Oral P2Y12 inhibitors represent the cornerstone of treatment for patients undergoing percutaneous coronary interventions (PCI) [Citation1]. In patients undergoing PCI, oral P2Y12 inhibitors are generally used in addition to aspirin therapy, a regimen known as dual antiplatelet therapy (DAPT) [Citation1]. While traditionally patients undergoing PCI would discontinue P2Y12 inhibiting therapy after a minimum required duration of treatment and maintain single antiplatelet therapy with aspirin, more recent evidence has suggested the benefit of dropping aspirin and maintaining P2Y12 inhibitor monotherapy as a more advantageous regimen compared with aspirin monotherapy or standard DAPT [Citation2]. However, the term ‘oral P2Y12 inhibitor’ embraces a number of compounds with different pharmacokinetic (PK) and pharmacodynamic (PD) profiles which translate into different clinical outcomes [Citation3]. Oral P2Y12 inhibitors may belong to either the family of thienopyridines (i.e. clopidogrel and prasugrel) or cyclopentyltriazolopyrimidines (i.e. ticagrelor) [Citation3]. Compared with clopidogrel, prasugrel and ticagrelor are characterized by more ‘potent’ platelet inhibitory effects, resulting in lower rates of high platelet reactivity (HPR), a marker of thrombotic events such as stent thrombosis (ST) or myocardial infarction (MI) [Citation4]. However, more potent platelet inhibition inevitably translates into increased bleeding which carries important prognostic implications, including increased mortality, underscoring the importance of bleeding reduction strategies among patients undergoing PCI [Citation3,Citation5]. Indeed, tailoring antiplatelet treatment regimens to an individual patient to optimize their safety and efficacy has represented a field of extensive investigation over the past decade [Citation6].
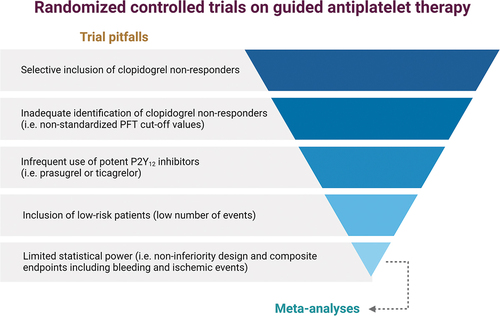
Growing evidence supports the notions that the superior efficacy of the more ‘potent’ P2Y12 inhibitors over clopidogrel is not solely attributable to their enhanced reduction of platelet reactivity but also to their more predictable PK/PD effects compared with clopidogrel. In fact, while 10% to 40% of clopidogrel-treated patients have impaired platelet inhibition resulting in HPR, also known as ‘poor responders,’ the rates of ‘poor responders’ to prasugrel of ticagrelor are trivial (<5%) [Citation7]. The pharmacological differences behind the disparities between platelet inhibitory effects of oral P2Y12 inhibitors are discussed in detail elsewhere [Citation8]. In brief, the hepatic cytochrome P450 (CYP) system, particularly the CYP2C19 enzyme, is responsible in large part for the biotransformation of clopidogrel into its active metabolite. Of note, CYP2C19 is transcripted by a gene characterized by multiple genetic variants affecting the function of this enzyme with carriers of loss-of-function (LoF) alleles associated with impaired clopidogrel metabolism and reduced clopidogrel-induced platelet inhibition [Citation8,Citation9]. These observations have prompted box warning on the product label of clopidogrel suggesting the use of alternative agents (i.e. prasugrel or ticagrelor) among carriers of LoF alleles requiring oral P2Y12 inhibiting therapy [Citation10]. However, evidence from randomized controlled trials (RCTs) on the use of a guided selection of oral P2Y12 inhibiting therapy has failed to reach unequivocal results. An important determinant of this failure lies in the fact that early RCTs, which used platelet function testing (PFT) rather than genetic testing to guide the selection of antiplatelet therapy, were compromised by pitfalls in trial design, such as the inclusion of low-risk patients, inadequate definitions of poor clopidogrel responders, and the infrequent use of potent P2Y12 inhibitors to overcome clopidogrel non-responsiveness (Figure) [Citation11]. Such disappointing results of early trials of a PFT-guided strategy inevitably led to skepticism toward personalized treatment approaches. However, RCTs using genetic testing to guide the selection of oral P2Y12 inhibiting therapy in patients undergoing PCI were designed in light of the limitations of earlier RCTs using PFT, leading to more promising results.
The differentiation of two lines of research reflecting different strategies to guide the selection of oral P2Y12 inhibiting therapy has played a key role in the appraisal of the available evidence on this complex and interwoven topic [Citation12]. The first strategy compared the use of potent P2Y12 inhibitors versus clopidogrel selectively among clopidogrel non-responders, and the second strategy compared the use of a guided approach among the totality of patients undergoing PCI. While the first strategy played a role in supporting the rationale for the use of a guided selection of P2Y12 inhibitors among PCI patients [Citation13], trials from the second line of research were key to answer to the fundamental clinical question of whether or not a guided selection of P2Y12 inhibiting therapy may be associated with better outcomes compared to a non-guided approach [Citation11,Citation12].
Promising trials from the second line of research adopting a genotype-guided strategy include PHARMCLO, which was the first to explore the potential benefit of a genotype-guided selection of antiplatelet therapy among 888 acute coronary syndrome (ACS) patients, as well as a recent study by Al-Rubaish et al. that included 755 ST-segment elevation ACS (STE-ACS) patients [Citation14,Citation15]. Nevertheless, the most relevant contributions in the field are represented by the POPular Genetics and the TAILOR-PCI trials. POPular Genetics was a RCT including 2488 STE-ACS patients randomized to either genotype-guided or standard therapy (mainly ticagrelor) within 48 hours after PCI [Citation16]. The trial met both the non-inferiority primary endpoint of net adverse clinical events (a composite of death from any cause, MI, definite ST, stroke, or major bleeding defined according to Platelet Inhibition and Patient Outcomes (PLATO) criteria) and found a significant 22% reduction of the co-primary endpoint of PLATO major and minor bleeding at 12 months favoring the guided arm [Citation16]. Importantly, ischemic events were not increased but numerically reduced in the guided as compared to standard therapy arm (2.7% vs 3.3% for the combined composite endpoint of CV death, MI, ST, or stroke) [Citation16]. TAILOR-PCI is the largest (n = 5302) trial comparing guided versus standard (mainly ticagrelor) antiplatelet therapy in both ACS (69%) and chronic coronary syndrome (31%) [Citation17]. The primary analysis was conducted among patients with CYP2C19 LoF variants, and secondary analysis included all randomized patients (corresponding to the first and second lines of research, respectively, as discussed above).
With regard to the more clinically relevant analysis focusing on all randomized patients, the ischemic endpoint (a composite of CV death, MI, stroke, ST, and severe recurrent ischemia) at 12 months was non-significantly reduced in the guided as compared to standard therapy arm (4.4% vs 5.3%; HR, 0.84 [95% CI, 0.65–1.07]; p = 0.16) [Citation17]. However, the trial was underpowered to show any significant differences between these groups, also because of the lower than expected incidence of events and the use of a rather ambitious 85% power to detect a minimum HR of 0.50. Collectively, the main limitations of recent RCTs in the setting of a genotype-guided selection of antiplatelet therapy lie in the low statistical power for hard endpoints, the sample size, the use of non-inferiority designs, and the adoption of composite endpoints including both ischemic and bleeding outcomes (). To this extent, meta-analyses may be useful in improving statistical power. Recent comprehensive meta-analyses found that a guided selection of antiplatelet therapy may improve outcomes compared to a standard therapy and provide the best performance in terms of safety and efficacy compared to other regimens such as DAPT with ‘potent’ P2Y12 inhibitors or unguided use of clopidogrel [Citation18,Citation19].
Although genetic testing has the advantages of being easy-to-use and identifying a genetic makeup that does not vary over time, they also have the disadvantage that CYP2C19 genotypes represent only one of the factors contributing to clopidogrel response and that the incidence of LoF alleles varies widely across different ethnicities [Citation8]. Integrating genetic data with clinical variables (age, body mass index, chronic kidney disease, and diabetes mellitus) such as in the ABCD-GENE score can enhance the accuracy in identifying individuals with impaired clopidogrel response (i.e. HPR status) [Citation20,Citation21]. Moreover, the individual response to P2Y12 inhibitors may be affected not onlyby clinical variables but also by sex-related and demographic characteristics [Citation6]. Specifically, females, who are often underrepresented in RCTs, may display a different response to antiplatelet therapy compared to males [Citation22]. Moreover, Asian presents are at increased risk of bleeding and reduced risk of ischemic events despite the higher prevalence of CYP2C19 LoF alleles compared to the general population, contributing to the so-called ‘Asian Paradox.’ These observations limit the application of the evidence from RCTs conducted in Asian populations to non-Asian patients [Citation6].
In conclusion, oral P2Y12 inhibitors are not all born equal, and results from RCTs and meta-analysis exploring the impact of implementing tools for a guided selection of these agents, particularly genotype testing, call for a larger use of a precision medicine approach for the selection of antiplatelet therapy after PCI. Nevertheless, the available data still suggest the need for more evidence to support the benefit of using a guided selection of oral P2Y12 inhibiting therapy with future studies requiring more details in trial design and sample size calculation to enhance the statistical power for hard endpoints. Studies also taking into account economic and practical limitations surrounding the use of tools for a guided selection of antiplatelet therapy are warranted.
Declaration of interest
M Galli declares that he has received consulting fees or honoraria from Terumo, outside the present work. DJ Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura, outside the present work. DJ Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R MacKenzie Foundation.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Angiolillo DJ, Galli M, Collet JP, et al. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371–96.
- Galli M, Capodanno D, Andreotti F, et al. Safety and efficacy of P2Y(12) inhibitor monotherapy in patients undergoing percutaneous coronary interventions. Expert Opin Drug Saf. 2021;20(1):9–21.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12(1):30–47.
- Aradi D, Kirtane A, Bonello L, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36(27):1762–1771. DOI:10.1093/eurheartj/ehv104
- Capodanno D, Bhatt DL, Gibson CM, et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol. 2022;19(2):117–132. DOI:10.1038/s41569-021-00598-1
- Galli M, Ortega-Paz L, Franchi F, et al. Precision medicine in interventional cardiology: implications for antiplatelet therapy in patients undergoing percutaneous coronary intervention. Pharmacogenomics. 2022;23(13):723–737.
- Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(16):1521–1537. DOI:10.1016/j.jcin.2019.03.034
- Galli M, Franchi F, Rollini F, et al. Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations. Expert Rev Clin Pharmacol. 2021;14(8):1–16. DOI:10.1080/17512433.2021.1927709
- Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–1830.
- Simon T, Bhatt DL, Bergougnan L, et al. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin Pharm Therap. 2011;90(2):287–295.
- Galli M, Franchi F, Rollini F, et al. Role of platelet function and genetic testing in patients undergoing percutaneous coronary intervention. Trends Cardiovasc Med. 2021 Dec 20;S1050-1738(21):00157.
- Galli M, Franchi F. Guided selection of antiplatelet therapy in acute coronary syndrome: impact on outcomes and resource utilization. Int J Cardiol. 2021 Dec 15;345:36–38.
- Pereira NL, Rihal C, Lennon R, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y(12) inhibitor therapy: a meta-analysis. JACC Cardiovasc Interv. 2021;14(7):739–750.
- Notarangelo FM, Maglietta G, Bevilacqua P, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLOPHARMCLO trial. J Am Coll Cardiol. 2018;71(17):1869–1877.
- Al-Rubaish AM, Al-Muhanna FA, Alshehri AM, et al. Bedside testing of CYP2C19 vs. conventional clopidogrel treatment to guide antiplatelet therapy in ST-segment elevation myocardial infarction patients. Int J Cardiol. 2021;343:15–20.
- Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N Engl J Med. 2019;381(17):1621–1631.
- Pereira NL, Farkouh ME, So D, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324(8):761–771.
- Galli M, Benenati S, Capodanno D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet (London, UK). 2021;397(10283):1470–1483.
- Galli M, Benenati S, Franchi F, et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur Heart J. 2022 Mar 7;43(10):959–967.
- Angiolillo DJ, Capodanno D, Danchin N, et al. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: the ABCD-GENE score. JACC Cardiovasc Interv. 2020;13(5):606–617.
- Capodanno D, Angiolillo DJ, Lennon RJ, et al. ABCD-GENE score and clinical outcomes following percutaneous coronary intervention: insights from the TAILOR-PCI trial. J Am Heart Assoc. 2022;11(4):e024156.
- Laborante R, Borovac JA, Galli M, et al. Gender-differences in antithrombotic therapy across the spectrum of ischemic heart disease: time to tackle the Yentl syndrome? Front Cardiovasc Med. 2022;9:1009475.