Abstract
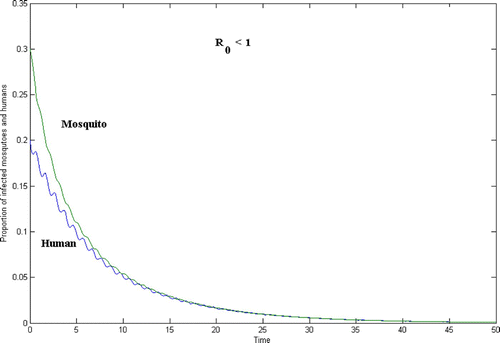
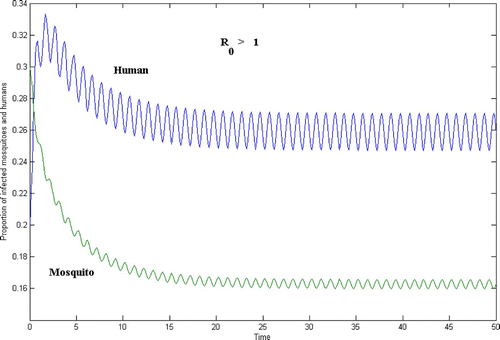
In this paper, we introduce a model of malaria, a disease that involves a complex life cycle of parasites, requiring both human and mosquito hosts. The novelty of the model is the introduction of periodic coefficients into the system of one-dimensional equations, which account for the seasonal variations (wet and dry seasons) in the mosquito birth and death rates. We define a basic reproduction number R 0 that depends on the periodic coefficients and prove that if R 0<1 then the disease becomes extinct, whereas if R 0>1 then the disease is endemic and may even be periodic.
1. Introduction
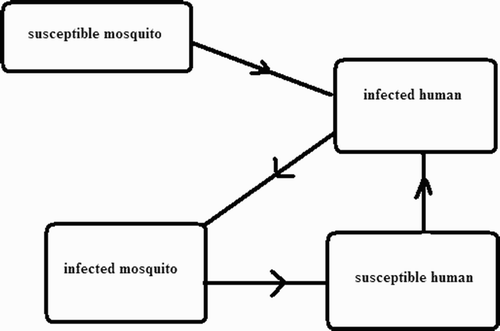
Malaria is a disease caused by a parasite called plasmodium sporozoite. Humans become infected by bites from infected mosquitoes, and mosquitoes become infected by biting infected humans. Animals can also get infected by malaria but they do not spread it to humans or to other animals Citation2–6,Citation8 Citation9 Citation11,Citation14–23,Citation25 Citation27. The human–mosquito interaction is described in .
Figure 1. Susceptible mosquitoes bite infected humans and become infected; infected mosquitoes bite susceptible humans and these humans then become infected.
In this paper, we study the effect of seasonal variations (wet and dry seasons) on the spread of malaria. Since the mosquito population varies periodically, we introduce a seasonal variation into the birth rate of the mosquito population and into the death rates of mosquito and human. In the literature, the malaria disease is generally modelled as SIRS (susceptible, infected, recovered, susceptible) disease for the humans and SIS (susceptible, infected, susceptible) disease for the vectors, i.e. for the mosquitoes. Some studies also include the incubation period Citation11. Further malaria model developments have appeared in the work of Bailey Citation2, Bekessey et al. Citation3, Dietz Citation7 Citation8, Macdonald Citation18, McKenzie and Bossert Citation20, and Struchiner et al. Citation25. The most general deterministic model of malaria, which includes both human and mosquito interactions, is due to Gideon and Shu Citation11. Their model is described by a system of one-dimensional equations (ODEs) with constant coefficients. In spite of all the work on malaria models, little has been done with regard to seasonal variations Citation5. However, there are several papers in the literature which discuss the role of seasonal variations in other disease models Citation14 Citation17 Citation26. For example, Greenhalgh and Moneim Citation13 studied an SIRS epidemic model with a general seasonal variation in the contact rate; their model is motivated by diseases such as measles, chickenpox, mumps, and rubella.
2. Experimental motivation and main results
Our mathematical model of malaria is based on previously published extensions of the classical Macdonald–Ross model Citation18 Citation23. In our model, the total human population (N h) is split into susceptibles (S h), infectives (I h) and recovered (R h) while the total mosquito population (N m) is split into susceptibles (S m) and infectives (I m). The non-autonomous model has time-dependent periodic coefficients which account for the seasonal variations (wet and dry seasons) in the birth rate of the mosquito population, and the death rates of both mosquito and human populations.
Our model is given by the following system of ODEs:
Table 1. Parameters for the malaria model.
To analyse the model, we introduce the following variables:
The variables of the model are defined in .
Table 2. Variables for the rescaled malaria model.
By definition, the variables in should satisfy the equations and
this is indeed proved in Lemma 3.2.
All the parameters in are positive constants except the T-periodic functions , μm(t) and μh(t). The parameter βh relates to the period of time during which the recovered humans are immune, and it represents the rate of loss of immunity. The parameter
represents birth rate; children of infected and of recovered individuals are born as susceptible individuals, and thus there is a loss of the infected and recovered populations at rate
. On the other hand, it is assumed that birth rate does not increase or reduce the relative proportions of susceptibles. Newborn mosquitoes of the infected population are assumed to be susceptible and thus new births decrease i
m at a rate
and increase s
m at the same rate
. We assume that birth rate of susceptible mosquitoes does not increase or reduce their relative proportion. Worldwide, there are about 300–500 million annual cases of malaria with 1–3 million deaths Citation5. For example, in Mali more than 50,000 people die from malaria annually. A large percentage of these malaria deaths occur during the wet season. To capture this in our model, we assume that the mosquito and human death rates (and thus their longevity), μm(t) and μh(t), may vary periodically with time. Furthermore, the birth rate of mosquitoes,
, is also periodic. Our model does not include a latent period for bitten humans or mosquitoes; including these classes of populations would introduce a time delay into the system. To make the ratio of mosquito to human populations
periodic, we assume throughout the paper that
The function varies with the season. It is a periodic function which takes larger values during the wet season and smaller values during the dry season. In this paper, we focus on the following question: Under what assumptions on the model coefficients is the malaria disease endemic?
In the case where , μm(t) and μh(t) are constant functions, the answer can be given in terms of the so-called basic reproduction number R
0: When R
0<1 the disease goes extinct, whereas when R
0>1 the disease persists Citation1
Citation2
Citation7
Citation18.
The novelty of the present paper is that it provides an answer to the above question when , μm(t) and μh(t) are non-constant, namely when
, μm(t) and μh(t) are T-periodic. We shall compute the basic reproduction number as
In , γ=0.9, whereas in , γ=2.5. In both figures, the initial population size is
In and , the time t=50 on the horizontal axis corresponds to 50 years. Since and
are periodic of period 1, each period corresponds to one season.
The ideas involved in our analysis could be applied to other systems of ODEs with periodic coefficients. Examples of previous work on disease models with periodic coefficients include Bacaër and Guernaoui Citation1, Chitnis et al. Citation5, Franke and Yakubu Citation10, Grassly and Fraser Citation12, Greenhalgh and Moneim Citation13, Hoshen and Morse Citation14, Ma and Ma Citation17, Smith Citation24, Wang and Zhao Citation26, and Wyse et al. Citation27.
According to the 2006 UNICEF report, malaria is one of the most life-threatening tropical diseases for which no successful vaccine has been developed. Sulphadoxine-pyrimethamine (SP) is an effective drug for a person infected with the malaria disease. In a 2002 experimental study of the effectiveness of SP as a temporary vaccine, Coulibaly et al. Citation6 considered two groups of people at a site in Bandiagara (Mali), a region of endemic malaria. The first group was administered SP at the beginning of the wet season, and the second group was not given the drug. The two groups were monitored during the entire wet season for the first malaria disease episode. Coulibaly et al. observed that in the first four weeks much fewer first episodes occurred in the first group. However, this was slowly reversed between the fourth week and eighth week. The experimental data of Coulibaly et al. over the entire wet season in Mali (May–August) showed no significant advantage to the group that received SP. We believe that our mathematical malaria model framework could be used to explore possible SP administration protocols that would optimize its effectiveness as malaria vaccine.
The paper is organized as follows: preliminary technical results on our model are given in Section 3. In Section 4, the basic reproduction number R 0 is introduced and is used to determine the global extinction of the infective population when R 0<1. Sections 5 and 6 deal with the persistence of the disease and with existence of a periodic solution when R 0>1.
3. Preliminary results
In this section, we establish the invariance of the first quadrant,
the plane
Lemma 3.1
Let A(t) and B(t) be n×n matrices of bounded measurable functions on [0, ∞). If
, B(t)≥0 for 0<t<t
0
and r(0)>0, or r(0)=0 but B(0)>0, then r(t)>0 for all 0<t<t
0.
Indeed, this follows from the integrated form of the differential equation
Lemma 3.2
The following identities hold:
Proof
Adding EquationEquations (2)–(4), we obtain
Lemma 3.3
The following inequalities hold:
Proof
Consider first the case where i h(0)>0. Let {0<t<t 0} be any interval such that i h(t)>0 for 0<t<t 0. By EquationEquations (4)–(6) we have,
Next, equations by Equation(2) and Equation(13)
,
We next show that i
h(t) remains positive for all t>0. Proceeding by contradiction we suppose that i
h(t)>0 for 0≤t<t
0 and . Then
. On the other hand, by EquationEquation (3)
,
Lemma 3.4
Proof
From model Equation(1),
and
. Hence,
whenever N
m(0)>0. Similarly, N
h(t)>0 whenever N
h(0)>0. Let
. Then,
. Hence,
4. Global stability of disease-free equilibrium
In this section, we define a basic reproduction number R 0 and prove that, independent of the initial population size, if R 0<1 then the disease will die out.
In view of Lemma 3.2, it suffices to consider the sub-system of EquationEquations (2)–Equation(6)
for s
h, i
h and i
m which we conveniently write in the form
The following matrix will play a fundamental role in the sequel:
R
0 is defined by EquationEquation (11). Note that the average infectious period of a single person is
, since (when i
m=0)
. Also, the average infectious period of a single mosquito (when i
h=0) is
. Hence, R
0 may be viewed as the average value of the expected number of secondary infection cases produced by a single infected individual entering the population at the DFE. R
0 is called the basic reproduction number.
We note that if R 0<1 then the two eigenvalues of the matrix A have negative real parts, whereas if R 0>1 then one eigenvalue is positive and the other is negative.
Theorem 4.1
If R 0<1 then the disease-free equilibrium point, DFE=(1, 0, 0), is globally asymptotically stable.
Proof
Since and
, using Lemma 3.4 we have that
and
.
Let be the solution of
Since R 0<1, the eigenvalues of A have negative real parts and the same is true for A+δ I provided δ is small enough. Hence,
5. Endemic malaria
Here, we use a series of auxiliary results to prove that R 0>1 implies the persistence of malaria disease in the periodic environment.
Theorem 5.1
If R
0>1 then there exist numbers δ0>0 and δ1>0 such that for any initial values
there is a time
such that
and
for all
.
We first establish several lemmas.
Lemma 5.2
Let a and b be any positive numbers.
-
If
andthen
for all T 0<t<ξ, where
-
If
andthen
for all T 1<t<ξ, where
The proof follows from the integrated form of the differential inequalities Equation(22) and Equation(23)
.
Lemma 5.3
Let η0 be any positive number such that
Proof
By EquationEquation (4),
and
. Applying Lemma 5.2(i) with
, the assertion follows. ▪
If ,
and x
1≥y
1, x
2≥y
2, then we write X≥Y.
Lemma 5.4
Let η0 and η1 be any small positive numbers, and assume that
Proof
By EquationEquations (13), Equation(16)
and Equation(17)
we have
Claim. For t>T 0,
Similarly, we derive a contradiction in case (ii). This completes the proof of the claim.
Taking , we conclude that the claim holds even when
. Set
Lemmas 5.2 and 5.3 did not use the assumption R
0>1. But from now on we shall use this assumption. We shall also always choose η0 and η1 sufficiently small so that one eigenvalue of the matrix in EquationEquation (26) is positive and bounded from below by a positive constant. Setting
Corollary 5.5
Under the assumptions of Lemma 5.4, if η0 and η1 are sufficiently small, then
Lemma 5.6
For any sufficiently small positive η0, set
Proof
Since , we have from EquationEquation (17)
Lemma 5.7
For any sufficiently small η1>0, set
Proof
From EquationEquation (15), we have
For future reference we state the following fact which follows immediately from the differential equations for i h and i m.
Lemma 5.8
There exists a positive number B such that, for any
Lemma 5.9
Let η0 and η1 be sufficiently small positive numbers and set
Proof
Suppose, to the contrary, that
We shall now use Lemmas 5.2–5.4 Lemmas 5.6–5.9 and Corollary 5.5 to prove Theorem 5.1.
Proof of Theorem 5.1
Consider first the case where, in Lemma 5.9, . Then, by Lemma 5.8,
We now repeat the above process starting at time t=t
1+t
2. We note, however, that whereas in EquationEquation (34)
if
, in the present case the time
is uniformly bounded by a constant which depends on η0 and η1 but not on i
−(0). Indeed this is a consequence of the estimates Equation(37)–(40). We conclude that either
or
where t
3∈
. Thus, as before, there exists a time t
4 such that
and
,
. Proceeding in this way step by step, we obtain a monotone increasing sequence of points t
k
such that
,
,
,
,
, and
, where
depends on η0 and η1, but ĉ is independent of η0 and η1. Using Lemma 5.8 to estimate i
h(t), i
m(t) in the intervals t
k
<t<t
k+1, the proof of Theorem 5.1 is complete. ▪
6. Endemic malaria on periodic solution
In this section, we prove that if R
0>1 then there exists at least one periodic solution of the system Equation(2)–Equation(6)
. The proof is based on Theorem 5.1 and on the following fixed point theorem of Horn.
Theorem 6.1
(Horn's fixed point theorem Citation13) Let
be non-empty convex sets in a Banach space X such that X
0
and X
2
are compact and X
1
is open relative to X
2. Let W be a continuous mapping
such that, for some positive integer m,
for
and
for
, where
. Then W has a fixed point in X
0.
Theorem 6.2
If R 0>1, then there exists at least one T - periodic solution of the system Equation(12)–(14). That is, R 0>1 implies the persistence of the infective human and mosquito populations on a periodic solution.
Proof
The proof uses the same argument as Greenhalgh and Moneim Citation13. We write a variable point in R
5 in the form and we write z≥0 if all the component of z are nonnegative. We introduce the convex sets
Acknowledgements
We thank the referees for useful comments and suggestions that improved our manuscript. This work was partially written when the first author was visiting the Mathematical Biosciences Institute (MBI) at Ohio State University; he wishes to thank the MBI for its hospitality. He also wishes to thank Drs. Winston Anderson, Frances Mather and Ousmane Koita for their support throughout this study. This work was partially supported by the National Sciences Foundation upon grant No. 0112050. Abdul-Aziz Yakubu was partially supported by Howard University MHIRT Program 2006 and NIH Award No. 9T37MD001582-09.
References
- Bacaër , N. and Guernaoui , S. 2006 . The epidemic threshold of vector-borne diseases with seasonality: the case of cutaneous leishmaniasis in Chichaoua, Morocco . J. Math. Biol. , 53 : 421 – 436 .
- Bailey , N. T.J. 1957 . The Mathematical Theory of Epidemics , 1 , London : Griffin .
- Bekessey , A. , Molineaux , L. and Storey , J. 1976 . Estimation of incidence and recovery rates of Plasmodium falciparium parasitaemia from longitudinal data . Bull. Wld. Hlth. Org. , 54 : 685 – 693 .
- Carnevale , P. , Mouchet , J. , Coosemans , M. , Julvez , J. , Manguin , S. , Lenoble , R. D. and Sircoulou , J. 2004 . Biodiversité du Paludisme Dans le Monde , Paris : John Libbey Eurotext .
- Chitnis , N. , Hyman , J. M. and Cushing , J. M. 2008 . Determining important parameters in the spread of malaria through the sensistivity analysis of a mathematical model . Bull. Math. Biol. , 70 : 1272 – 1296 .
- Coulibaly , D. 2002 . Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali . Am. J. Trop. Med. Hyg. , 67 ( 6 ) : 604 – 610 .
- Diekmann , O. , Heesterbeek , J. A.P. and Metz , J. A.J. 1990 . On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogenous populations . J. Math. Biol. , 28 : 365 – 382 .
- Dietz , K. Mathematical models for malaria in different ecological, zones . Presented to the 7th International Biometric Conference . August , Hannover. pp. 16 – 21 .
- Dietz , K. 1988 . “ Mathematical models for transmission and control of malaria ” . In Principles and Practice of Malariology , Edited by: Wernsdorfer , W. H. and McGregor , I. 1091 – 1113 . London : Churchill Livingstone .
- Franke , J. and Yakubu , A. A. 2006 . Discrete–time SIS epidemic model in a seasonal environment . SIAM J. Appl. Math. , 66 : 1563 – 1587 .
- Gideon , A. N. and Shu , W. S. 1999 . A mathematical model for endemic malaria with variable human and mosquito populations , 1 – 27 . United Nations Educational Scientific and Cultural Organization and International Atomic Energy Agency IC . Available at http://www.ictp.trieste.it/~pub_off
- Grassly , N. C. and Fraser , C. 2006 . Seasonal infectious disease epidemiology . Proc. R. Soc. B , 273 : 2541 – 2550 .
- Greenhalgh , D. and Moneim , I. A. 2003 . SIRS epidemic model and simulations using different types of seasonal contact rate . Syst. Anal. Modell. Simul. , 43 : 573 – 600 .
- Hoshen , M. B. and Morse , A. P. 2004 . A weather-driven model of malaria transmission . Malaria J. , 3 : 32
- Kermack , W. O. and McKendrick , A. G. 1927 . Contributions to the mathematical theory of epidemics (Part I) . Proc. Roy. Soc. A. , 115 : 700 – 721 .
- Lokta , A. J. 1923 . Contributions to the analysis of malaria epidemiology . Am. J. Hyg. , 3 : 1 – 121 .
- Ma , J. and Ma , Z. 2006 . Epidemic threshold conditions for seasonality forced SEIR models . Math. Biosci. Eng. , 3 : 161 – 172 .
- Macdonald , G. 1950 . The analysis of infection rates in diseases in which superinfection occurs . Trop. Dis. Bull. , 47 : 907 – 915 .
- Martini , E. 1921 . Berechnungen und Beobachtungen zur Epidemiologie und Bekam¨pfung der Malaria , Hamburg : Gente .
- McKenzie , F. E. and Bossert , H. W. 2005 . An integrated model of Plasmodium falciparum dynamics . J. Theor. Biol. , 232 : 411 – 426 .
- National Institute of Allergy and Infectious Diseases, Publication No. 02-7139, Malaria, 2002
- Norman , T. J. and Baley , M. A. 1982 . The Biomathematics of Malaria , New York : Oxford University Press .
- Ross , R. 1911 . The Prevention of Malaria , 2 , London : Murray . with Addendum on the Theory of Happenings
- Smith , H. L. 1983 . Subharmonic bifurcation in an S-I-R model . J. Math. Biol. , 17 : 163 – 177 .
- Struchiner , C. J. , Halloran , M. E. and Spielman , A. 1989 . Modeling malaria vaccines I: new uses for old ideas . Math. Biosci. , 94 : 87 – 113 .
- Wang , W. and Zhao , X.-Q. 2008 . Threshold dynamics for compartmental epidemic models in periodic environments . J. Dyn. Diff. Equat. , 20 : 699 – 717 .
- Wyse , A. P.P. , Bevilacqua , L. and Rafikov , M. 2007 . Simulating malaria model for different treatment intensities in a variable environment . Ecol. Model. , 206 : 322 – 330 .