ABSTRACT
Introduction
Chronic kidney disease-associated pruritus (CKD-aP) is often experienced by patients with CKD receiving dialysis. Approximately 40% of hemodialysis patients are ‘moderately’ to ‘extremely bothered’ by itching, associated with reduced quality of life, poor sleep quality, and depression as well as worse clinical outcomes, including increased medication use, infections, hospitalizations, and mortality.
Areas covered
This review covers the pathophysiology and treatment landscape of CKD-aP, and the development, clinical efficacy, and safety profile of difelikefalin. We summarize the existing evidence, and discuss both the position of difelikefalin in the treatment pathway and potential future developments.
Expert opinion
Difelikefalin is a kappa opioid receptor agonist, with a primary mode of action that is outside of the central nervous system and provides an improved safety profile compared with other opioid agonists, with limited potential for abuse and dependency. Difelikefalin has demonstrated efficacy, tolerability, and safety profile in several large-scale clinical trials in more than 1,400 hemodialysis patients with CKD-aP treated for up to 64 weeks. Difelikefalin is the only approved treatment for CKD-aP in the U.S.A and Europe; other treatments are used off-label, have limited proof of efficacy in large-scale clinical trials in this patient population, and may present an increased risk of toxicity in patients with CKD.
1. Introduction
Chronic kidney disease-associated pruritus (CKD-aP) (previously referred to as uremic pruritus; however, due to the involvement of multiple factors in addition to uremic toxins, CKD-aP was found to be a more inclusive nomenclature) is a frequent and particularly bothersome symptom experienced by many patients with CKD receiving maintenance dialysis [Citation1] [Citation2]. Up to 40% of hemodialysis (HD) patients reported being ‘moderately’ to ‘extremely bothered’ by itching in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [Citation3]. However, the prevalence of CKD-aP is likely to be significantly underreported; data from DOPPS reported that approximately 1 in 6 HD patients with severe pruritus did not report their symptoms to a healthcare professional (HCP) [Citation4]. A survey of recognition of symptoms and their severity in HD patients reported that nephrologists underestimated the severity of the majority of symptoms in their patients [Citation5]. Underreporting is potentially due to a combination of patients not associating their symptoms with CKD and a lack of awareness by HCPs [Citation6], as well as a lack of approved, effective treatments. This means that CKD-aP may also be undertreated when diagnosed [Citation7,Citation8]; more than two-thirds of HD unit medical directors surveyed by DOPPs underestimated the prevalence of CKD-aP among their patients [Citation4]. Additionally, tools to identify and quantify the severity of CKD-aP are frequently not used in the clinical setting; there is currently no universally accepted method to quantify CKD-aP [Citation9].
The importance of CKD-aP extends beyond the symptom itself because CKD-aP is associated with reduced quality of life (QoL), poor sleep quality, and depression among other outcomes [Citation10,Citation11]. Patients with severe pruritus are also more likely to withdraw from or miss dialysis sessions [Citation3]. CKD-aP is also associated with worse clinical outcomes, including increased medication use (intravenous [IV] antibiotics, erythropoiesis-stimulating agents, and iron supplementation) infections, hospitalizations, and mortality [Citation2,Citation3,Citation8].
The mechanism of chronic itch (lasting 6 weeks or longer) is markedly different from that of acute itch. Chronic itch develops from activation of pruriceptors after exposure to pruritogens or inflammatory mediators, enhancing the responsiveness of pruriceptive nerve fibers. Immune modulation results in further interactions with sensory fibers, transmitting both histamine-dependent and histamine-independent itch.
Several mechanisms appear to contribute to the occurrence of CKD-aP and is most likely multifactorial (). These include activation of the non-histaminergic itch pathway, as well as the accumulation of uremic toxins [Citation19]. A combination of these mechanisms results in opioid imbalance, peripheral neuropathy, or immune system dysregulation, with subsequent microinflammation and xerosis [Citation9,Citation20,Citation21].
Figure 1. Pathophysiology of CKD-aP [Citation2,Citation12–18]. CKD-aP, chronic kidney disease-associated pruritus; IL, interleukin; KOR, kappa opioid receptor; MOR, mu opioid receptor.
![Figure 1. Pathophysiology of CKD-aP [Citation2,Citation12–18]. CKD-aP, chronic kidney disease-associated pruritus; IL, interleukin; KOR, kappa opioid receptor; MOR, mu opioid receptor.](/cms/asset/96205168-f72f-4486-9fba-7da5751a507b/ierj_a_2197209_f0001_oc.jpg)
CKD-aP is commonly thought to be driven by inadequate control of metabolic bone disease parameters such as phosphate, parathyroid hormone and calcium, or poor dialysis adequacy [Citation3]. However, recent studies have not shown consistent associations between biochemical markers and pruritus [Citation1,Citation4,Citation22–24]. Serum levels of inflammatory markers, including interferon gamma (IFNγ), interleukin (IL)-2, and granulocyte macrophage colony-stimulating factor (GM-CSF), were significantly correlated with itch intensity and markers of pruritus (e.g. IL-31) in HD patients with moderate-to-severe pruritus [Citation25].
Until the approval of difelikefalin (CR845), there were no approved therapies for CKD-aP in the U.S.A or Europe (nalfurafine hydrochloride is approved in Japan for the treatment of resistant pruritus in HD patients). Various off-label treatments have therefore been used to treat CKD-aP, including moisturizers, antihistamines, ultraviolet light, corticosteroids, gabapentinoids, and opioids; however, these agents have not been studied in large-scale, high-quality randomized trials [Citation26]. Besides doubtful efficacy, they might be associated with significant adverse events.
Difelikefalin is approved in both the U.S.A (as CR845/KORSUVA™) and Europe (as Kapruvia®) for the treatment of moderate-to-severe pruritus in adults undergoing HD [Citation27].
Difelikefalin is a kappa opioid receptor (KOR) agonist, which acts mainly on peripheral neurons and cells of the immune system. The primary mode of action is therefore outside of the central nervous system [Citation28,Citation29].
In phase 3 clinical trials (KALM-1 and KALM-2) in HD patients with moderate-to-severe pruritus, treatment with difelikefalin resulted in significantly greater reduction in itch intensity and improvement in itch-related QoL, compared with placebo. Additionally, difelikefalin was shown to have an acceptable safety profile [Citation30–32].
2. Overview of the treatment landscape
The underlying etiology of CKD-aP is distinct to other causes of itching seen within the general population [Citation33]. Historically, toxin accumulation and deposition have been implicated in CKD-aP, particularly inadequate phosphate and calcium control [Citation34–37]. Peripheral neuropathy, immune system dysregulation, or opioid dysregulation have also been suggested as pathways implicated in CKD-aP [Citation9], or potentially a combination of these factors [Citation38]. Therefore, control of phosphate and optimization of dialysis adequacy should be initially investigated: changing dialyzer type e.g. to polymethyl methacrylate, may also be beneficial, as this has shown reductions in ‘dialysis itchiness,’ potentially through the removal of inflammatory cytokines [Citation22,Citation39]). Current treatment algorithms then recommend a stepwise approach starting with emollients and topical anti-inflammatories, or alternatively systemic treatment with gabapentin or κagonists (difelikefalin, or nalfurafine in refractory cases), followed by drugs with an antiinflammatory action, then phototherapy, or acupuncture [Citation40]. In addition, successful kidney transplantation will relieve patients from CKD-aP [Citation12] (). However, until the recent approval of difelikefalin, no therapies were approved for CKD-aP in the U.S.A or Europe, and difelikefalin remains the only approved therapy within the treatment algorithm [Citation80].
Table 1. Overview of CKD-aP treatments.
Oral, topical, or IV antihistamines are frequently prescribed as off-label treatments for pruritus [Citation6], despite data indicating that histamines are not a major pruritogen in CKD-aP [Citation12], with any efficacy likely contributed to by a combination of sedative and placebo effects.
Off-label treatment with gabapentin has shown efficacy in small clinical trials (14–54 patients) with relatively limited follow-up (1–8 weeks) [Citation60], as well as following meta-analysis [Citation7,Citation60]. However, gabapentinoids require reduced doses in patients on HD because the risk of altered mental status, falls, and fractures increases in doses greater than 100 mg (the maximum recommended dose in post-dialysis patients is 300 mg) [Citation61,Citation81]. Gabapentin toxicity is a particular concern in patients with CKD because gabapentinoids are exclusively eliminated renally [Citation82]. According to data from DOPPS, 5%, 19%, and 21% of medical directors use gabapentin as a first-, second-, or third-choice treatment in patients with CKD-aP; however, approximately 50% of medical directors never prescribe gabapentin for pruritus [Citation4]. Non-pharmacological therapies include phototherapy with ultraviolet B light, which has demonstrated efficacy in some small, open-label trials [Citation55,Citation56]. However, regular ultraviolet B therapy is time-consuming for patients, of limited availability, and may present an increased risk of skin cancer, particularly in immunosuppressed patients [Citation14], although a systematic review did not report an increased risk of skin cancer [Citation13].
3. Introduction to the drug
3.1. Chemistry and pharmacokinetics
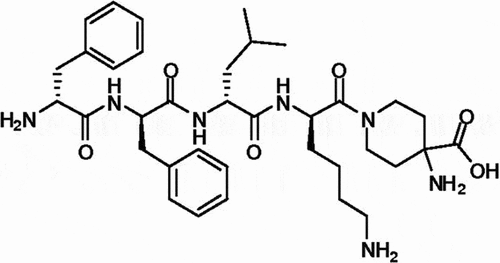
Difelikefalin has a unique pharmacology. It is a synthetic D-amino acid tetra-peptide with a single stereoisomer [Citation44] () that was developed as an analogue of an endogenous opioid peptide, Dynorphin A, a neuromodulator of pruritus [Citation41].
Difelikefalin acetate is a white to off-white powder with a molecular weight of 0.68kDa (monoisotopic; free base). It is soluble in water, is not sensitive to light, and can be stored at room temperature. The chemical name of difelikefalin acetate is 4-amino-1-(D-phenylalanyl-D-phenylalanyl-D-leucyl-D-lysyl)piperidine-4-carboxylic acid, acetate salt [Citation44].
Difelikefalin is a hydrophilic peptide with very limited, if any, blood–brain barrier penetration, meaning it is peripherally restricted [Citation41,Citation42]. The mean volume of distribution of difelikefalin is approximately 238 mL/kg [Citation44], and difelikefalin is non protein-bound (approximately 23–28% in plasma) [Citation44]. Difelikefalin is not metabolized to any appreciable extent and is not a substrate for cytochrome P450 enzymes; therefore, interactions of difelikefalin with other medicinal products are unlikely [Citation27,Citation44]. Additionally, the structure of difelikefalin is not a substrate for drug uptake transporters and is not significantly metabolized [Citation83,Citation84]. Difelikefalin is renally excreted and is also removed by the dialyzer membrane during HD [Citation27,Citation44].
The half-life of difelikefalin in patients undergoing HD ranges between 23 and 31 hours; HD reduced plasma concentrations of difelikefalin by 70–80%, with difelikefalin undetectable in plasma following two dialysis cycles. For this reason, difelikefalin is administered via bolus IV injection at the end of HD treatment, during or after rinse back [Citation44]; this also means dialysis efficiency will not impact the efficacy of difelikefalin. Clinically relevant pharmacokinetics of difelikefalin were not affected by age (25–80 years of age), sex, race/ethnicity, or mild-to-moderate hepatic impairment [Citation44].
3.2. Pharmacodynamics and mechanism of action
The proposed mechanism of action of difelikefalin in alleviating itching is thought to be through selectively activating KORs on peripheral sensory neurons and immune cells, which are thought to play an important role in CKD-aP [Citation41,Citation42].
Difelikefalin is a selective and full agonist at the KOR, with no or negligible activity at mu or delta opioid receptors or other receptors, ion channels, or transporters (based on nonclinical studies conducted by Cara Therapeutics), with at least a 10,000 times greater affinity for KORs than mu opioid receptors [Citation28]. The unique peptidic structure of difelikefalin differs significantly from the other small-molecule KOR agonists developed to date, which for the most part are active in the central nervous system, whereas difelikefalin was designed to activate KORs located primarily in the peripheral nervous system without producing side effects associated with the activation of mu opioid receptors, such as respiratory depression and abuse. Additionally, difelikefalin is a hydrophilic peptide with limited membrane permeability by passive diffusion, thereby limiting access of the drug to the central nervous system [Citation41,Citation42]. Consequently, difelikefalin is expected to have an improved safety and tolerability profile compared with other opioid agonists, including centrally acting KOR agonists.
To assess the abuse potential of difelikefalin, a double-blind, randomized, placebo-controlled, four-way crossover study of volunteers was carried out (n = 44; 18–55 years, mean = 28 years; 35 males). The study concluded that difelikefalin has a low risk for abuse potential in humans [Citation85]. An additional single‐center, randomized, double‐blind, placebo‐controlled, three‐way crossover study also reported that difelikefalin did not produce respiratory depression [Citation84].
Difelikefalin therefore has low abuse potential and is not categorized as a controlled substance according to the Controlled Substance Act Scheduling. Furthermore, no events of dysphoria or euphoria have been reported [Citation44].
Reduction in itch intensity with DFK correlated with reductions in serum levels of inflammatory markers, including IFNγ, IL-2, and GM-CSF. These results identify a potential mechanism of action of DFK through significantly reducing markers of pruritus-associated inflammation [Citation25]; however, elucidation of the mechanism of action requires further investigation in well-designed studies.
4. Clinical efficacy
4.1. Assessment of clinical efficacy in research settings
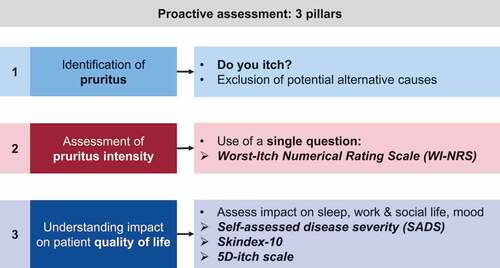
The clinical efficacy and safety profile of difelikefalin have been assessed through a large-scale clinical trial program, recruiting more than 1,400 HD patients who were treated for up to 64 weeks, with assessments of pruritus severity incorporating evaluations of both itch intensity and the impact of itch on patient QoL () [Citation86].
Table 2. Key clinical trial results of difelikefalin in moderate-to-severe pruritus associated with CKD in adults undergoing hemodialysis.
Patient-reported outcomes (PROs) assessed during the trial program include ():
Kidney Disease Quality of Life Instrument, a 36-item health-related QoL measure for dialysis patients [Citation96].
The Worst-Itch Numerical Rating Scale (WI-NRS), an 11-point scale ranging from 0 to 10, which was validated in patients with CKD-aP [Citation17,Citation22,Citation95]. Clinically meaningful changes in itch intensity have been associated with a reduction of ≥ 3 points on the WI-NRS for patients with moderate-to-severe pruritus undergoing HD [Citation95].
The 5-D Itch scale, which utilizes five separate itch-related domains to assess itch-related QoL and itch intensity [Citation39]. Clinically meaningful changes in itch intensity have been associated with a ≥ 5-point reduction from baseline in the total 5-D itch score [Citation86].
Skindex-10, a multidimensional tool evaluating itch-related QoL across three domains: Disease, Mood/Emotional Distress, and Social Functioning [Citation22]. Clinically meaningful changes in itch intensity have been associated with a reduction from baseline of ≥ 15 points [Citation86].
Sleep quality, assessed by a Sleep Quality Numerical Rating Scale (NRS) with responses ranging from 0 (‘did not interfere’) to 10 (‘completely interfered’) to indicate how much itch interfered with sleep over the preceding 24 hours [Citation91].
The Patient Global Impression of Change, a global patient-reported outcome measure assessing overall perception of change in itch (ranging from ‘Very much improved’ to ‘Very much worse’) compared with the start of the study [Citation97].
Table 3. Overview of itch-related patient-reported outcome measures.
4.2. Phase 2 studies
4.2.1. Difelikefalin phase 2 study: intravenous administration [Citation86]
A randomized, double-blind, placebo-controlled trial in 174 HD patients with moderate-to-severe pruritus was the largest phase 2 clinical trial of difelikefalin. Participants were randomized to IV difelikefalin (0.5, 1.0, or 1.5 µg/kg) or placebo, following HD, three times per week for 8 weeks. The primary endpoint was the change from baseline to week 8 in the weekly mean of the 24-hour WI-NRS score, while secondary and other endpoints included change in itch-related QoL (measured by the Skindex-10 questionnaire and the 5-D itch scale), safety profile, and sleep quality.
Treatment with difelikefalin resulted in a significant reduction from baseline in WI-NRS scores at week 8 versus placebo (P = 0.002). Significant improvement with difelikefalin compared with placebo was also reported for Skindex-10, 5-D itch, and sleep disturbance scores (P < 0.005). Treatment-emergent adverse events (TEAEs) were reported in more patients receiving difelikefalin (78%), compared with 42% of patients receiving placebo. Diarrhea, dizziness, nausea, somnolence, and falls were the most commonly reported adverse events.
The overall conclusion of the trial was that difelikefalin effectively reduced itching intensity and improved sleep and itch-related QoL.
4.3. Phase 3 studies
The pivotal phase 3 trials of difelikefalin were the KALM-1 and KALM-2 double-blind, placebo-controlled studies (), followed by the open-label 3105 single-arm study.
Figure 3. KALM trial design. *1 patient withdrew post-randomization and before first dose of placebo. HD, hemodialysis; IV, intravenous; U.S., United States; WI-NRS, Worst-Itch Numerical Rating Scale.
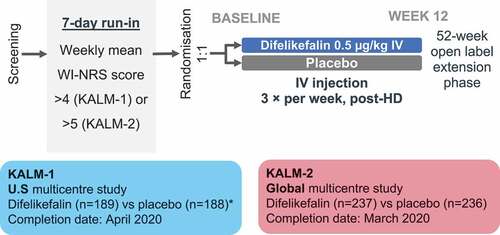
4.3.1. KALM-1 and KALM-2 studies [Citation30]
KALM-1 was a double-blind, placebo-controlled, phase 3 trial. Hemodialysis patients with moderate-to-severe pruritus were randomly assigned to receive either IV difelikefalin (0.5 µg/kg) or placebo three times per week for 12 weeks, with an additional open-label extension of 52 weeks. The primary outcome of the double-blind treatment period was the proportion of patients with an improvement of at least 3 points from baseline at week 12 in the weekly mean of the 24-hour WI-NRS. Secondary outcomes included change from baseline in itch-related QoL, proportion of patients with an improvement in WI-NRS score ≥4 points at week 12, and safety profile.
The overall conclusion of the trial was that HD patients with moderate-to-severe pruritus treated with difelikefalin had a significant, clinically meaningful reduction in itch intensity and improved itch-related QoL compared with patients who received placebo.
The KALM-2 phase 3 study had an almost identical study design to KALM-1; however, KALM-1 was conducted in the U.S.A whereas KALM-2 was conducted in North America, Europe, and Asia-Pacific region (Australia, New Zealand, South Korea, Taiwan). Inclusion criteria differed slightly between the two trials, with KALM-1 requiring a weekly mean WI-NRS score of >4 during the run-in period, whereas KALM-2 required a WI-NRS weekly mean score of ≥5. The results of both the KALM studies have therefore been combined, with further publications reporting on the pooled population.
4.3.2. KALM-1 and KALM-2 pooled efficacy [Citation31]
Overall, in KALM-1 and -2, 851 patients were randomized (426 patients received difelikefalin and 425 received placebo). Significantly more patients achieved a clinically meaningful (≥3-point) reduction in weekly mean WI-NRS score with difelikefalin versus placebo at week 12 (51.1% vs. 35.2%, P < 0.001). Difelikefalin also demonstrated greater improvements in itch intensity compared with placebo in patient subgroups based on age, sex, race, geographic region, medical conditions, and baseline use of anti-itch medication (including gabapentin or pregabalin). At week 12, significantly more patients reported complete WI-NRS response (≥80% of weekly scores equal to 0 or 1) with difelikefalin versus placebo (12.0% vs. 6.7%, P = 0.006). Significantly greater proportions of difelikefalin-treated patients also achieved clinically meaningful decreases in Skindex-10 total score (≥15 points; 55.5% vs. 40.5%, P < 0.001) and in 5-D itch total score (≥5 points; 52.1% vs. 42.3%, P = 0.012).
Overall, difelikefalin provided clinically meaningful improvements in itch intensity and QoL in patients with moderate-to-severe pruritus undergoing HD. Additionally, findings were consistent across diverse patient subgroups.
4.3.3. KALM-1 and KALM-2 pooled safety [Citation32]
Pooled safety findings were reported for two separate cohorts: a placebo-controlled cohort from the 12-week pivotal studies, KALM-1 and KALM-2, and an all-difelikefalin-exposure cohort including subjects who received one or more IV doses of difelikefalin (0.5 µg/kg) for up to 64 weeks. Differences in TEAEs are descriptive only, as p values are generally not ascribed to AE data; AEs are already noted in the label and this study was not powered for safety data.
Total exposure in the all-difelikefalin-exposure cohort was 811.3 person-years (n = 1,306 subjects); exposure was ≥6 months in 711 patients, with 400 patients exposed for ≥12 months. In the placebo-controlled cohort, the most commonly reported TEAEs occurring in more patients in the difelikefalin group were diarrhea (9.0% and 5.7%), dizziness (6.8% and 3.8%), nausea (6.6% and 4.5%), gait disturbance including falls (6.6% and 5.4%), hyperkalemia (4.7% and 3.5%), headache (4.5% and 2.6%), somnolence (4.2% and 2.4%), and mental status changes (3.3% and 1.4%), in the difelikefalin and placebo groups, respectively. Adverse events were mostly mild to moderate in severity, with most patients continuing study drug. Long-term exposure did not increase incidence rates of TEAEs, serious TEAEs, or study drug discontinuations caused by TEAEs. The incidence of death was higher in the placebo group than in the difelikefalin group, and death rates in the all-difelikefalin-exposure cohort were consistent with those for patients undergoing HD documented in the US Renal Data System 2020 report [Citation98].
Overall, IV difelikefalin demonstrated an acceptable safety profile and was generally well tolerated with long-term use. Difelikefalin therefore has the potential to address the unmet need for a safe and effective treatment for CKD-aP in patients undergoing HD.
4.3.4. Study 3105 [Citation91]
Study 3105 was an open-label, multicenter, single-arm intervention trial of maintenance HD patients with moderate-to-severe CKD-aP (baseline WI-NRS ≥5). Patients received IV difelikefalin (0.5 µg/kg) after each HD session for 12 weeks. The primary outcome of the study was to assess the safety profile of difelikefalin, while secondary outcomes included the effectiveness of difelikefalin to reduce itch intensity (assessed by the WI-NRS); improving itch-related QoL (assessed by the 5-D itch and Skindex-10 scales); and improvement of sleep (assessed with the Sleep Quality NRS). Clinically meaningful thresholds for improvement in itch and QoL were previously established in this population as ≥3-point reduction in WI-NRS, ≥15-point reduction in Skindex-10, and ≥5-point reduction in 5-D itch. A clinically relevant reduction in Sleep Quality NRS score has not yet been validated.
Among 222 patients, mean [standard deviation] baseline WI-NRS was 7.6 [1.3], mean age was 58 years, 55% were male, and mean dialysis duration was 5.9 years. Treatment was completed by 197 patients (89%). Adverse events were reported in 7.2% of patients. The most common TEAEs related to study drug were somnolence (1.8%), hypoesthesia (1.4%), nausea (0.9%) and dizziness (0.9%); no deaths or serious TEAEs were considered related to treatment. Efficacy of difelikefalin was clearly demonstrated, with 74% of patients reporting clinically meaningful reductions in itch intensity, and 70% and 63% also reporting clinically relevant improvements in QoL (measured by 5-D itch and Skindex-10, respectively). Improvements in sleep quality (≥3-point NRS reduction) were reported in 66% of patients.
Study 3105 concluded that difelikefalin was well tolerated overall and resulted in clinically meaningful improvements in itch intensity, as well as improvements in itch-related QoL and sleep quality in HD patients who had moderate-to-severe CKD-aP. Additionally, Study 3105 provided evidence regarding the potential real-world effectiveness of difelikefalin.
4.3.5. Difelikefalin phase 3 study: oral administration [Citation99]
An additional phase 3, multicenter, double-blind, randomized, placebo-controlled study in 400 patients of oral difelikefalin is currently underway in CKD patients with moderate-to-severe pruritus. The study aims to evaluate the safety and efficacy of oral difelikefalin (1 mg once-daily tablet) in reducing the intensity of itch compared with placebo in patients with advanced CKD with moderate-to-severe pruritus who are not on dialysis. The development of an oral formulation of difelikefalin will enable treatments of patients with CKD-aP who are not undergoing HD, or who are undergoing dialysis at home [Citation99].
5. Regulatory status
In the U.S.A, difelikefalin is indicated for the treatment of moderate-to-severe pruritus associated with CKD in adults undergoing HD [Citation44]. In Europe, a Pediatric Investigation Plan was agreed in May 2020 by the European Medicines Agency [Citation100]. In May 2022, difelikefalin was also approved as the first therapy in Europe for the treatment of CKD-aP in HD patients [Citation27]. Subsequent approvals followed in 2022 in Switzerland as part of an Access Consortium procedure, together with Canada, Australia and Singapore [Citation101,Citation102]. Additionally, a new drug application was submitted in Japan in September 2022.
6. Conclusion
CKD-aP is an underreported, underdiagnosed, and undertreated condition, which is common in patients with CKD, including those undergoing HD. CKD-aP is a particularly bothersome symptom, affecting patients’ QoL, as well as resulting in worse clinical outcomes for patients on HD, including higher rates of mortality.
Difelikefalin has demonstrated efficacy, tolerability, and safety profile in several large-scale clinical trials in more than 1,400 HD patients with CKD-aP, who were treated for up to 64 weeks.
Difelikefalin activates peripherally located KORs without producing adverse effects associated with the activation of mu opioid receptors, such as respiratory depression and abuse. Consequently, difelikefalin presents an improved safety profile compared with other opioid agonists, with limited potential for abuse and dependency.
Difelikefalin is the only approved treatment for CKD-aP in the U.S.A and Europe; other treatments are used off-label, have limited proof of efficacy in large-scale clinical trials in this patient population, and may present an increased risk of toxicity in patients with CKD.
7. Expert opinion
Currently, CKD-aP is underreported [Citation4], as well as undertreated [Citation7,Citation8]. This may partly be due to a previous lack of approved treatments, as well as a lack of standardized assessment and monitoring tools. However, difelikefalin, now approved in multiple countries worldwide [Citation27,Citation44,Citation103,Citation104], has demonstrated efficacy in a large-scale clinical-trial program in more than 1,400 HD patients with CKD-aP, who were treated for up to 64 weeks [Citation31,Citation86,Citation91], and has a well-demonstrated safety profile, with no serious adverse events related to study drug reported even with up to triple the intended dose [Citation32,Citation86]. The most common TEAEs reported following difelikefalin treatment are mostly mild in nature and manageable; diarrhea, dizziness, somnolence, and vomiting are the most common adverse events reported, rarely requiring discontinuation of treatment [Citation30,Citation32,Citation91]. Additionally, TEAEs of somnolence tended to subside with continued dosing [Citation27]. Of particular note, unlike other opioid medications, difelikefalin has a low abuse potential [Citation84,Citation85]. Of further note, the lack of serious adverse events at higher concentrations may be relevant for assessing the efficacy of increased dosing more than three times per week in patients receiving dialysis more frequently, or those receiving at-home HD. An associated future development in the difelikefalin pipeline is the development of an oral formulation for non-dialysis and home-dialysis patients [Citation99], which again is possible due to the safety profile and lack of abuse potential of difelikefalin [Citation84,Citation85].
Difelikefalin represents a potential step-change for patients with CKD-aP: other treatments for CKD-aP are only available as off-label treatments, and have limited evidence of efficacy in much smaller, shorter-term studies, with the safety profile and lack of toxicity not demonstrated in large-scales trials in this patient population (). Additionally, the clinical development program has demonstrated the importance of the use of validated PROs to diagnose and monitor the course of CKD-aP. Therefore, the combination of the availability of an approved, safe, and effective therapy along with increased recognition of the value of utilizing itch-specific PROs has the potential to dramatically increase both the recognition and treatment of this debilitating condition.
However, it is vital that HCPs change their view of CKD-aP as a mild or rare symptom associated with CKD, and understand that it has a significant impact on patients’ QoL [Citation10,Citation11], in addition to impacting clinical outcomes, such as attendance at dialysis sessions, hospitalizations, infections, and mortality [Citation2,Citation3,Citation8]. The lack of recognition by HCPs that CKD-aP is a common, debilitating condition appears to be one of the major barriers to patient education regarding the association of their symptoms to CKD, so that patients are aware that treatments are available.
The importance of HCP awareness of the impact of symptoms associated with CKD on patient QoL and the prioritization of symptom management have recently been highlighted [Citation105,Citation106], including the importance of the use of PROs. The tools chosen for assessing itch intensity and impact on QoL in the clinical trial program are based on tools that can be applied in real-world clinics, in order to address the underreporting and undertreatment of CKD-aP. Further avenues of exploration therefore include whether increasing use of these tools (), as well as the availability of an approved treatment with demonstrated efficacy and a tolerable safety profile, can reduce the underreporting and undertreatment of CKD-aP.
As the only approved treatment of CKD-aP in the U.S.A and Europe, difelikefalin has been recommended as first-line treatment for patients with moderate to severe pruritus (). It is important that the treatment pathway includes screening, diagnosis, and assessment of itch severity, as well as treatment options. Gabapentinoids are also recommended as a first-line treatment; however as gabapentoids are not approved for this indication and their use is off-label, it may be preferable to reserve treatment with gabapentinoids to patients found to be refractory to difelikefalin. Other treatments such as phototherapy and selective serotonin reuptake inhibitors are recommended as third-line (again, off-label) treatments. Skin moisturization and barrier-function therapy with emollients, moisturizing ointments, and bath oils remain a universal approach to the management of xerosis related to CKD-aP, particularly for relief of mild disease.
Figure 5. Treatment algorithm for management of CKD-aP [Citation2,Citation3,Citation30,Citation107–110]. CKD-aP, chronic kidney disease-associated pruritus; PTH, parathyroid hormone; SSRI, selective serotonin reuptake inhibitor; UVB, ultraviolet B.
![Figure 5. Treatment algorithm for management of CKD-aP [Citation2,Citation3,Citation30,Citation107–110]. CKD-aP, chronic kidney disease-associated pruritus; PTH, parathyroid hormone; SSRI, selective serotonin reuptake inhibitor; UVB, ultraviolet B.](/cms/asset/cad316b5-aa32-4118-ba7b-1abfcbe69e05/ierj_a_2197209_f0005_oc.jpg)
To improve implementation of difelikefalin within the CKD-aP treatment pathway, further research is required into real-world effectiveness and safety profile: the anticipated real-world effectiveness of difelikefalin has already been suggested through the open-label Study 3105 [Citation91]; however, confirmation of the safety profile and effectiveness of treatment within a real-world population (without additional inclusion/exclusion criteria) will also be important. Additionally, the safety profile and efficacy of difelikefalin in patients undergoing HD more than three times per week remain to be confirmed (although given the tolerable safety profile observed at treble doses of difelikefalin [Citation86], it is not expected that additional treatments at the current dose of difelikefalin will result in any additional safety signals).
Furthermore, although the clinical trial program included continued use of other itch therapies (approximately one-third of participants were receiving an anti-itch medication at baseline) [Citation31,Citation91], elucidation of any benefits of combination treatments will be beneficial. Further investigation of combining difelikefalin with other therapies may avoid the potential for additional adverse effects from additional treatments (particularly off-label treatments), if these do not substantially increase symptom relief, compared with the use of difelikefalin alone, as well as to ascertain any potential benefits of improving control of CKD-aP.
Elucidating the driving factors behind CKD-aP (e.g. predictors of disease severity and predictors of response to difelikefalin) is another exciting avenue of research, which will further improve QoL for patients.
Additionally, in real-world management of CKD-aP, many patients may be referred to dermatologists rather than nephrologists, and it is important to assess the real-world use of difelikefalin in this setting.
When these research queries have been answered, difelikefalin has the potential to significantly advance the treatment of CKD-aP, as well as utilizing the lessons learned through its clinical development program to advance the use of appropriate, validated PROs as part of routine clinical management of CKD.
Article highlights
CKD-aP is quite prevalent in hemodialysis patients but is still underrecognized, underreported, underdiagnosed, and undertreated.
The pathogenesis of CKD-aP is complex and likely to be multifactorial, with the dysregulated endogenous opioid pathway playing a significant role.
Difelikefalin is the first and only approved therapy for CKD-aP in the U.S.A and Europe.
Difelikefalin is a peripherally acting KOR agonist.
Difelikefalin’s unique pharmacology contributes to its safety profile, efficacy, and tolerability.
Difelikefalin has been studied widely and has not been found to be associated with any dependency or withdrawal.
Declaration of interest
A Rastogi reports advisory board participation and speakers bureau fees from AstraZeneca and Relypsa Inc, and research grants from AstraZeneca. E Lerma reports speaker/advisory board/steering committee involvements with Akebia, AstraZeneca, Bayer, Glaxo Smith Kline, Otsuka, Travere, and CSL Vifor. S Frisbane reports receipt of grants from Cara Therapeutics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed involvement with CSL Vifor’s advisory board on difelikefalin. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Information resources
Sukul, N., et al., Self-reported Pruritus and Clinical, Dialysis-Related, and Patient-Reported Outcomes in Hemodialysis Patients. Kidney Med, 2021. 3(1): p. 42–53.e1. - Describes self-reported pruritus severity from the DOPPS study.
Shirazian S, et al., Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis, 2017. 10: p. 11–26. - Reviews the quality-of-life instruments available, recent studies examining the prevalence, characteristics and outcomes of CKD-aP, current treatments and clinical trials.
Rayner, H.C., et al., International Comparisons of Prevalence, Awareness, and Treatment of Pruritus in People on Hemodialysis. Clin J Am Soc Nephrol, 2017. 12(12): p. 2000–2007. - Describes the prevalence of CKD-aP in over 35,000 patients from the DOPPS study.
Fishbane, S., et al., A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. New England Journal of Medicine, 2020. 382(3): p. 222–232. - Describes the key placebo-controlled phase 3 clinical trial of difelikefalin efficacy and safety.
Topf, J., et al., Efficacy of Difelikefalin for the Treatment of Moderate to Severe Pruritus in Hemodialysis Patients: Pooled Analysis of KALM-1 and KALM-2 Phase 3 Studies. Kidney Medicine, 2022: p. 100512. - Describes the pooled efficacy data from the key placebo-controlled phase 3 clinical trials of difelikefalin.
Fishbane, S., et al., Safety and Tolerability of Difelikefalin for the Treatment of Moderate to Severe Pruritus in Hemodialysis Patients: Pooled Analysis From the Phase 3 Clinical Trial Program. Kidney Medicine, 2022: p. 100513. - Describes the pooled safety data from the key placebo-controlled phase 3 clinical trials of difelikefalin.
Agarwal, R., et al., Alleviating symptoms in patients undergoing long-term hemodialysis: a focus on chronic kidney disease-associated pruritus. Clinical Kidney Journal, 2022: p. sfac187. - Describes the proposed treatment algorithm for difelikefalin and positioning within the treatment pathway, as well as the pathway to development and regulatory approval of difelikefalin.
Weiner, D.E., et al., Safety and Effectiveness of Difelikefalin in Patients With Moderate-to-Severe Pruritus Undergoing Hemodialysis: An Open-Label, Multicenter Study. Kidney Medicine. - Describes the safety and efficacy data from the key open-label phase 3 clinical trial of difelikefalin, providing important insights into expected real-world safety and effectiveness.
US Food and Drug Administration. Korsuva Prescribing Information. 2021; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214916s000lbl.pdf.
Agency, E.M. European Medicines Agency decision. 2020; Available from: https://www.ema.europa.eu/en/documents/pip-decision/p/0172/2020-ema-decision-13-may-2020-agreement-paediatric-investigation-plan-granting-deferral-granting_en.pdf.
Acknowledgments
Medical writing support was provided by AXON Communications (London, United Kingdom) and funded by CSL Vifor.
Additional information
Funding
References
- Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495–3505. DOI:10.1093/ndt/gfl461
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11–26.
- Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2021;3(1):42–53.e41. DOI:10.1016/j.xkme.2020.08.011
- Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. DOI:10.2215/CJN.03280317.
- Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960–967. DOI:10.2215/CJN.00990207
- Aresi G, Rayner HC, Hassan L, et al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manage. 2019;58(4):578–586.e572. DOI:10.1016/j.jpainsymman.2019.06.010
- Hercz D, Jiang SH, Webster AC. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev. 2020;12(12):Cd011393.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70(5):638–655. DOI:10.1053/j.ajkd.2017.05.018
- Verduzco HA, Shirazian S. CKD-Associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387–1402.
- Weiss M, Mettang T, Tschulena U, et al. Health-related quality of life in haemodialysis patients suffering from chronic itch: results from GEHIS (German Epidemiology Haemodialysis Itch Study). Qual Life Res. 2016;25(12):3097–3106.
- Plewig N, Ofenloch R, Mettang T, et al. The course of chronic itch in hemodialysis patients: results of a 4-year follow-up study of GEHIS (German Epidemiological Hemodialysis Itch Study). J Eur Acad Dermatol Venereol. 2019;33(7):1429–1435.
- Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87(4):685–691.
- Wang E, Sasaki J, Nakamura M, et al. Cutaneous carcinogenic risk of phototherapy: an updated comprehensive review. J Psoriasis Psoriatic Arthritis. 2015;1(1):44–51.
- Kuypers DR. Skin problems in chronic kidney disease. Nat Clin Pract Nephrol. 2009;5(3):157–170.
- Patel TS, Freedman BI, Yosipovitch G. An update on pruritus associated with CKD. Am J Kidney Dis. 2007;50(1):11–20.
- Zakrzewska-Pniewska B, Jedras M. Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol Clin. 2001;31(3):181–193.
- Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502–507. DOI:10.2340/00015555-1246
- Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21(3):749–755. DOI:10.1093/ndt/gfi204
- Reddy VB, Iuga AO, Shimada SG, et al. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28(17):4331–4335.
- Lanot A, Kottler D, Béchade C. Pruritus associated chronic kidney disease. Nephrol Ther. 2021;17(7):488–495.
- Namer B, Carr R, Johanek LM, et al. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100(4):2062–2069.
- Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1410–1419. DOI:10.2215/CJN.00100110
- Shirazian S, Kline M, Sakhiya V, et al. Longitudinal predictors of uremic pruritus. J Ren Nutr. 2013;23(6):428–431. DOI:10.1053/j.jrn.2013.08.002
- Weisshaar E, Weiss M, Passlick-Deetjen J, et al. Laboratory and dialysis characteristics in hemodialysis patients suffering from chronic itch–results from a representative cross-sectional study. BMC Nephrol. 2015;16(1):184.
- Shirazian S, Spencer RH, Kilfeather SA, et al. 137 reduction of pruritus by difelikefalin correlates with reductions in markers for pruritus and inflammation in subjects undergoing hemodialysis. Am J Kidney Diseases. 2022;79(4):S42.
- Rayner H, Baharani J, Smith S, et al. Uraemic pruritus: relief of itching by gabapentin and pregabalin. Nephron Clin Pract. 2012;122(3–4):75–79.
- Electronic Medicines Compendium. Kapruvia summary of product characteristics. 2022. [cited 2021 Apr]. Available from: https://www.medicines.org.uk/emc/product/13735/smpc/print
- Gardell LR, Spencer RH, Chalmers DT, et al. Preclinical profile of CR845: a novel, long-acting peripheral kappa opioid receptor agonist. Poster Presentation at the International Association for the Study of Pain, Glasgow, UK. https://ir.caratherapeutics.com/static-files/396df363-8031-47f6-b903-eca1df1df26f, (2008).
- Menzaghi F, Spencer R, Abrouk N, et al. (422) CR845, a peripheral kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy. J Pain. 2015;16(4):S81.
- Fishbane S, Jamal A, Munera C, et al. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. . DOI:10.1056/NEJMoa1912770
- Topf J, Wooldridge T, McCafferty K, et al. Efficacy of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis of KALM-1 and KALM-2 Phase 3 Studies. Kidney Medicine. 2022;4(8):100512. DOI:10.1016/j.xkme.2022.100512.
- Fishbane S, Wen W, Munera C, et al. Safety and tolerability of difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis from the phase 3 clinical trial program. Kidney Medicine. 2022;4(8):100513. DOI:10.1016/j.xkme.2022.100513.
- Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142(5):1375–1390.
- Manenti L, Tansinda P, Vaglio A. Uraemic pruritus: clinical characteristics, pathophysiology and treatment. Drugs. 2009;69(3):251–263.
- Blachley JD, Blankenship DM, Menter A, et al. Uremic pruritus: skin divalent ion content and response to ultraviolet phototherapy. Am J Kidney Dis. 1985;5(5):237–241.
- Chou FF, Ho JC, Huang SC, et al. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2000;190(1):65–70.
- Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632. DOI:10.1038/sj.ki.5000251
- Hashimoto T, Yosipovitch G. Itching as a systemic disease. J Allergy Clin Immunol. 2019;144(2):375–380.
- Elman S, Hynan LS, Gabriel V, et al. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593.
- Lipman ZM, Paramasivam V, Yosipovitch G, et al. Clinical management of chronic kidney disease-associated pruritus: current treatment options and future approaches. Clin Kidney J. 2021;14(Supplement_3):i16–22.
- Kardon AP, Polgár E, Hachisuka J, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82(3):573–586. DOI:10.1016/j.neuron.2014.02.046
- Cowan A, Kehner GB, Inan S. Targeting Itch with Ligands Selective for κ Opioid Receptors. Handb Exp Pharmacol. 2015;226:291–314.
- Fishbane S, Wen W, Munera C, et al. Long-term safety and efficacy of difelikefalin in patients with chronic kidney disease-associated pruritus: analysis from KALM-1 and KALM-2. Am J Kidney Diseases. 2021;77(4):593–594.
- US Food and Drug Administration. Korsuva Prescribing Information. 2021. [cited 2021 Apr]. Available from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214916s000lbl.pdf
- Lipman ZM, Yosipovitch G. An evaluation of difelikefalin as a treatment option for moderate-to-severe pruritus in end stage renal disease. Expert Opin Pharmacother. 2021;22(5):549–555.
- Kozono H, Yoshitani H, Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch(®) capsules 2.5 μg) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9–24.
- Mathur VS, Kumar J, Crawford PW, et al. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine er tablets for uremic pruritus. Am J Nephrol. 2017;46(6):450–458.
- Duque MI, Yosipovitch G, Fleischer AB Jr., et al. Lack of efficacy of tacrolimus ointment 0.1% for treatment of hemodialysis-related pruritus: a randomized, double-blind, vehicle-controlled study. J Am Acad Dermatol. 2005;52(3 Pt 1):519–521.
- Pour-Reza-Gholi F, Nasrollahi A, Firouzan A, et al. Low-dose doxepin for treatment of pruritus in patients on hemodialysis. Iran J Kidney Dis. 2007;1(1):34–37.
- Smith PF, Corelli RL. Doxepin in the management of pruritus associated with allergic cutaneous reactions. Ann Pharmacother. 1997;31(5):633–635.
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41(4):533–539.
- Bigliardi PL, Stammer H, Jost G, et al. Treatment of pruritus with topically applied opiate receptor antagonist. J Am Acad Dermatol. 2007;56(6):979–988.
- Phan NQ, Bernhard JD, Luger TA, et al. Antipruritic treatment with systemic μ-opioid receptor antagonists: a review. J Am Acad Dermatol. 2010;63(4):680–688.
- Szepietowski JC, Morita A, Tsuji T. Ultraviolet B induces mast cell apoptosis: a hypothetical mechanism of ultraviolet B treatment for uraemic pruritus. Med Hypotheses. 2002;58(2):167–170.
- Sherjeena PB, Binitha MP, Rajan U, et al. A controlled trial of narrowband ultraviolet B phototherapy for the treatment of uremic pruritus. Indian J Dermatol Venereol Leprol. 2017;83(2):247–249. DOI:10.4103/0378-6323.198464
- Ada S, Seçkin D, Budakoğlu I, et al. Treatment of uremic pruritus with narrowband ultraviolet B phototherapy: an open pilot study. J Am Acad Dermatol. 2005;53(1):149–151.
- Sharma D, Kwatra SG. Thalidomide for the treatment of chronic refractory pruritus. J Am Acad Dermatol. 2016;74(2):363–369.
- Silva S, Viana P, Lugon N, et al. Thalidomide for the treatment of uremic pruritus: a crossover randomized double-blind trial. Nephron. 1994;67(3):270–273.
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris). 1997;153 Suppl 1:S39–45.
- Eusebio-Alpapara KMV, Castillo RL, Dofitas BL. Gabapentin for uremic pruritus: a systematic review of randomized controlled trials. Int J Dermatol. 2020;59(4):412–422.
- Ishida JH, McCulloch CE, Steinman MA, et al. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. 2018;29(7):1970–1978.
- Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch Dermatol. 1993;129(5):577–581.
- Amirkhanlou S, Rashedi A, Taherian J, et al. Comparison of gabapentin and ketotifen in treatment of uremic pruritus in hemodialysis patients. Pak J Med Sci. 2016;32(1):22–26.
- Tarng DC, Cho YL, Liu HN, et al. Hemodialysis-related pruritus: a double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron. 1996;72(4):617–622.
- Breneman DL, Cardone JS, Blumsack RF, et al. Topical capsaicin for treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992;26(1):91–94.
- Young TA, Patel TS, Camacho F, et al. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatol Treat. 2009;20(2):76–81. DOI:10.1080/09546630802441218
- Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97–103.
- Pakfetrat M, Malekmakan L, Hashemi N, et al. Sertraline can reduce uremic pruritus in hemodialysis patient: a double blind randomized clinical trial from Southern Iran. Hemodial Int. 2018;22(1):103–109.
- Chan KY, Li CW, Wong H, et al. Use of sertraline for antihistamine-refractory uremic pruritus in renal palliative care patients. J Palliat Med. 2013;16(8):966–970. DOI:10.1089/jpm.2012.0504
- Electronic Medicines Compendium. Serotonin prescribing information. 2020. [cited 2023 Apr]. Available from: https://www.medicines.org.uk/emc/product/7162/smpc
- Min S, Kim KW, Jung WM, et al. Acupuncture for histamine-induced itch: association with increased parasympathetic tone and connectivity of putamen-midcingulate cortex. Front Neurosci. 2019;13:215.
- Panahi Y, Dashti-Khavidaki S, Farnood F, et al. Therapeutic effects of omega-3 fatty acids on chronic kidney disease-associated pruritus: a literature review. Adv Pharm Bull. 2016;6(4):509–514.
- Ghanei E, Zeinali J, Borghei M, et al. Efficacy of omega-3 fatty acids supplementation in treatment of uremic pruritus in hemodialysis patients: a double-blind randomized controlled trial. Iran Red Crescent Med J. 2012;14(9):515–522.
- Morton CA, Lafferty M, Hau C, et al. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant. 1996;11(10):2031–2036.
- Hiroshige K, Kabashima N, Takasugi M, et al. Optimal dialysis improves uremic pruritus. Am J Kidney Dis. 1995;25(3):413–419.
- Liakopoulos V, Krishnan M, Stefanidis I, et al. Improvement in uremic symptoms after increasing daily dialysate volume in patients on chronic peritoneal dialysis with declining renal function. Int Urol Nephrol. 2004;36(3):437–443. DOI:10.1007/s11255-004-8788-9
- Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS ONE. 2013;8(8):e71404. DOI:10.1371/journal.pone.0071404
- Uchiumi N, Sakuma K, Sato S, et al. The clinical evaluation of novel polymethyl methacrylate membrane with a modified membrane surface: a multicenter pilot study. Renal Replacement Ther. 2018;4(1):32. DOI:10.1186/s41100-018-0170-y
- Lin HH, Liu YL, Liu JH, et al. Uremic pruritus, cytokines, and polymethylmethacrylate artificial kidney. Artif Organs. 2008;32(6):468–472. DOI:10.1111/j.1525-1594.2008.00568.x
- Agarwal R, Burton J, Gallieni M, et al. Clin Kidney J, Sfac. 2022;16(1):30–40. DOI:10.1093/ckj/sfac187.
- Raouf M, Atkinson TJ, Crumb MW, et al. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J Pain Res. 2017;10:275–278.
- Zand L, McKian KP, Qian Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am j med. 2010;123(4):367–373.
- Shram MJ, Spencer RH, Qian J, et al. Evaluation of the abuse potential of difelikefalin, a selective kappa-opioid receptor agonist, in recreational polydrug users. Clin Transl Sci. 2021;15(2):535–547.
- Viscusi ER, Torjman MC, Munera CL, et al. Effect of difelikefalin, a selective kappa opioid receptor agonist, on respiratory depression: a randomized, double-blind, placebo-controlled trial. Clin Transl Sci. 2021;14(5):1886–1893.
- Albert-Vartanian A, Boyd MR, Hall AL, et al. Will peripherally restricted kappa-opioid receptor agonists (pKoras) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Therapeutics. 2016;41(4):371–382. DOI:10.1111/jcpt.12404
- Fishbane S, Mathur V, Germain MJ, et al. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep. 2020;5(5):600–610. DOI:10.1016/j.ekir.2020.01.006
- Ahdoot R, Kalantar-Zadeh K, McCafferty K, et al. POS-601 IMPROVEMENT in SLEEP QUALITY from REDUCTION of ITCH INTENSITY in PATIENTS with MODERATE-TO-SEVERE PRURITUS UNDERGOING HEMODIALYSIS. Kidney Int Rep. 2022;7(2):S258. DOI:10.1016/j.ekir.2022.01.634
- Fishbane S, Clegg DJ, Lerma EV, et al. Safety and efficacy of difelikefalin in black or African American Patients on Hemodialysis with CKD-Associated Pruritus: pooled Analysis of KALM-1 and KALM-2. Abstract PO0805; American Society of Nephrology Kidney Week; November 4, 2021; Virtual
- Fishbane S, Walpen S, Schaufler T, et al. 405 effect of treatment with difelikefalin on itch severity in patients with chronic kidney disease-associated pruritus measured by the patient global impression of change. Am J Kidney Diseases. 2022;79(4):S123.
- Csiky B, Walpen S, Schaufler T, et al. MO467: time to Improvement of Itch Intensity in Patients with Chronic Kidney Disease-Associated Pruritus Treated with Difelikefalin. Nephrol Dialysis Transplantation. 2022;37(Supplement_3):gfac070.081.
- Weiner DE, Vervloet MG, Walpen S, et al. Kidney Medicine. 2022;4(10):100542. DOI:10.1016/j.xkme.2022.100542.
- Weiner DE, Walpen S, Schaufler T, et al. Itch Reduction with Difelikefalin Correlates with Improved Sleep Quality in Hemodialysis Patients with Pruritus. In: Abstract FR-OR26; American Society of Nephrology Kidney Week; November 5, 2021; Virtual.
- Weiner D, Budden J, Woods S, et al. 406 Improvement in Itch-Related Sleep Disruption with Difelikefalin in Patients with Moderate to Severe Chronic Kidney Disease Associated Pruritus Undergoing Hemodialysis: a Post Hoc Analysis of an Open-Label, Multicenter Study. Am J Kidney Diseases. 2022;79(4):S124.
- Weiner DE, Vervloet MG, Menzaghi F, et al. Improvement of Itch with Difelikefalin in CKD Patients on Dialysis by Baseline Itch Severity. Abstract SA-PO286; American Society of Nephrology Kidney Week; November 5, 2022; Florida, U.S.A.
- Vernon M, Ständer S, Munera C, et al. Clinically meaningful change in itch intensity scores: an evaluation in patients with chronic kidney disease-associated pruritus. J Am Acad Dermatol. 2021;84(4):1132–1134.
- Hays RD, Kallich JD, Mapes DL, et al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–338.
- Scott W, McCracken LM. Patients’ impression of change following treatment for chronic pain: global, specific, a single dimension, or many? J Pain. 2015;16(6):518–526.
- Johansen KL, Chertow GM, Foley RN, et al. US Renal Data System 2020 Annual Data Report: epidemiology of Kidney Disease in the United States. Am J Kidney Diseases. 2021;77(4):A7–8. DOI:10.1053/j.ajkd.2021.01.002
- ClinicalTrials.gov. A study to evaluate the safety and efficacy of difelikefalin in advanced chronic kidney disease patients with moderate-to-severe pruritus and not on dialysis. 2019. [cited 2023 Apr]. Available from: https://clinicaltrials.gov/ct2/show/NCT05342623
- European Medicines Agency. European Medicines Agency decision. 2020. [cited 2023 Apr]. Available from: https://www.ema.europa.eu/en/documents/pip-decision/p/0172/2020-ema-decision-13-may-2020-agreement-paediatric-investigation-plan-granting-deferral-granting_en.pdf
- Australian Government Department of Health and Aged Care. Prescription medicine decision summary. 25 Nov 2022. [cited 2023 Apr]. Available from: https://www.tga.gov.au/resources/auspmd/korsuva
- Health Sciences Authority. New drug approvals - August 2022. [cited 2023 Apr]. Avaialble from: https://www.hsa.gov.sg/announcements/new-drug-approval/new-drug-approvals---august-2022
- Swissmedic. Kapruvia prescribing information. 2022. [cited 2023 Apr]. Availble from: https://www.swissmedicinfo.ch/ViewMonographie
- Health Canada. Summary basis of decision- Korsuva. 2022. [cited 2023 Apr]. Available from: https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?lang=en&linkID=SBD00615&lang=en
- SONG Executive Committee. Standardised Outcomes in Nephrology Initiative. 2021. [cited 2023 Apr]. Available from:https://songinitiative.org/
- Kalantar-Zadeh K, Lockwood MB, Rhee CM, et al. Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat Rev Nephrol. 2022;18(3):185–198. DOI:10.1038/s41581-021-00518-z
- Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–459. DOI:10.1038/ki.2015.110
- Combs SA, Teixeira JP, Germain MJ. Pruritus in Kidney Disease. Semin Nephrol. 2015;35(4):383–391.
- Weisshaar E, Szepietowski JC, Dalgard FJ, et al. European S2k Guideline on Chronic Pruritus. Acta Derm Venereol. 2019;99(5):469–506. DOI:10.2340/00015555-3164
- Kalantar-Zadeh K, Kam-Tao Li P, Tantisattamo E, et al. Living well with kidney disease by patient and care-partner empowerment: kidney health for everyone everywhere. Kidney Int. 2021;99(2):278–284. DOI:10.1016/j.kint.2020.11.004