ABSTRACT
Introduction
Childhood asthma is a complex heterogenous inflammatory disease that can pose a large burden on patients and their caregivers. There is a strong need to adapt asthma treatment to the individual patient taking into account underlying inflammatory profiles, moving from a ‘one size fits all’ approach toward a much-needed personalized approach.
Areas covered
This review article aims to provide an overview of recent advances in the management and treatment of pediatric asthma, including novel insights on the molecular heterogeneity of childhood asthma, the emergence of biologicals to treat severe asthma, and innovative e-health and home monitoring techniques to make asthma management more convenient and accessible.
Expert opinion
Molecular technologies have provided new treatment leads. E-health and home monitoring technologies have helped to gain more insights into disease dynamics and improve adherence to treatment while bringing health care to the patient. However, uncontrolled childhood asthma is still a major unmet clinical need and precision-medicine approaches are still scarce in clinical practice. Advanced omics methods may help researchers or clinicians to more accurately phenotype and treat subtypes of childhood asthma and gain more insight into the complexity of the disease.
1. Introduction
Asthma is one of the most common chronic respiratory diseases. In 2019, an estimated 262 million individuals were affected by asthma, and 461,000 people died as a result of the disease [Citation1]. The prevalence of asthma in European children has been estimated between 5% to over 20% [Citation2].
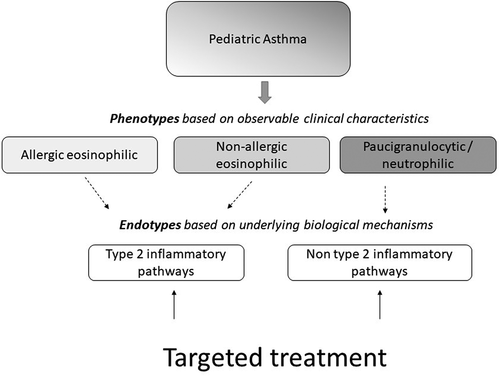
The presence of airway wall inflammation is a key pathophysiological mechanism and activated immune cells and their mediators can be found in the airways of asthma patients, yet different inflammatory patterns have been recognized [Citation3,Citation4]. A common subclassification is based on the presence or absence of Type 2 inflammation in the blood and/or airways. Type 2 inflammatory patterns seem to be most common in childhood asthma and are characterized by type 2 mediators produced by Thelper2 (Th2) and/or innate lymphoid cells type 2 (ILC2s). This may result in infiltration of activated eosinophils [Citation5]. In contrast, non-type 2 inflammatory asthma is characterized by the lack of Type 2 inflammatory mediators or airway eosinophils [Citation6].
Asthma is a heterogeneous inflammatory disorder of the airways that can have a large impact on the quality of life of patients and families. The disease is characterized by variable airflow limitation and hyperresponsiveness of the airways. Reversible airway obstruction is the most common asthma feature [Citation7]. In children with asthma, another pathological mechanism may play a role: remodeling of the airways through hyperplasia in the airway’s smooth muscles, excessive collagen deposition in subepithelial and goblet cell metaplasia [Citation8,Citation9]. The exact relation between airway inflammation and airway remodeling remains unclear [Citation10]. Although airway inflammation is thought to drive airway remodeling, these structural changes may also occur due to other processes. Since these structural changes lead to irreversible lung function loss, reducing airway inflammation and preventing airway remodeling are both important treatment goals. The inflammatory biomarkers are particularly important since novel targeted treatments have been approved to treat severe asthma. For example, biologicals such as mepolizumab and omalizumab specifically target type-2 mediated inflammatory pathways, which may be differentially activated among different patients. However, in childhood asthma detecting the underlying inflammatory status may be challenging since collecting sputum specimens can be difficult in children and performing venipunctures to collect blood can cause distress. In addition, inflammatory phenotypes based on sputum samples seem to be less stable in children compared to adults [Citation11].
The risk of asthma development is influenced by various factors e.g. atopy, the composition of the gut and lung microbiome, genetic variations and environmental factors (such as living environment, air pollution, exposure to cigarette smoke) [Citation12]. All these factors may contribute to the observed heterogeneity of pediatric asthma. Clinical criteria and/or biomarker measurements are used to identify asthma subgroups or phenotypes. Asthma is often treated in a ‘one size fits all’ approach. However, the heterogeneity of the disease supports the idea that phenotyping and individualized therapy could be beneficial in the diagnosis, treatment, and prediction of the disease [Citation5].
This review highlights the latest developments in the management and treatment of childhood asthma, including the shift from the classical stepwise treatment approach to precision medicine approaches. We also highlight new ways to make asthma management more convenient and accessible to patients and their caregivers using e-health and home monitoring techniques.
2. Advances in childhood asthma management
2.1. Literature search
For this narrative review, we performed a literature search in PubMed (including the MEDLINE database) to identify relevant studies on childhood asthma management up to April 2022. There were no time limitations, but we prioritized recent original articles and reviews). Abstracts from conferences, studies written in languages other than English, and papers not conducted in humans were excluded.
2.2. The classical stepwise approach to a childhood asthma treatment
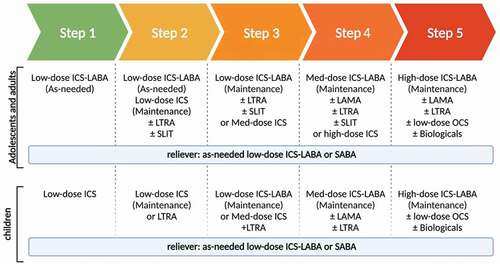
Asthma treatment is generally directed against bronchoconstriction and/or underlying airway inflammation. The main aim of asthma treatment is to obtain asthma control and decrease the risk of asthma exacerbations. Clinical guidelines often follow the yearly updated recommendations of The Global INitiative for Asthma (GINA). The GINA recommends a stepwise (step 1–5) approach to control symptoms and decrease the risk of exacerbations ().
Figure 1. Stepwise approach of asthma treatment based on the GINA 2021 recommendations. ICS: Inhaled corticosteroid; LABA: Long-acting beta-agonists; LTRA: leukotriene receptor antagonists; SLIT: Sublingual immunotherapy; Med: medium; LAMA: Long-acting muscarinic antagonists; SABA: Short-acting beta 2 agonist. Created with BioRender.com.
2.3. Asthma medications’ mechanism of action
The most powerful bronchodilators recommended for therapeutic use in pediatric asthma are β2 agonists. Short-acting β2 agonists are the bronchodilator of choice for asthma acute attacks [Citation13,Citation14]. The main function of the β2 agonists is to relax the smooth muscle of the airways by β2 adrenergic receptor stimulation. This leads to increased levels of cyclic adenosine monophosphate (cAMP), which mediates the control of the smooth muscle tone [Citation15]. Short-acting β2 agonist drugs (SABAs), like salbutamol, provide a 4–6 hour bronchodilator effect. Long-acting beta-agonists (LABAs) such as salmeterol, formoterol, and vilanterol, on the other hand, provide bronchodilator effects for 12–24 hours [Citation16]. Long-acting muscarinic antagonists (LAMAs) like tiotropium are also bronchodilators, but through a different mechanism that involves acetylcholine competition at muscarinic receptor sites. Hence, LAMA agents can directly reduce airway smooth muscle contraction by blocking the acetylcholine function at muscarinic receptors [Citation17,Citation18].
In addition to the bronchodilators to treat bronchoconstriction, anti-inflammatory maintenance treatment is commonly prescribed to treat the underlying chronic airway inflammation. Inhaled corticosteroids (ICSs) such as beclomethasone, fluticasone, budesonide, mometasone, and ciclesonide can bind to intracellular corticosteroid receptors and suppress inflammation both directly and indirectly through activation of anti-inflammatory genes, reducing the number of inflammatory cells like eosinophils, T-lymphocytes, mast cells, and dendritic cells, and turning off the expression of inflammation genes in airways. Furthermore, ICSs may enhance the expression and function of β2 receptors [Citation19–21].
Another class of asthma medicines are the leukotriene receptor antagonists (LTRAs). Zafirlukast and montelukast are two types of LTRAs that antagonize the type 1 cysteinyl leukotriene receptors (CysLT1Rs). These receptors are responsible for cysteinyl leukotrienes’ bronchoconstriction and immune cells’ activation such as Th2, mast cells, and ILC2s. LTRAs can inhibit bronchoconstriction, reduce mucus production, and downregulate the generation of proinflammatory cytokines [Citation22–24].
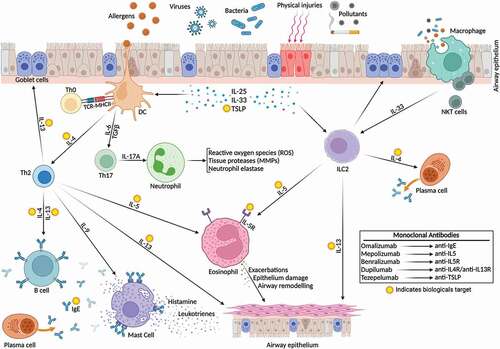
In the past years, biologics have emerged as an add-on medication to treat uncontrolled severe asthmatic children. Different types of biological agents have been studied in these patients, such as anti-IgE (omalizumab), anti-IL-5 (mepolizumab/reslizumab), anti-IL-5R (benralizumab), anti-IL-4R/IL-13 (dupilumab) [Citation13] (). Recently anti-TSLP (tezepelumab) was approved for adults and children aged 12 years and older with severe asthma . Omalizumab (anti-IgE, approved for children ≥6 years) reduces the inflammation related to allergic asthma through inhibition of IgE binding to inflammatory cells (mast cells, dendritic cells, and basophils), and decreasing the number of eosinophils and multiple inflammatory mediators in the airways [Citation25]. Th2 lymphocytes and type 2 innate lymphoid cells (ILC2) are the major producers of IL-5 [Citation26–30]. IL-5 is produced by Th2 lymphocytes when antigen-presenting dendritic cells activate them. Whereas IL-5 is produced by ILC2 cells when activated by epithelium-derived alarmins in the airways [Citation31]. The IL-5 cytokine plays a role in regulating eosinophil differentiation, proliferation, recruitment, activation, life cycle, chemoattraction, and degranulation [Citation32,Citation33]. IL-5 is the main responsible cytokine for cytotoxic proteins production, including eosinophil peroxidase, eosinophil cationic protein, major basic protein, and eosinophil-derived neurotoxin. The epithelial cell layer is harmed by cytotoxic proteins. Hence, increased eosinophil counts can be detected in induced sputum, bronchial specimen, and peripheral blood of asthmatics driven by type-2 inflammation [Citation33–37]. Mepolizumab binds to IL-5 and blocks the IL-5 signaling path. Mepolizumab inhibits eosinophil production and survival, which may prevent severe exacerbations [Citation32]. IL-5 receptor is a membrane protein with two subunits α (IL-5 Rα) and βc chain. IL-5 Rα is selective for IL-5 binding, whilst the βc chain has an ability to bind IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor or GM-CSF [Citation38,Citation39]. Benralizumab is an anti-IL-5Rα monoclonal antibody, it has a selective affinity to α-subunit of the IL-5 receptor by its Fab regions [Citation40,Citation41]. In addition, benralizumab has an affinity to bind to the FcγRIIIa receptor of natural killer (NK) cells [Citation42,Citation43]. As a result, benralizumab is an effective inducer of eosinophil death by two pathways: blocking the IL-5 receptors and binding to the FcγRIIIa receptors of the NK cells [Citation33]. Clinical studies, show that benralizumab decreases the eosinophil level both in the blood and airways of asthma patients [Citation30]. In comparison to other anti-IL-5 agents, benralizumab is able to eliminate eosinophils in blood and sputum more quickly and nearly completely, including eosinophil-lineage committed progenitor cells [Citation44]. It seems to be more effective in severe eosinophil asthma than reslizumab and mepolizumab [Citation45]. Another biological agent approved to treat children with uncontrolled asthma is dupilumab which is directed against IL4Rα. Dupilumab binds to the IL4 receptor and blocks IL4 and IL13 signaling, down-regulating the Th2 inflammation pathway in eosinophilic asthma and allergic diseases [Citation46–48]. GINA 2021 recommended using benralizumab and dupilumab in severe asthmatic children ≥12 years [Citation13]. Recently (12–2021), the FDA approved another biological agent, tezepelumab, for adults and children ≥12 years with severe asthma. Tezepelumab is the first approved medicine that targets thymic stromal lymphopoietin (TSLP), which plays a role in airway inflammation. Furthermore, tezepelumab is also the first approved biological agent that has an indication in all inflammatory types of severe asthma. According to a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase 3 clinical study among 1061 severe uncontrolled asthmatics aged 12–80 years, tezepelumab had a significant decrease in annual asthma exacerbations [Citation49]. Another randomized, double-blind, placebo-controlled clinical study in 584 adults with inadequately controlled severe asthma, also demonstrated the efficacy of tezepelumab in reducing the annual asthma exacerbation rate in the severe asthmatics [Citation50].
Figure 2. Biologics target type 2 airway inflammation in childhood asthma. External triggers (e.g. allergens) trigger the inflammatory cascade in previously sensitized children. Biologics target specific inflammatory compounds to reduce airway inflammation. Th0: naïve T helper cell; Th2: T helper 2; Th17: T helper 17; NKT cells: natural killer T cells; IL: interleukin; ILC2: innate lymphoid cell type 2; B cell: Basophil cell; TSLP: thymic stromal lymphopoietin; IgE: Immunoglobulin E; DC: dendritic cell. Created with BioRender.com.
2.4. Immune modulation therapies to treat allergies
Children often suffer from allergic asthma. Allergens play an important role in triggering symptoms and exacerbations and other allergic diseases (e.g. rhinitis, food allergy, eczema) might co-exist. Allergen immunotherapy might therefore be indicated for certain allergic asthma patients. Allergen immunotherapy is an add-on therapy option for allergic asthmatics aged ≥12 years during GINA step 2–4 treatment regimens, according to GINA version 2021 [Citation13].
Allergen immunotherapy attempts to stimulate allergen-specific regulatory T cells, which have suppressive actions by producing cytokines like IL-10 and TGF-β, as well as using inhibitory surface molecules like CTLA-4 and PD1 [Citation51]. T-cell tolerance, Th2 response reduction, IgE-blocking IgG antibodies induction, and allergen-specific IgG4 induction are the other mechanisms mentioned in the allergen immunotherapy [Citation52]. Different indications are recommended for children for allergen immunotherapy, including asthma, rhinitis, venom, and food allergies. Different routes of administration are available for immunotherapies agents, such as subcutaneous and sublingual (drop and tablet) [Citation51]. House dust mites and grass pollens are the most widely used allergens in the allergy immunotherapy [Citation53]. House dust mites like Dermatophagoides pteronyssinus or Dermatophagoides farinae are the most common indoor allergens linked to asthma, which stimulate the production of allergen-specific IgE [Citation52]. According to UpToDate version 2022 (a clinical-decision resource for physicians), clinical indications for allergen immunotherapy are: 1) the incidence of symptoms following allergen exposure, like the incidence of symptoms after exposure to grass pollen; 2) the presence of specific IgE after allergen tests (allergen skin/serum tests); 3) and at least one of the following conditions: a) poor response to pharmacotherapy, b) allergen avoidance, c) unacceptable adverse drug reactions, d) aim to eliminate long-term pharmacotherapy and medication costs, e) possible prevention of asthma in allergic rhinitis patients [Citation54]. In a Cochrane review study among 88 randomized trials on diverse allergen immunotherapy (dust mite, pollens, animal dander, cladosporium mold, and mixed allergens) in adults and children, Abramson et al. discovered that allergen immunotherapy decreased asthma symptoms and medication use significantly, whereas it improved bronchial hyperreactivity. In addition, subcutaneous immunotherapy has been linked to a decrease in symptom severity and medication prescription in asthmatic patients, as well as improved airway hyperresponsiveness in both allergen-specific and nonspecific types [Citation55]. A randomized study in 65 children aged 6–17 who were in GINA step 2 and 3 asthma treatment showed that subcutaneous immunotherapy against house dust mites for two years significantly decreased the requirement of ICS [Citation56]. Sublingual allergen immunotherapy includes the administration of tablets or drops under the tongue. According to a randomized, double-blind, placebo-controlled trial among 812 children aged 5–12 years who suffered from grass pollen-induced allergic rhinoconjunctivitis without asthma diagnosed at the time of inclusion, grass sublingual allergen immunotherapy (active treatment or placebo for 3 years + 2 years of follow-up without treatment) significantly decreased the risk of future asthma symptoms and asthma medication requirements. Sublingual allergen immunotherapy was found to be effective in preventing the development of asthma both during and after treatment [Citation57]. Severe asthma was an absolute contraindication for allergen immunotherapy in guidelines for many years, but it was revised and now only uncontrolled asthma is an absolute contraindication for allergen immunotherapy, whereas the use of severe controlled asthma has been reduced to a relative contraindication [Citation51]. Although serious adverse drug reactions for subcutaneous immunotherapy are uncommon, life-threatening anaphylactic reactions may appear during or after treatment [Citation58]. Adverse drug reactions reported for the sublingual type are mostly limited to the oral cavity and gastrointestinal tract. Like other treatments, cost-effectiveness should be considered before ordering allergen immunotherapy for the individual patient.
2.5. Triple inhaler therapy for childhood asthma
In 2015 the EMA (European Medicines Agency) and two years later the FDA (US Food and Drug Administration) approved the use of add-on LAMA to treat moderate to severe asthmatic children (≥6 years old) who still have uncontrolled symptoms despite proper ICS and LABA treatment [Citation13]. According to GINA 2021, LAMA can be prescribed as add-on therapy in separate devices for children ≥6 years and in a single device/triple therapy (ICS/LABA + LAMA) for asthmatics ≥18 years if their symptoms cannot be controlled by medium/high ICS-LABA [Citation13]. As addressed previously, LAMAs have a different bronchodilatory action than LABAs [Citation59], and therefore adding the two could have a complementary effect. Although studies in adults showed that triple therapy (ICS/LABA + LAMA) successfully reduced exacerbations and led to a minor improvement in lung function compared to dual therapy in moderate to severe asthmatics, there was no significant association with the quality of life or asthma symptoms status [Citation60]. A post hoc analysis of two parallel randomized, double-blind, active-controlled, multi-central phase 3 trials that compared triple therapy versus dual therapy (TRIMARAN: 1155 participants from 16 countries, and TRIGGER: 1437 participants from 17 countries) discovered that adding a LAMA to the combination of ICS-LABA reduced the exacerbation rate (TRIMARAN: with a 15% decrease in the rate of moderate to severe exacerbation (RR 0 · 85, 95% CI 0 · 73–0 · 99; p = 0 · 033), TRIGGER: with 12% (0 · 88, 0 · 75–1 · 03; p = 0 · 11)) and improved lung function (TRIMARAN: with 57 mL improvement in FEV1 (95% CI 15–99; p = 0 · 0080), TRIGGER: with 73 mL (26–120; p = 0 · 0025) [Citation61].
If triple therapy can control asthma symptoms, patients may avoid using additional systemic medications such as OCSs, which have a higher risk of side effects [Citation62–64], or biological treatment, which can increase healthcare costs and drug adverse reactions [Citation65]. Triple therapy could be a bridge between the therapies currently available in clinics and biologicals, but more research in the pediatric population is needed.
2.6. From one size fits all toward precision medicine approaches
With the rapid advances in molecular technologies, it has become possible to more accurately identify distinct inflammatory asthma profiles. Different asthma phenotypes might respond differently to specific treatment options. By developing personalized medicine strategies, we could select the optimal treatment for different patient groups at an early stage (). As a result, the quality of life of patients and their caregivers will be improved, ineffective treatment and unnecessary adverse drug reactions will be prevented, and also care costs may be reduced. However, so far only a few predictors for asthma treatment response have been established.
2.7. Predictors of ICS responsiveness
Most studies have focused on predictors of ICS response since this is the main building block of asthma management. Eosinophil levels have been reported as predictors for ICS responsiveness [Citation66]. Eosinophilic airway inflammation seems to be more responsive to treatment with inhaled corticosteroids (ICSs) compared to non-type 2 airway inflammation. ICS has a weak effect on the innate immunity-driven neutrophilic inflammation [Citation67]. However, a meta-analysis showed that guiding treatment based on eosinophilic markers (sputum eosinophils and FeNO) had no significant impact on lung function or asthma control in adults or children [Citation68]. On the other hand, monitoring the number of eosinophils in sputum did lead to a decrease in the frequency and severity of asthma exacerbations in adults [Citation69]. Therefore, further investigation is required to determine whether using sputum eosinophils in the management and treatment of childhood asthma is effective.
More recently circulating miRNA’s have been implicated as potential predictors of ICS response in children. Using randomized clinical trial data of 462 moderate to severe asthmatic children on ICS or placebo treatment, Li et al. demonstrated that there was a significant difference in specific circulating serum miRNAs between the group who received ICS and the control group without using ICS. Furthermore, the levels of two functionally validated miRNA at baseline were significantly associated with ICS response (based on lung function outcomes) during the trial (combined AUROC of 0.86), suggesting that circulating miRNA before ICS start might be a biomarker for ICS responsiveness in asthmatic children [Citation70].
2.8. Predictors of LABA responsiveness
When focusing on treatment response, genetics seems to be especially valuable in predicting LABA response in children. A meta-GWAS analysis, including 1425 asthmatics aged 6–21, found 8 separated genetic variants to be suggestively linked to exacerbation rate despite regular LABA intake [Citation71]. Another meta-analysis focusing on the ADRB2 gene (which encodes the β2 adrenergic receptors) found that children carrying a variant ADRB2 gene were more at risk of exacerbations despite LABA use [Citation72]. A genotype-guided randomized clinical trial on 241 asthmatic children aged 12–18 years who took ICS showed that prescribing LABA based on the ADRB2 genotype could significantly improve the quality of life compared to standard care (95%CI 0.02–0.81; p = 0.041) [Citation73].
2.9. Predictors of LAMA responsiveness
Predicting treatment response to LAMA is still a challenge. Sputum and blood eosinophil levels seem to be poor predictors for tiotropium (LAMA) responsiveness in terms of control status, lung function, and first exacerbation incidence [Citation74–77]. High serum IgE level (>430 µg/L) seems to be a better biomarker for poor tiotropium responsiveness in adult asthma patients [Citation76]. However, according to a study among children aged 6–17, total-IgE and blood eosinophil levels did not play a role in predicting LAMA responsiveness [Citation78]. Although some research is done on triple therapy in asthmatic adults and children, GINA has not recommended any predictor criteria that indicate triple therapy responsiveness.
2.10. Predictors of biologics responsiveness
The selection of suitable biological agents due to the patient’s characteristics for severe asthmatics is still challenging, especially in children. GINA recommends using increased blood eosinophil (≥150/μL) and/or sputum eosinophils (≥2%) and/or FeNO (≥20ppb) as cutoff values in severe, uncontrolled adult and adolescent asthmatics to initiate treatment with biologics targeted to type 2 inflammation (15). However, it remains unclear how to define biologics treatment response, and data in pediatrics are scarce ().
Table 1. Potential predictors of biologics response in children.
* currently only approved for the treatment of adults with severe asthma Furthermore, the immune system is still under development in children. This may affect observed inflammatory profiles, including eosinophil levels. A study among 589 asthmatics aged 5–21 years who had at least one exacerbation and had taken systemic corticosteroids used during a year before the inclusion date, investigated that type 2 inflammation features associated with exacerbations may differ by the participants’ age [Citation79]. Higher levels of blood eosinophils were associated with exacerbations in adolescents compared to children.
Some factors may predict good anti-IgE responsiveness in asthmatic children ≥12 years and adults: blood eosinophils ≥260/μL [Citation80,Citation81], FeNO≥20ppb [Citation80], childhood asthma onset, and allergens-driven symptoms. Whereas baseline IgE may not predict anti-IgE responsiveness in asthmatic children ≥12 years and adults [Citation82]. Potential factors that may be used as a predictor for anti-IL-5 or anti-IL-5R agents’ responsiveness in asthmatic children ≥12 years and adults are the number of yearly exacerbations, blood eosinophils, onset of asthma, nasal polyposis, and predicted FEV1 [Citation83–86]. In two post hoc analyses, an blood eosinophil level of 150 cells/μL was defined as a cutoff; severe asthmatics ≥12 years who had blood eosinophil levels over this cutoff showed better mepolizumab responsiveness due to exacerbation rate improvement [Citation86]. Another post hoc study among 477 severe asthmatics aged 12–75 from two phase 3 trials in order to evaluate reslizumab responsiveness found that participants with a lower BMI, greater baseline ACQ, history of nasal polyposis, and greater age of onset were more likely to be super responders [Citation87]. According to a recent study, eosinophil levels can be used as an exacerbation rate predictor in eosinophilic asthmatic patients using benralizumab. However, the level of serum IgE is not [Citation88]. Castro et al. showed that baseline blood eosinophils (≥150/μL or ≥300/μL) and FeNO (≥25ppb or ≥ 50ppb) could be used as predictors of dupilumab responsiveness in moderate to severe uncontrolled asthmatics older than 12 years because the exacerbation rate was significantly different between those who were upper and lower than these cutoffs [Citation89].
2.11. Home administration of biologicals
In order to successfully integrate personalized medicine approaches in clinical practice, it is important that health services are also adapted to the patient’s needs. One of the recent advancements in this area is the development of home administration approaches for biologics use, bringing health care to the patient. Biological asthma treatments consist of monoclonal antibodies and administrated through injections. Hypersensitivity and anaphylaxis are potential serious adverse drug reactions of biological therapy. The evaluation of clinical measures before the start of biological therapy and during treatment is therefore warranted; this limits the administration of these drugs to hospitals with careful monitoring of safety and efficacy. Recently, home administration approaches have become available for these treatments, which may be beneficial for patients that have been successfully treated with these drugs for a longer period already. Self-administration of medications provides some advantages such as patient/caregiver convenience improvement, reduction in healthcare costs, and reduced pressure on the healthcare professionals through decreasing hospital visits. In addition, during the COVID-19 pandemic, it was recommended to reduce unnecessary hospital visits [Citation90]. A study that assessed the safety of biological administration at home for asthmatic children (6–18 years of age) showed that biologicals self-administration, which was supervised remotely and monitored using a spirometer and an electronic monitoring device, is safe and well-received by children and caregivers [Citation91].
2.12. Electronic health (e-Health) and home monitoring
Digital health technologies have been rapidly expanded in the past years and might also help to adapt asthma treatment better to the patient’s needs. One of the major causes of uncontrolled asthma, especially in children, is poor adherence and incorrect inhaler technique during treatment. Different methods that can be used in digital health in pediatric asthma treatment are symptoms and treatment home monitoring, medication time reminder, adherence and inhaler technique tracking by using specific electronic devices, providing education materials like serious games, mobile health applications, interactive websites, physician supportive routs (e.g. telemedicine and text messaging), and analyzing gathered data in order to improve treatments and find new potential biomarkers [Citation92].
For children, serious game approaches might be especially suitable to improve self-management. ‘Asthma control’ is one of the first serious games in which players strive to assist the superhero with taking asthma treatments for his symptoms while also receiving guidance from doctors. This game was used in a randomized case-control clinical among 137 children aged 3–12 years old with physician-diagnosed asthma and demonstrated that all participants enjoyed playing the game and learned more about asthma treatment than the control group. After this game, other serious games are developed in order to educate asthmatic patients, both children and adolescents, such as ‘bronkie’s asthma adventure,’ ‘secret agents,’ ‘wee willie wheezie,’ and ‘asthma files’ [Citation93–96].
Electronic drug monitoring devices might be useful to monitor and improve adherence. These devices collect data that can be used to assess medication adherence and asthma symptoms without bias in patient reports. Smart inhalers are the gold standard devices now available in the market to monitor the date and time of inhalations taken in order to evaluate adherence. Smart inhalers are compatible with many types of inhalers [Citation97]. Tracking medication adherence and providing positive feedback leads to more use of preventive medication [Citation98]. A recent systematic review (including 1123 children from 10 randomized controlled trials) showed that using electronic adherence monitoring devices could improve inhaler adherence but had no significant effect on exacerbations, symptoms, and lung function [Citation99].
Home monitoring of symptoms and biomarkers to better understand disease dynamics has also gained more attention lately. Especially in children, it might be difficult to assess asthma control during scheduled health care visits. Children might find it difficult to express the burden of the disease and they may adapt their behavior to their symptoms. This may influence how they perceive their symptoms and may lead to underreporting of symptoms [Citation100,Citation101]. Longitudinal monitoring over time at home might improve clinical decision-making. A proof of concept study among 60 pediatrician-diagnosed asthmatics and 30 non-asthmatics aged 4–14 years in the Netherland showed that short-term intense home monitoring was feasible and correlated with asthma control determined by a pediatrician [Citation102]. Participants were tracked for 14 days at their homes via wearable devices, physical activity trackers, handheld spirometers, smart inhalers, and ambulatory electrocardiography devices to monitor heart and respiratory rate to evaluate asthma control [Citation102]. For long-term monitoring, such an intense monitoring approach might not be suitable. Therefore, it is important to develop a home monitoring tool in collaboration with the end-users to ensure usability.
2.13. Lifestyle interventions
Exercise interventions may improve lung function, reduce symptoms, and enhance life quality in children with asthma [Citation103,Citation104]. In addition, for the prevention and management of asthma, the available literature supports recommending a higher intake of fruits and vegetables [Citation105]. A systematic review and meta-analysis study found that obesity in children was associated with asthma risk and vitamin D and iron deficiency [Citation106]. A meta-analysis showed vitamin D supplementation reduced the risk of asthma exacerbation rate reduction and improved pulmonary function in patients with vitamin D insufficiency [Citation107]. In contrast, a more recent randomized, double-blinded clinical trial study, did not find a significant improvement in the time to severe asthma exacerbation in 192 children with persistent asthma and low vitamin D levels [Citation108]. The effectiveness of vitamin D on the outcomes of asthma in children, therefore, requires further investigation.
3. Conclusion
In the past 15 years, several new drugs (e.g. biologics) and medication regimens (triple therapy) have become available to treat moderate to severe childhood asthma. In addition, molecular technologies have provided new insights into the heterogeneity of childhood asthma and provided new leads to predict medication responsiveness. E-health and home monitoring technologies have helped gain more insights into disease dynamics, improve adherence to treatment, and track symptoms and response to medication. However, uncontrolled childhood asthma is still a major unmet clinical need and precision-medicine approaches are still scarce in clinical practice. Most studies have focused on adults. However, it is challenging to extrapolate findings in adults to the pediatric population because molecular pathways, clinical effects, biomarkers, and biological response predictors seem to be age-dependent. In order to adequately diagnose and phenotype children with asthma, improve drug responsiveness and patients’ adherence, and select the optimal treatment with the best outcome, more research is needed to evaluate pediatric asthma treatment and discover novel biomarkers for precision medicine.
4. Expert opinion
Asthma is one of the most common chronic respiratory diseases that influence the quality of life of children and their families. Atopy, the composition of the gut and lung microbiome, genetic and environmental factors (such as living environment, air pollution, and exposure to cigarette smoke) all increase the likelihood of developing asthma [Citation12]. Asthma is often still treated in a ‘one size fits all.’ However, the heterogenicity of asthma (in onset, symptoms, disease dynamics, and treatment response) strongly suggest that different asthma subtypes would benefit from alternative management strategies. Especially with the emergence of novel targeted biologicals that might need to be administrated for life, it is important to understand which patient will optimally benefit from which treatment. Furthermore, it remains to be assessed what the effects of long-term eosinophil suppression or depletion in a developing immune system are. Hence, more research is urgently needed.
Asthma in adults is often classified into different inflammatory subgroups (eosinophilic, neutrophilic, mixed granulocytic, and paucigranulocytic) based on eosinophil and neutrophil cell counts in the induced sputum [Citation109]. In children, phenotyping based on sputum samples is challenging, the technique is difficult to perform in younger children and inflammatory sputum profiles are less stable in the pediatric population [Citation11]. Advanced omics methods, including (pharmaco)genomics, epigenomics, transcriptomics, proteomics, metabolomics, microbiomics, and exposomics (environmental exposures), may help researchers or clinicians to develop alternative phenotyping strategies and gain more insight into the complexity of the disease. One of the omics fields that have gained attention lately is microbiomics. Although the full influence of the microbiome on disease and treatment response remains largely unclear, various studies have suggested that the microbiome composition plays an important role in asthma pathophysiology. According to a longitudinal clinical trial among 254 children aged 5–11 years who were followed up for one year, there was an association between upper airway microbiota and severe exacerbation rate [Citation110]. In terms of inflammatory phenotypes of asthma, neutrophilic asthma appears to be the most linked to microbial profile in the sputum [Citation111]. In a longitudinal multicenter cohort study on 100 adult severe asthmatics, the microbiota of the sputum could be used as a biomarker to better characterize the neutrophilic asthma phenotype [Citation112]. Furthermore, according to the ‘gut-lung-axis,’ the gut microbiota may influence asthma development. Microbiota that exist in the gut convert dietary carbohydrates to short-chain fatty acids via fermentation; these short-chain fatty acids are able to bypass the metabolism system and affect distal organs, such as the lungs [Citation113]. Furthermore, it was shown that the microbiota influences the generation of several cytokines and chemokines, which involve inflammatory pathway regulation in the lung [Citation114]. This makes the composition of the microbiome an interesting therapeutical target, but further studies are needed to fully understand how the microbiome composition affects asthma dynamics.
Azithromycin showed some anti-inflammatory and immunomodulatory effects [Citation115], and it is recommended as an add-on therapy for adult asthma patients who are in step 5 of the GINA guidelines [Citation13]. However, some studies have been conducted to evaluate azithromycin efficacy in pediatric asthma. A recent open-labeled randomized controlled trial on 120 children with poorly controlled asthma showed that adding oral azithromycin to standard therapy decreased exacerbations and enhanced asthma control [Citation115]. Another randomized controlled trial in asthma children showed that long-term azithromycin use significantly reduced bronchial hyperresponsiveness and sputum neutrophils, but had no effect on lung function [Citation116].
Metabolomics measurements in exhaled breath could be an easy, noninvasive approach to identify distinct asthma phenotypes and guide treatment in children. Volatile organic compounds (VOCs) exist in the exhaled breath, which come with different origins (endogenous [cellular] and exogenous [viruses or bacteria]). Exhaled breath profiles, including VOCs, can be detected via gas chromatography-mass spectrometry (GC–MS), identifying a single compound, or via the eNose method, focusing on profiles of VOCs [Citation117]. In a prospective study of 521 asthma patients (children and adults), Schleich et al. demonstrated that the VOCs in exhaled breath could be used to distinguish between two different phenotypes of asthma (neutrophilic and eosinophilic) [Citation118]. A prospective study on 40 asthmatic children showed that VOCs detected by GC-MS can be a predictor of exacerbations [Citation119]. Studies to assess the value of exhaled breath to assess biologics response are ongoing.
Integrating two or more different omics methods through a systems medicine approach might provide a more accurate molecular categorization for the phenotyping or subgrouping asthmatics. Nevertheless, multi-omics approaches are expensive and demand computational and human resources. Hence, an international multidisciplinary collaboration among different centers and professionals is needed to reach this goal [Citation111]. U-BIOPRED (Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes) [Citation120], SARP (Severe Asthma Research Program) [Citation121], and SysPharmPediA [Citation122] are large consortia focusing on unraveling the complex pathophysiology of uncontrolled asthma. Hopefully, these multi-omics studies will help to provide leads for the future of precision medicine in childhood asthma.
Advances in multi-omics approaches combined with home monitoring could lead to a better understanding of the underlying pathobiological mechanisms (asthma endotypes) of the broad childhood asthma spectrum (represented in various asthma phenotypes) and could uncover new biomarkers and potential treatment targets for precision medicine (). This is of utmost importance to optimally treat children with uncontrolled asthma at an early stage, improve their quality of life and prevent poor health outcomes later in life.
Article highlights
Asthma management is shifting from a ‘one size fits all’ to a precision medicine approach based on novel insights on the heterogeneity in underlying inflammatory patterns.
Biologics targeting inflammatory pathway mediators have emerged as an add-on medication to treat specific subgroups of children with severe asthma.
E-health and home monitoring technologies have helped gain more insights into disease dynamics, improve adherence to treatment, and track symptoms and response to medication.
Advances in omics methods, including (pharmaco)genomics, epigenomics, transcriptomics, proteomics, metabolomics, microbiomics, and exposomics (environmental exposures), provide more insight into the complexity of the disease and potential therapeutic targets.
Studying the effect of asthma treatment in children is of utmost importance, since molecular pathways, clinical effects, biomarkers, and biological response predictors seem to be age-dependent.
Declaration of interest
SJH Vijverberg has received research funding from ERAPerMed, ZonMW and Lung Foundation NL for her research on severe pediatric asthma. AHM van der Zee received unrestricted research grants from Vertex and Boehringer Ingelheim; received consulting fees (paid to institution) from AstraZeneca and Boehringer Ingelheim, and received honoraria (paid to institution) for lectures by GSK. She is also the PI of a P4O2 (Precision Medicine for more Oxygen) public–private partnership sponsored by Health Holland involving many private partners who contribute in cash and/or in kind (Boehringer Ingelheim, Breathomix, Fluidda, Ortec Logiqcare, Philips, Quantib-U, Smartfish, SODAQ, Thirona, TopMD, and Novartis), and she is the president of the federation of innovative drug research in the Netherlands (FIGON) (unpaid) and President of the European Association of Systems Medicine (EASYM). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed performing consulting, serving on advisory boards or receiving travel reimbursements from Amphastar, AstraZeneca, Chiesi, Connect Biopharma, GlaxoSmithKline, Mylan, Novartis, Sunovion and Theravance; and conducting multicenter clinical research trials for some 40 pharmaceutical companies. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222.
- Wirl C, Puklova V. Prevalence of asthma and allergies in children. WHO Fact Sheet. 2007. [cited 2022 Aug 5]. Available from: https://www.euro.who.int/__data/assets/pdf_file/0012/96996/3.1.pdf
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches Nat Med. 2012 May 4;18(5):716–725.
- Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999 Sep;160(3):1001–1008.
- Diamant Z, Vijverberg S, Alving K, et al. Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy. 2019 Oct;74(10):1835–1851.
- Hudey SN, Ledford DK, Cardet JC. Mechanisms of non-type 2 asthma. Curr Opin Immunol. 2020 Oct;66:123–128.
- Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet. 2018 Feb 24;391(10122):783–800.
- Hammad H, Lambrecht BN. The basic immunology of asthma Cell. 2021Mar18;184(6):1469–1485.
- Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018 Mar 1;128(3):997–1009.
- Banno A, Reddy AT, Lakshmi SP, et al. Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clin Sci (Lond). 2020 May 15;134(9):1063–1079.
- Fleming L, Tsartsali L, Wilson N, et al. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012 Aug;67(8):675–681.
- Schoettler N, Strek ME. Recent advances in severe asthma: from phenotypes to personalized medicine. Chest. 2020 Mar;157(3):516–528.
- Reddel HK, Bacharier LB, Bateman ED, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med. 2022 Jan 1;205(1):17–35.
- (MD) B. National asthma education and prevention program, third expert panel on the diagnosis and management of asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007.
- Johnson M. The β-adrenoceptor. Am J Respir Crit Care Med. 1998;158(supplement_2):S146–S153.
- Robert F, Lemanske J. Beta agonists in asthma: benefits and risks. UpToDate2022.
- Roux E, Molimard M, Savineau JP, et al. Muscarinic stimulation of airway smooth muscle cells. Gen Pharmacol. 1998 Sep;31(3):349–356.
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents Respir Res. 2010 Oct 29;11(1):149.
- PJ B. Inhaled Corticosteroids Pharmaceuticals (Basel). 2010 Mar 8;3(3):514–540.
- Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011 May;163(1):29–43.
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013 Nov;132(5):1033–1044.
- Holgate ST, Bradding P, Sampson AP. Leukotriene antagonists and synthesis inhibitors: new directions in asthma therapy. J Allergy Clin Immunol. 1996 Jul;98(1):1–13
- Drazen JM. Pharmacology of leukotriene receptor antagonists and 5-lipoxygenase inhibitors in the management of asthma. Pharmacotherapy. 1997 Jan-Feb;17(1 Pt 2):22s–30s.
- Rachelefsky G. Childhood asthma and allergic rhinitis: the role of leukotrienes. J Pediatr. 1997 Sep;131(3):348–355.
- Thomson NC, Chaudhuri R. Omalizumab: clinical use for the management of asthma. Clin Med Insights Circ Respir Pulm Med. 2012;6:27–40.
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009 Sep 1;180(5):388–395.
- Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013 Aug;19(8):977–979.
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol. 2013 Feb;13(2):75–87.
- Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016 Jan;137(1):75–86.e8.
- Yanagibashi T, Satoh M, Nagai Y, et al. Allergic diseases: from bench to clinic - Contribution of the discovery of interleukin-5. Cytokine. 2017 Oct;98:59–70.
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015 Jan;16(1):45–56.
- Fala L. Nucala (Mepolizumab): first IL-5 antagonist monoclonal antibody FDA approved for maintenance treatment of patients with severe asthma. Am Health Drug Benefits. 2016 Mar;9(Spec Feature):106–110.
- Pelaia C, Calabrese C, Vatrella A, et al. Benralizumab: from the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. Biomed Res Int. 2018;2018:4839230.
- Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039.
- Howarth PH, Bradding P, Montefort S, et al. Mucosal inflammation and asthma. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 2):S18–22.
- Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000 Apr;105(4):651–663.
- McBrien CN, Menzies-Gow A. The biology of eosinophils and their role in asthma. Front Med (Lausanne). 2017;4:93.
- Rossjohn J, McKinstry WJ, Woodcock JM, et al. Structure of the activation domain of the GM-CSF/IL-3/IL-5 receptor common beta-chain bound to an antagonist. Blood. 2000 Apr 15;95(8):2491–2498.
- Murphy JM, Young IG. IL-3, IL-5, and GM-CSF signaling: crystal structure of the human beta-common receptor. Vitam Horm. 2006;74:1–30.
- Ishino T, Pasut G, Scibek J, et al. Kinetic interaction analysis of human interleukin 5 receptor alpha mutants reveals a unique binding topology and charge distribution for cytokine recognition. J Biol Chem. 2004 Mar 5;279(10):9547–9556.
- Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010 Jun;125(6):1344–1353.e2.
- Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002 Jul 26;277(30):26733–26740.
- Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003 Jan 31;278(5):3466–3473.
- Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol. 2018 Apr;141(4):1529–1532.e8.
- Hillas G, Fouka E, Papaioannou AI. Antibodies targeting the interleukin-5 signaling pathway used as add-on therapy for patients with severe eosinophilic asthma: a review of the mechanism of action, efficacy, and safety of the subcutaneously administered agents, mepolizumab and benralizumab. Expert Rev Respir Med. 2020 Apr;14(4):353–365.
- Del Rosso JQ. MONOCLONAL ANTIBODY THERAPIES for atopic dermatitis: where are we now in the spectrum of disease management? J Clin Aesthet Dermatol. 2019 Feb;12(2):39–41.
- Thibodeaux Q, Smith MP, Ly K, et al. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. 2019;15(9):2129–2139.
- Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy. 2020 Jan;50(1):5–14.
- Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020 Oct 13;21(1):266.
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017 Sep 7;377(10):936–946.
- Alvaro-Lozano M, Akdis CA, Akdis M, et al. EAACI allergen immunotherapy user’s guide. Pediatr Allergy Immunol. 2020 May;31 Suppl 25(Suppl25):1–101.
- Agache I, Lau S, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. 2019 May;74(5):855–873.
- Di Bona D, Frisenda F, Albanesi M, et al. Efficacy and safety of allergen immunotherapy in patients with allergy to molds: a systematic review. Clin Exp Allergy. 2018 Nov;48(11):1391–1401.
- Peter S, Creticos M. Subcutaneous immunotherapy (SCIT) for allergic disease: indications and efficacy. UpToDate2022.
- Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010 Aug;4(8):Cd001186.
- Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010 Nov;126(5):942–949.
- Valovirta E, Berstad AK, de Blic J, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ-standardized grass allergy immunotherapy tablet in children with grass pollen-induced allergic rhinoconjunctivitis. Clin Ther. 2011 Oct;33(10):1537–1546.
- James C, Bernstein DI. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017 Feb;17(1):55–59.
- Buels KS, Fryer AD. Muscarinic receptor antagonists: effects on pulmonary function. Handb Exp Pharmacol. 2012;208:317–341.
- Kim LHY, Saleh C, Whalen-Browne A, et al. Triple vs dual inhaler therapy and asthma outcomes in moderate to severe asthma: a systematic review and meta-analysis. Jama. 2021 Jun 22;325(24):2466–2479.
- Virchow JC, Kuna P, Paggiaro P, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019 Nov 9;394(10210):1737–1749.
- Canonica GW, Colombo GL, Bruno GM, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the severe asthma network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1):100007.
- Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. Bmj. 2017 Apr;12(357):j1415.
- Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015 Dec;136(6):1488–1495.
- Anderson WC 3rd, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019 Apr;122(4):367–372.
- Green RH, Brightling CE, Woltmann G, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002 Oct;57(10):875–879.
- Louis R, Schleich F, Barnes PJ. Corticosteroids: still at the frontline in asthma treatment? Clin Chest Med. 2012 Sep;33(3):531–541.
- Petsky HL, Cates CJ, Kew KM, et al. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax. 2018 Dec;73(12):1110–1119.
- Choi BS. Eosinophils and childhood asthma Clin Exp Pediatr. 2021 Feb;64(2): 60–67.
- Li J, Panganiban R, Kho AT, et al. Circulating microRNAs and treatment response in childhood asthma. Am J Respir Crit Care Med. 2020 Jul 1;202(1):65–72.
- Slob EMA, Richards LB, Vijverberg SJH, et al. Genome-wide association studies of exacerbations in children using long-acting beta2-agonists. Pediatr Allergy Immunol. 2021 Aug;32(6):1197–1207.
- Turner S, Francis B, Vijverberg S, et al. Childhood asthma exacerbations and the Arg16 β2-receptor polymorphism: a meta-analysis stratified by treatment. J Allergy Clin Immunol. 2016 Jul;138(1):107–113.e5.
- Ruffles T, Jones CJ, Palmer C, et al. 2021Aug Asthma prescribing according to Arg16Gly beta-2 genotype: a randomised trial in adolescents, Eur Respir J;58:2004107.
- Peters SP, Bleecker ER, Kunselman SJ, et al. Predictors of response to tiotropium versus salmeterol in asthmatic adults. J Allergy Clin Immunol. 2013 Nov;132(5):1068–1074.e1.
- Casale TB, Bateman ED, Vandewalker M, et al. Tiotropium respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2018 May-Jun;6(3):923–935.e9.
- Cheng WC, Wu BR, Liao WC, et al. Clinical predictors of the effectiveness of tiotropium in adults with symptomatic asthma: a real-life study. J Thorac Dis. 2018 Jun;10(6):3661–3669.
- Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med.2019 May 23;380(21):2009–2019.
- Szefler SJ, Vogelberg C, Bernstein JA, et al. Tiotropium is efficacious in 6- to 17-Year-Olds with asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2019 Sep-Oct;7(7):2286–2295.e4.
- Shah SP, Grunwell J, Shih J, et al. Exploring the utility of noninvasive type 2 inflammatory markers for prediction of severe asthma exacerbations in children and adolescents. J Allergy Clin Immunol Pract. 2019 Nov-Dec;7(8):2624–2633.e2.
- Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013 Apr 15;187(8):804–811.
- Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018 Feb;73(2):490–497.
- Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009 Nov;103(11):1633–1642.
- Brusselle G, Germinaro M, Weiss S, et al. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther. 2017;43:39–45.
- Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):4.
- FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51–64.
- Albers FC, Licskai C, Chanez P, et al. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806.
- Wechsler M, McDonald M, Garin M.C. Reslizumab high-responder and super-responder asthma patients. Am J Respir Crit Care Med. 2018:197.
- Jackson DJ, Humbert M, Hirsch I, et al. Ability of serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Adv Ther. 2020 Feb;37(2):718–729.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496.
- Lombardi C, Bagnasco D, Passalacqua G. Biological agents for severe asthma: the evolution of the at-home self-injection approach. Curr Opin Allergy Clin Immunol. 2020 Aug;20(4):421–427.
- Makhecha S, Jamalzadeh A, Irving S, et al. Paediatric severe asthma biologics service: from hospital to home. Arch Dis Child. 2021 Sep;106(9):900–902.
- Bonini M, Usmani OS. Novel methods for device and adherence monitoring in asthma. Curr Opin Pulm Med. 2018 Jan;24(1):63–69.
- Huss K, Winkelstein ML, Crosbie K, et al. ’Backpack adventures in asthma’: interactive multimedia computer game piques childrens’ interest in asthma. J Allergy Clin Immunol. 2001;107(2):239.
- McPherson A, Forster D, Glazebrook C, et al. The asthma files: evaluation of a multimedia package for children’s asthma education. Paediatr Nurs. 2002 Mar;14(2):32–35.
- Shames RS, Sharek P, Mayer M, et al. Effectiveness of a multicomponent self-management program in at-risk, school-aged children with asthma. Ann Allergy Asthma Immunol. 2004 Jun;92(6):611–618.
- McPherson AC, Glazebrook C, Forster D, et al. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics. 2006 Apr;117(4):1046–1054.
- Vrijens B, Dima AL, Van Ganse E, et al. What we mean when we talk about adherence in respiratory medicine. J Allergy Clin Immunol Pract. 2016 Sep-Oct;4(5):802–812.
- Burgess SW, Sly PD, Devadason SG. Providing feedback on adherence increases use of preventive medication by asthmatic children. J Asthma. 2010 Mar;47(2):198–201.
- Lee JR, Leo S, Liao S, et al. Electronic adherence monitoring devices for children with asthma: a systematic review and meta-analysis of randomised controlled trials. Int J Nurs Stud. 2021 Oct;122:104037.
- Lammers N, van Hoesel MHT, Kamphuis M, et al. Assessing exercise-Induced bronchoconstriction in children; the need for testing. Front Pediatr. 2019;7:157.
- McQuaid EL, Kopel SJ, Nassau JH. Behavioral adjustment in children with asthma: a meta-analysis. J Dev Behav Pediatr. 2001 Dec;22(6):430–439.
- van der Kamp MR, Klaver EC, Thio BJ, et al. WEARCON: wearable home monitoring in children with asthma reveals a strong association with hospital based assessment of asthma control. BMC Med Inform Decis Mak. 2020 Aug 14;20(1):192.
- Carew C, Cox DW. Laps or lengths? The effects of different exercise programs on asthma control in children. J Asthma. 2018 Aug;55(8):877–881.
- Abdelbasset WK, Alsubaie SF, Tantawy SA, et al. Evaluating pulmonary function, aerobic capacity, and pediatric quality of life following a 10-week aerobic exercise training in school-aged asthmatics: a randomized controlled trial. Patient Prefer Adherence. 2018;12:1015–1023.
- Lu KD, Forno E. Exercise and lifestyle changes in pediatric asthma. Curr Opin Pulm Med. 2020 Jan;26(1):103–111.
- Malden S, Gillespie J, Hughes A, et al. Obesity in young children and its relationship with diagnosis of asthma, vitamin D deficiency, iron deficiency, specific allergies and flat-footedness: a systematic review and meta-analysis. Obes Rev. 2021 Mar;22(3):e13129.
- Wang M, Liu M, Wang C, et al. Association between vitamin D status and asthma control: a meta-analysis of randomized trials. Respir Med. 2019 Apr;150:85–94.
- Forno E, Bacharier LB, Phipatanakul W, et al. Effect of Vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low Vitamin D levels: the VDKA randomized clinical trial. Jama. 2020 Aug 25;324(8):752–760.
- Simpson JL, Scott R, Boyle MJ, et al. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006 Jan;11(1):54–61.
- Zhou Y, Jackson D, Bacharier LB, et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun. 2019 Dec 16;10(1):5714.
- Abdel-Aziz MI, Neerincx AH, Vijverberg SJ, et al. Omics for the future in asthma. Semin Immunopathol. 2020 Feb;42(1):111–126.
- Abdel-Aziz MI, Brinkman P, Vijverberg SJH, et al. Sputum microbiome profiles identify severe asthma phenotypes of relative stability at 12 to 18 months. J Allergy Clin Immunol. 2021 Jan;147(1):123–134.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003 Feb;62(1):67–72.
- Frati F, Salvatori C, Incorvaia C, et al. The Role of the Microbiome in Asthma: the Gut⁻Lung Axis. Int J Mol Sci. 2018 Dec 30;20(1):123.
- Ghimire JJ, Jat KR, Sankar J, et al. Azithromycin for poorly controlled asthma in children: a randomized controlled trial. Chest. 2022 Jun;161(6):1456–1464.
- Piacentini GL, Peroni DG, Bodini A, et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc. 2007 Mar-Apr;28(2):194–198.
- Neerincx AH, Vijverberg SJH, Bos LDJ, et al. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr Pulmonol. 2017 Dec;52(12):1616–1627.
- Schleich FN, Zanella D, Stefanuto PH, et al. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am J Respir Crit Care Med. 2019 Aug 15;200(4):444–453.
- Robroeks CM, van Berkel JJ, Jöbsis Q, et al. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur Respir J. 2013 Jul;42(1):98–106.
- Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J. 2015 Nov;46(5):1322–1333.
- Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018 Mar-Apr;6(2):545–554.e4.
- Abdel-Aziz MI, Neerincx AH, Vijverberg SJH, et al. A system pharmacology multi-omics approach toward uncontrolled pediatric asthma. J Pers Med. 2021 May 28;11(6):484.