Abstract
A discrete-time susceptible and infected (SI) epidemic model, with less than 100% vertical disease transmission, for the spread of a fungal disease in a structured amphibian host population, is analysed. Criteria for persistence of the population as well as the disease are established. Stability results for host extinction and for the disease-free equilibrium are presented. Bifurcation theory is used to establish existence of an endemic equilibrium.
1. Introduction
Amphibian populations have been declining worldwide for decades Citation11 Citation15 Citation17 due to habitat loss and disease. According to Skerratt Citation11, the relatively recent global emergence of the fungal disease chytridiomycosis has resulted in ‘the most spectacular loss of vertebrate biodiversity due to disease in recorded history’. Individual frogs contract this disease either when their skin comes into contact with water that contains spores from infected animals, or by direct contact with any infected animal. After infection, the fungus invades the surface layers of the frog skin, causing damage to the keratin layer.
A family of discrete-time models that incorporate the life-stage structure of an amphibian population, which may include larva (L), juvenile (J) and adult (A) stages, afflicted with a fungal disease were formulated by Emmert and Allen Citation6. The most general models were of LJA-SI and LJA-susceptible, infected and recovered (SIR) type, meaning that they included all three stages and were of SI or SIR epidemic type. A notable feature of the models was the assumption of 100% efficient vertical transmission of disease from infected adults to their larval offspring. The authors also considered several sub-models characterized by simpler population stage structure (JA or even simply A). Their mathematical results establish persistence and extinction of the population, not of the disease, but persistence is established in a somewhat narrow sense – the existence of a disease-free equilibrium. Numerous numerical simulations and bifurcation diagrams show that quite complicated dynamic behaviour is possible in the models, especially, with the Ricker recruitment nonlinearity and large intrinsic birth rates.
Emmert and Allen extended their models to seasonal models Citation7, by considering the birth functions, as well as the survival probabilities within stages, and transition probabilities from one stage to another to be periodic functions. In the same paper, they also analyse some stochastic versions of the models (discrete-Markov chains branching processes). Using the ‘next generation matrix approach’, Allen and van den Driessche Citation1 obtained explicit formulae for the basic reproductive ratio for the adult model with infection included.
Motivated by Citation6, the authors in Citation10 applied theoretical ideas from persistence theory to the model of Allen and Emmert to establish that: Equation(1) the disease cannot lead to the extinction of the amphibian population (persistence of the host) and Equation(2)
the disease can become endemic in the population (persistence of the disease) under suitable conditions. Persistence here is used in the stronger, dynamical sense of persistence theory Citation14
Citation18. Along with the natural condition for persistence of the disease, namely, that the disease-free state is unstable to invasion of the disease, we were forced to assume that the disease-free dynamics was convergent. More precisely, we assumed that in the absence of the disease, the population settled into a globally attracting fixed point. While we could give sufficient conditions for global convergence, and although simulations suggested it occurred for a much larger parameter set, nonetheless, it is a quite restrictive hypothesis, as simulations in Citation6 clearly show.
The paper of Thieme et al. Citation16 examines the question of whether a disease can drive a host population to extinction or not. They proposed a traditional ordinary differential equation model with mass action incidence but with general disease-free host dynamics that may include an Allee effect. Among many interesting outcomes, one is host eradication due to the disease. In recent models of chytridiomycosis, Briggs et al. Citation3 and Mitchell et al. Citation9 also show through numerical simulations that the extinction of the amphibian host can occur. Mitchell et al. use a deterministic model, in which extinction is interpreted in the sense of total population decreasing below a given threshold. In Citation3 extinction may occur due to the stochastic nature of the model.
The present paper builds on our earlier work Citation10. As noted above, the Emmert–Allen model assumes 100% efficient vertical transmission of the disease from infected host to their offspring. An unusual consequence of this assumption for the LJA-SI model is that, in addition to the usual disease-free invariant set, there exists a dynamical regime (invariant set) in which the host population consists entirely of infected individuals. It seems unlikely that this state could be an attractor for realistic parameter regions and therefore it probably can be ignored on biological grounds since a viable ‘disease-free’ host population is a prerequisite for the emergence of disease. However, this feature certainly complicated the mathematics. Moreover, from a biological point of view, the hypothesis of completely faithful vertical transmission seems unlikely and from the mathematical point of view it leads to a structurally unstable system. For, if the model is modified by assuming that a (very small) proportion of offspring of infected adults is born susceptible, then the ‘all-infectives dynamical regime’ disappears. For this reason, we are motivated to modify the Emmert–Allen model as just described, and to reconsider our analysis in Citation10. Furthermore, motivated by issues of mathematical tractability, we restrict attention here to the JA-SI model, dropping consideration of the larval stage.
Our earlier results Citation10 continue to hold for the modified model and we can sharpen some of these. We establish that the disease cannot drive the host to extinction, as we did in Citation10, and we provide sufficient conditions for the disease to persist in the population which are completely similar to those in Citation10, requiring the restrictive hypothesis of a globally attracting steady state for the disease-free subsystem. We hope to relax this assumption in future work. However, we can obtain somewhat better sufficient conditions for this global convergence to hold due to the lower dimensionality of the disease-free system in the absence of a larval stage. Finally, we employ an extension of the Rabinowitz global bifurcation theorem to establish the existence of an endemic disease steady state for all values of the disease transmission rate exceeding the critical value at which the disease-free state loses stability. The illuminating application of the Rabinowitz bifurcation theorem to a broad class of models in population dynamics has been championed by Cushing Citation4 Citation5. We dedicate this paper to him on the occasion of his 65th birthday.
In the next section, we state our results without proofs. Unless otherwise specified, proofs can be found in Section 4.
2. The SI juvenile and adult model
The Emmert–Allen model Citation6 considers Juvenile J, and Adult A stages of the host population, labelled with subscripts S, I indicating their status as susceptible or recovered. F denotes density of fungus in the environment. We consider here the case when we have less than 100% vertical transmission. In addition, all parameters in the model are assumed to be positive, except for and
(see below), which are assumed to be non-negative (we allow them to be zero). The JA-SI model is
| • | p JK , p AK are the probabilities of survival within a stage; | ||||
| • | p F is the probability that fungi survive in the environment without a live host; | ||||
| • |
q
LK
, q
JK
are the probabilities of transition among stages, but remaining in the same class (susceptible or infected) – for examples | ||||
| • | b K φ(T) is the number of eggs/adults that survive to larval stage; | ||||
| • | b F is the birth of fungal zoospores from growth on dead larvae, juveniles or adults; | ||||
| • |
| ||||
The probability of becoming infected in stage N:(N=J, A), that is, , increases with increasing values of infection in the population and environment, infection represented by the weighted sum of the infected stages and fungus:
The following inequalities among parameters are assumed:
Let
Also, we denote by x, in short. In our next result, we analyse the boundedness of Equation(1)
, as well as the existence of boundary fixed points.
Proposition 2.1
The following hold:
| a. |
If c
AK
>0, K=S, I then
Equation(1) | ||||
| b. |
| ||||
| c. |
There exists a unique non-trivial boundary fixed point E
S
of
Equation(1) | ||||
Parts (b) and (c) are trivial to check, and for a proof of (a) see an analogous result in [Citation10, Proposition 2.1].
represents no disease states, and the F-axis represents no host states.
Notice that 0 is always a fixed point of Equation(1).
2.1. Disease-free dynamics
When infection is not present, the model takes the form
Parameter Δ S distinguishes between extinction and survival of the host.
Theorem 2.2
The following hold:
| a. | If Δ S <1 then 0 is a globally asymptotically stable fixed point. | ||||
| b. |
If Δ
S
>1 then there exists
| ||||
The proof of this theorem is similar to that of one of [Citation10, Theorem 3.3], and we will omit it here. Part (a) can also be found in Citation6.
In the following theorem we give sufficient conditions for global stability of E S .
Theorem 2.3
Assume that Δ
S
>1, and
is asymptotically stable. Let
. In any of the following two cases,
attracts all solutions starting in
| a. |
| ||||
| b. |
| ||||
Conditions in Theorem 2.3 hold, for example, for the set of parameters given in the first column of except for b
S
=16, where , but we can extend the range of these parameters to nearby values (see Citation13). Although, numerical simulations suggest that the conclusion of Theorem 2.3 holds for a much larger set of parameters than required by our hypotheses. However, simulations in Citation7 suggest that the dynamics exhibit a period-doubling cascade as parameter b
S
is increased.
Table 1. Parameters used in the numerical simulations.
2.2. Main results
We begin by determining the stability of the boundary fixed points of Equation(1). Then we establish sufficient conditions for persistence of the host and of the disease, and finally we analyse the bifurcation of a positive, endemic equilibrium from E
S
.
The Jacobian of Equation(1) at 0 is
The relationship between Δ
K
(see Equation(3)) and
, is given in the following Lemma.
Lemma 2.4
, with equality if and only if Δ
K
=1.
One can view the Δ K s as basic reproductive numbers (see Citation1).
The matrix
The 5×5 Jacobian matrix J(E S ) evaluated at E S decomposes as
Below we give a result with regard to the stability of the boundary fixed points.
Theorem 2.5
The following hold:
| a. |
0
is asymptotically stable if
| ||||
| b. |
If E
S
is asymptotically stable in
| ||||
Now we give sufficient conditions for persistence of the host and for persistence of both the host and the disease.
Theorem 2.6
Persistence and positive fixed points for
Equation(1):
| I. |
If Δ
S
>1 then there exists an
| ||||||||||||||||
| II. | If one of the following holds:
| ||||||||||||||||
Under the hypotheses of Theorem 2.6(II) there exists a positive ‘endemic fixed point’, which may or may not be stable. The first two hypotheses in (IIa) are natural, E
S
should exist and be unstable, but the last hypothesis (‘E
S
is asymptotically stable in and attracts all initial data in
’) is too strong; hypotheses in (IIb) are biologically implausible as
is expected.
An alternative approach to obtain a positive fixed point is to use the bifurcation theory, but then we must select a ‘bifurcation parameter’. If we fix disease-free parameters () then E
S
is fixed and we may vary disease-associated parameters (
,
,
,
, b
I
, f, w
K
, v
K
, β
K
; K=J, A). The matrix
is monotone increasing in all these parameters, so by the Perron–Frobenius theory, its principal eigenvalue is monotone increasing in these parameters. Roughly speaking, stability of E
S
is reduced by the increase of any of these. We select the disease transmission rates
for special attention in our next result, but this choice is somewhat arbitrary. Other natural choices include the vertical transmission efficiency f, disease-related reduction in maximal reproduction rate b
I
=b
S
−μ, disease-related increase in adult death
, disease-related decrease in juvenile maturation
, or infection weighting factors w
K
, v
K
.
Theorem 2.7
Let
and assume that E
S
is a non-degenerate fixed point of the disease-free system and that
. Then
is continuous and strictly increasing on [0, ∞) with r(0)<1 and
. There exists a unique β0>0 such that
, E
S
is unstable for
and for each such β there exists at least one positive fixed point in
.
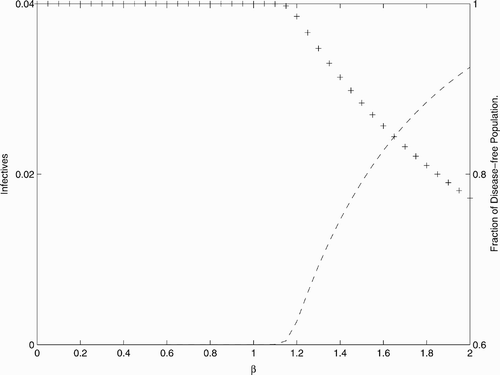
As our calculations indicate, for the values of parameters given in (, not given in the table, are all equal to 0.1), the bifurcation for the positive fixed point occurs at a value of β in the interval (1.16, 1.17) (see also ). By increasing the value of β (in a certain neighbourhood of the bifurcation value), the positive fixed point increases in the I components and decreases in the S components, as it can be observed from . Our last result shows that the population decreases at a fixed point from E
S
level to the endemic fixed point.
Proposition 2.8
Assume that
3. Discussion
As noted in the introduction, the Emmert–Allen model Citation6 assumes 100% efficient vertical transmission of the disease from the infected host to their offspring. Because this seems unlikely to be satisfied, we have introduced a vertical transmission efficiency parameter f∈[0, 1) in the JA-SI model of Citation6. When f=1 we recover the original model, while for f<1 the fraction 1−f of offspring of infected host begin life disease-free and susceptible.
Our previous work Citation11 provides sufficient conditions for persistence of the host and for persistence of the disease for both the LJA-SI and LJA-SIR models formulated by Emmert and Allen Citation6. These same results carry over to the JA-SI model, modified to include the vertical transmission efficiency parameter, considered here. Specifically, the disease cannot drive the host population to extinction (Theorem 2.6 (I)) and when the disease can invade the disease-free fixed point then the disease persists, provided the disease-free state attracts all non-trivial initial data for the disease-free system (Theorem 2.6 (IIa)).
By neglecting the larval stage of the full model, which we did here, we were able to obtain somewhat better sufficient conditions for this global convergence to hold in Theorem 2.3 than in the corresponding result in Citation10. Strictly speaking, the results are not comparable but here we were not forced to assume either that vanishes or that the system is monotone Citation12.
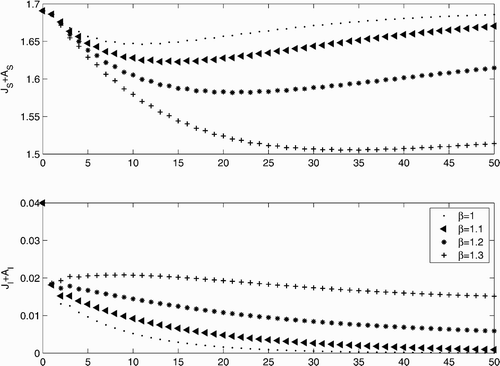
Our result guaranteeing persistence of the disease also implies the existence of a positive fixed point – the endemic equilibrium. An alternative approach to showing the existence of this equilibrium is to use bifurcation theory, which avoids any assumptions on the disease-free dynamics. We used an extension of the Rabinowitz global bifurcation theorem to establish the existence of an endemic disease steady state for all values of the disease transmission rate exceeding the critical value at which the disease-free state loses stability. Furthermore, we showed analytically and numerically that the host population at the endemic fixed point is less than the host population at the disease-free fixed point. Our numerical calculations show that this reduction can be substantial. The large reduction in host population due to the disease would leave the host vulnerable to extinction in a model that includes demographic stochasticity.
4. Proofs
We first introduce some notation that will be used throughout this section. We denote by A
T the transpose of matrix A, and by O
mn
the m×n zero matrix. As it is clear from the context, we will omit the subscripts, and simply write O for O
mn
. We call the matrix
-
positive, and write A>O if
and A≠O;
-
strictly positive, and write A≫O if
-
non-negative, and write A≥O if
.
We define the partial-order relation on the set of non-negative matrices as follows: . Assume analogous definitions for ‘<’ and ‘≪’. Also, we assume the same notation for vectors in ℝ
m
.
For a differentiable function , we denote the derivative of f at x by
.
We denote sequences that arise from the iteration of the difference equation in a model, by using superscripts (i.e., , etc.). Other sequences used have subscripts. The components of a vector
are denoted by
.
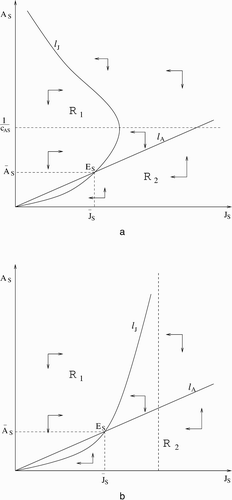
Proof of Theorem 2.3
Let and denote the right-hand side of Equation(5)
by
. Abusing notation, denote
also by E
S
.
(a) Let . Note that g is strictly increasing on ℝ+; hence
exists and it is strictly increasing. The curves
We now make the following assertion, which is trivial to check:
Now, based on (iii), we show that
| I. |
| ||||
| II. |
| ||||
| III. |
x
n
‘oscillates’ in between ℛ1 and ℛ2; x
n
belongs to each | ||||
-
(b) The J S and A S nullclines are
and l A the same as in Equation(9). It is trivial to check that u′(J S )>0 for
, hence u is strictly increasing. Also,
as
(see ), where ℛ1 and ℛ2 have the same definitions as in (a). The rest of the proof is completely analogous to the proof of (a).
Proof of Theorem 2.7
We may express Equation(1) as
where the 5×5 matrix A depends on both x and the parameter β. We want to explore the solution set of
, especially, near
so we set
. Define
. Then
is continuous. Let
, and note that S contains the ‘trivial branch’
. Also note that
can be written as
, where
, which is obviously differentiable.
We begin by establishing that a local bifurcation from the trivial branch can occur. We write the Jacobian matrix as
As has all positive entries and is strictly increasing in β, its spectral radius
is continuous and strictly increasing, by the Perron–Frobenius theory (see Citation2). r(β) lies between the column sums of
(see Citation2), and so
as
. r(0) is the spectral radius of
The nullspace of ,
, equals
, where
and v
I
is an eigenvector of
corresponding to the eigenvalue one. Hence
.
, where
, and such that
is an eigenvector of
. In order to show that a bifurcation occurs at β0 from the trivial branch 𝒯, it follows by the Crandall–Rabinowitz theorem that we must show
(see e.g. [Citation8, Theorem I.5.1]). Calculation gives
Therefore, in a neighbourhood of there are precisely two branches of fixed points of G, the trivial branch and
corresponding to
.
Now, we apply the extension of the Rabinowitz global bifurcation theorem in Kielhöfer; see [Citation8, Theorem II.5.9] for more details. Denote by the branch of solutions Equation(12)
corresponding to s≥0, and let
denote the branch of solutions Equation(12)
corresponding to s≤0. Let C denote the component of
containing
; Obviously,
. Following Citation8, let C
+ denote the maximal component containing
in
and C
− denote the maximal component containing
in
. Then
but C
+=C
− is not excluded. According to [Citation8, Theorem II.5.9], C
+ (and C
−) satisfies one of the alternatives: C
+ is unbounded (i), or it contains some
where
(ii), or it contains a pair of points
where
(iii).
Let , and note that it is open in S; then
. We claim that
is the only limit point of P not belonging to P. Let
be a limit point of P and let
. First note that
cannot hold since it is an isolated fixed point by Theorem 2.6(I). In fact, the same argument that establishes Theorem 2.6(I) implies that the persistence is robust, meaning that the same epsilon may be used for all parameters β in a neighbourhood of a given one (see Citation14). Therefore, no sequence
with
and
, such that
exists. More generally, if
then either
or
, since these are the only boundary equilibria. We have already shown that the latter cannot occur. We now show that if
then
. Indeed, the definition of
as a limit point of P implies that there exists a sequence
satisfying
and
, and where
and
. Then
The above arguments establish the claim that is the only limit point of P not belonging to P, so
. Therefore
and
can have at most the point
in common, but this point is missing from C
+. So, we may express the connected set C
+ as the disjoint union of two subsets:
; each subset is closed in C
+. One of these sets is empty since C
+ is connected, and clearly it is
. Thus C
+⊂P and, consequently, alternatives (ii) and (iii) cannot hold. We conclude that C
+ is unbounded. But the existence of a compact attracting set that is independent of β for the dynamics (see Proposition 2.1) implies that
is bounded. It follows that
is unbounded, and because it is connected and contains β0, our final assertion is proved. ▪
Proof of Proposition 2.8
Let be a fixed point and J=J
S
+J
I
and A=A
S
+A
I
. Then
Acknowledgements
Financial support was provided by a National Science Foundation Grant, DMS 0414270. The authors would like to acknowledge the useful comments of the reviewers.
References
- Allen , L. J.S. and van den Driessche , P. in press . The basic reproduction number in some discrete-time epidemic models . J. Difference Equ. Appl. ,
- Berman , A. and Plemmons , R. 1979 . Nonnegative Matrices in the Mathematical Sciences , New York : Academic Press .
- Briggs , C. J. , Vredenburg , V. T. , Knapp , R. A. and Rachowicz , L. J. 2005 . Investigating the population-level effects of chytridiomycosis: an emerging infectious disease of amphibians . Ecology , 86 : 3149 – 3159 .
- Cushing , J. M. 1977 . Integrodifferential Equations and Delay Models in Population Dynamics , New York : Springer .
- Cushing , J. M. 1998 . An Introduction to Structured Population Dynamics , Philadelphia : SIAM .
- Emmert , K. M. and Allen , L. J.S. 2004 . Population persistence and extinction in a discrete-time, stage-structured epidemic model . J. Difference Equ. Appl. , 10 : 1177 – 1199 .
- Emmert , K. M. and Allen , L. J.S. 2006 . Population extinction in Deterministic and stochastic discrete-time epidemic models with periodic coefficients with applications to amphibian populations . Natur. Resource Modeling , 19 : 117 – 164 .
- Kielhöfer , H. 2004 . Bifurcation Theory, An Introduction with Applications to PDEs , New York : Springer .
- Mitchell , K. M. , Churcher , T. M. , Garner , T. W. and Fisher , M. C. 2008 . Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction . Proc. Roy. Soc. Lond. Ser. B , 275 : 329 – 334 .
- Salceanu , P. L. and Smith , H. L. in press . Persistence in a discrete-time, stage-structured epidemic model . J. Difference Equ. Appl. ,
- Skerratt , L. F. 2007 . Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs . EcoHealth , 4 : 125 – 134 .
- Smith , H. L. 1995 . Monotone Dynamical Systems, an Introduction to the Theory of Competitive and Cooperative Systems , Providence, RI : Mathematical Surveys and Monographs, American Mathematical Society .
- Smith , H. L. and Waltman , P. 1999 . Perturbation of a globally stable steady state, with P. Waltman . Proc. Amer. Math. Soc. , 127 : 447 – 453 .
- Smith , H. L. and Zhao , X.-Q. 2001 . Robust persistence for semidynamical systems . Nonlinear Anal. , 47 : 6169 – 6179 .
- Stuart , S. N. , Chanson , J. S. , Cox , N. A. , Young , B. E. , Rodrigues , A. S.L. , Fischman , D. L. and Waller , R. W. 2004 . Status and trends of amphibian declines and extinctions worldwide . Science , 306 : 1783 – 1786 .
- Thieme , H. R. , Dhirasakdanon , T. , Han , Z. and Trevino , R. 2009 . Species decline and extinction: synergy of infectious disease and Allee effect? . J. Biol. Dyn. , 3 ( 2–3 ) : 305 – 323 .
- Weldon , C. , Dhirasakdanon , T. , Han , Z. and Trevino , R. 2004 . Origin of the amphibian chytrid fungus . Emerg. Infect. Dis. , 10 : 2100 – 2105 .
- Zhao , X.-Q. 2003 . Dynamical Systems in Population Biology , New York : Springer .