filled prescription does not necessarily imply that the medication was taken since actual consumption of medication could not be verified. However, given that we did observe an association for early exposure, misclassification of the exposure (i.e. false positives) is not a major concern. Finally, although our estimates were adjusted for several maternal traits, including demographic, social, medical, and therapeutic features, residual confounding cannot be excluded. Indeed, confounding variables are based on inpatient information only. Less severe comorbid conditions, that do not result in hospitalization or are not recorded as one of the patient diagnoses in hospitalizations for delivery or other medical problems, are therefore missed. Moreover, lifestyle factors (e.g. smoking, alcohol use, obesity) are known to be under-recorded in administrative databases.
5. Conclusion
Our data on antihypertensive drug utilization patterns in a real-world setting offer evidence that exposure to antihypertensive agents during the first 20 gestational weeks increases the risk of several maternal and neonatal outcomes. Our study supports the importance of increased antenatal surveillance and lifestyle for women with chronic hypertension to reduce maternal risks and achieve optimal perinatal survival. This objective can be achieved by an adequate preconception evaluation and counseling, early antenatal care, frequent antepartum visits to monitor both maternal and fetal well-being, timely delivery with intensive intrapartum monitoring, and a good education about nutritional requirements, weight gain, and sodium intake (maximum of 2.4 g of sodium per day) [Citation38,Citation41]. Thus, pregnancy in women with chronic hypertension needs to be carefully planned and managed to define the level of risk (low or high) in early pregnancy and to decide whether to initiate, continue or discontinue antihypertensive treatment during pregnancy. Nowadays, no agreement has been spotted regarding optimal treatment blood pressure target and the need for treating mild-to-moderate hypertension.
Declaration of interest
G Corrao received research support from the European Community (EC), the Italian Agency of Drug (AIFA), and the Italian Ministry for University and Research (MIUR). He took part in a variety of projects that were funded by pharmaceutical companies (i.e. Novartis, GSK, Roche, AMGEN and BMS). He also received honoraria as member of the Advisory Board from Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conceptualization: A Cantarutti and G Corrao; methodology, A Cantarutti, G Porcu and G Corrao; validation, A Cantarutti and G Corrao; formal analysis, G Porcu; writing—original draft preparation, A Cantarutti, G Porcu, A Locatelli and G Corrao; supervision, A Locatelli and G Corrao. All authors have read and agreed to the published version of the manuscript.
Ethical approval
According to the rules from the Italian Medicines Agency (available at: http://www.agenziafarmaco.gov.it/sites/default/files/det_20marzo2008.pdf) retrospective studies without direct contact with patients do not need written consent to process personal data when they are used for research aims.
Supplemental Material
Download MS Word (201.4 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17512433.2022.2072292
Additional information
Funding
References
- Borghi C, Ferri C, Sechi L. Italian society of hypertension. Clinical management of hypertension in pregnancy. Practical recommendations from the Italian Society of Hypertension (SIIA). High Blood Press Cardiovasc Prev. 2013 Sep;20(3):249. [corrected] [published correction appears in High Blood Press Cardiovasc Prev. 2013 Sep;20(3):249].
- Nzelu D, Dumitrascu-Biris D, Hunt KF, et al. Pregnancy outcomes in women with previous gestational hypertension: a cohort study to guide counselling and management. Pregnancy Hypertens. 2018;12:194–200.
- Al Khaja KA, Sequeira RP, Alkhaja AK, et al. Drug treatment of hypertension in pregnancy: a critical review of adult guideline recommendations. J Hypertens. 2014;32(3):454–463.
- Hitti J, Sienas L, Walker S, et al. Contribution of hypertension to severe maternal morbidity. Am J Obstet Gynecol. 2018;219:405.e1–7.
- Bortolotto MR, Francisco RPV, Zugaib M. Resistant hypertension in pregnancy: how to manage? Curr Hypertens Rep. 2018;20(8):63.
- Lodi E, Carollo A, Martinotti V, et al. Hypertension and pharmacological therapy in women. High Blood Press Cardiovasc Prev. 2018;25(2018):147–150.
- Magee LA, Pels A, Helewa M, et al. Canadian Hypertensive Disorders of Pregnancy (HDP) working group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4(2):105–145.
- Macdonald-Wallis C, Lawlor DA, Fraser A, et al. Blood pressure change in normotensive, gestational hypertensive, preeclamptic, and essential hypertensive pregnancies. Hypertension. 2012;59(6):1241–1248.
- Allen VM, Joseph K, Murphy KE, et al. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004;4:17.
- Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2006;3:CD001449. DOI:10.1002/14651858.CD001449.pub2
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254–1261.
- Macdonald-Wallis C, Tilling K, Fraser A, et al. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension. 2014;64(1):36–44.
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51(4):960–969.
- Cantarutti A, Franchi M, Rea F, et al. Use of nimesulide during early pregnancy and the risk of congenital malformations: a Population-based study from Italy. Adv Ther. 2018;35:981–992.
- Cantarutti A, Merlino L, Giaquinto C, et al. Use of antidepressant medication in pregnancy and adverse neonatal outcomes: a population-based investigation. Pharmacoepidemiol Drug Saf. 2017;26:1100–1108.
- Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
- Scott G, Gillon TE, Pels A, et al. Guidelines-similarities and dissimilarities: a systematic review of international clinical practice guidelines for pregnancy hypertension. Am J Obstet Gynecol. 2020;226(2S):S0002-9378(20)30846–2.
- Magee LA, von Dadelszen P. State-of-the-art diagnosis and treatment of hypertension in pregnancy. Mayo Clin Proc. 2018;93(11):1664‐77.
- Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344:467–471.
- Valero de Bernabé J, Soriano T, Albaladejo R, et al. Risk factor for low birth weight: a review. Eur J Obstet Gynecol. 2004;116:3–15.
- Lawn JE, Gravett MG, Nunes TM, et al. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10:S1.
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679.
- Desai RJ, Rothman KJ, Bateman BT, et al. A propensity‐score‐based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249‐57.
- Xu X, Wang Y, Xu H, et al. Association between proteinuria and maternal and neonatal outcomes in pre-eclampsia pregnancy: a retrospective observational study. J Int Med Res. 2020;48(4):300060520908114.
- Tesfalul M, Sperling J, Blat C, et al. Adverse perinatal outcomes associated with elevated blood pressure and stage 1 hypertension. AJOG. 2020;1(Supplement):S92–3.
- Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. discussion 575-577.
- Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- Sibai BM, Mabie WC, Shamsa F, et al. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162(4):960–966.
- Rezk M, Ellakwa H, Gamal A, et al. Maternal and fetal morbidity following discontinuation of antihypertensive drugs in mild to moderate chronic hypertension: a 4-year observational study. Pregnancy Hypertens. 2016;6(4):291–294.
- Abalos E, Duley L, Steyn DW, et al. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007;1:CD002252.
- Magee LA, von Dadelszen P, Singer J, et al. CHIPS Study Group. The CHIPS randomized controlled trial (Control of Hypertension in Pregnancy Study): is severe hypertension just an elevated blood pressure? Hypertension. 2016;68(5):1153–1159.
- Von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated meta regression analysis. J Obstet Gynaecol Can. 2002;24(12):941–945.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 125: chronic hypertension in pregnancy. Obstet Gynecol. 2012;119:396–407.
- Su CY, Lin HC, Cheng HC, et al. Pregnancy outcomes of anti-hypertensives for women with chronic hypertension: a population-based study. PloS One. 2013;8:e53844.
- Hypertension in pregnancy. Report of the American College of obstetricians and gynecologists’ task force on hypertension in pregnancy. American College of Obstetricians and Gynecologists. Task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Tsakiridis I, Giouleka S, Arvanitaki A, et al. Chronic hypertension in pregnancy: synthesis of influential guidelines. J Perinat Med. 2021;49(7):859–872.
- Abalos E, Duley L, Steyn D, et al. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10:CD002252.
- National Institute for Health and Care Excellence (UK). Hypertension in pregnancy: diagnosis and management. NICE guideline [NG133]. London: NICE. cited 2019 Jun 25. https://www.nice.org.uk/guidance/ng133] (ultimo accesso 27/07/2020
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002 Aug;100(2):369–377.

