Abstract
Forty years after the World Health Organization abandoned its eradication campaign, malaria remains a public health problem of the first magnitude with worldwide infection rates on the order of 300 million souls. The present paper reviews potential control strategies from the viewpoint of mathematical epidemiology. Following MacDonald and others, we argue in Section 1 that the use of imagicides, i.e., killing, or at least repelling, adult mosquitoes, is inherently the most effective way of combating the pandemic. In Section 2, we model competition between wild-type (WT) and plasmodium-resistant, genetically modified (GM) mosquitoes. Under the assumptions of inherent cost and prevalence-dependant benefit to transgenics, GM introduction can never eradicate malaria save by stochastic extinction of WTs. Moreover, alternative interventions that reduce prevalence have the undesirable consequence of reducing the likelihood of successful GM introduction. Section 3 considers the possibility of using seasonal fluctuations in mosquito abundance and disease prevalence to ‘slingshot’ GM mosquitoes into natural populations. By introducing GM mosquitoes when natural populations are about to expand, one can ‘piggyback’ on the yearly cycle. Importantly, this effect is only significant when transgene cost is small, in which case the non-trivial equilibrium is a focus (damped oscillations), and piggybacking is amplified by the system's inherent tendency to oscillate. By way of contrast, when transgene cost is large, the equilibrium is a node and no such amplification is obtained.
Where the Italian boot meets the island of Sicily lies the region called Calabria, the character of which, Douglas Citation24 observed, is incomprehensibly absent with reference to malaria. ‘Malaria’, he wrote, ‘is the key to a correct understanding of the landscape; it explains the inhabitants, their mode of life, their habits, their history’.
That was in 1915. Thirty years later, the American army, in its drive on Rome, responded to endemic malaria with marsh draining and DDT (), then a secret weapon. Eradication efforts continued after the war, and in 1970, the World Health Organization (WHO) declared the country malaria-free Citation75. The Italian success story was replicated in other parts of southern Europe, to a lesser extent in South America and southern Asia and hardly at all in Africa Citation9 Citation21 Citation25 Citation33 Citation64. Today, a century after Douglas wrote his travel book, worldwide infection rates are on the order of 300 million souls of whom at least a million die every year Citation78. Unsurprisingly, the social and economic costs are enormous Citation37 Citation58.
Figure 1. Home disinfection by American medical personnel, Pontine marshes, 1944. Painting by Sgt. Fred Toelle. Image used with permission from Central University Libraries, Southern Methodist University. Available in colour online.
Especially in Africa, a major-cause of resurgent malaria has been societal dysfunction: war, poverty and the collapse of health care systems following abandonment of the WHO's Eradicate Malaria Campaign Citation9 Citation21 Citation25 Citation33 Citation64 Citation78 Citation18 in the late 1960s. Other factors have also contributed. These include changing patterns of land use, expanding human populations Citation69, the evolution of Plasmodium resistance to anti-malarial drugs, Citation9 Citation34 Citation46 Citation89 Citation99 and the evolution of mosquito resistance and avoidance behaviour to insecticides Citation70 Citation73. Changing public health policies Citation18 Citation21 Citation33 Citation64 Citation78 – a shift in emphasis from vector control to treatment and the effective banning of DDT – have also taken their toll Citation8 Citation9 Citation25 Citation69 Citation73 Citation74 Citation99.
These developments have stimulated a search for new solutions: insecticide-treated bed nets (ITNs) Citation19 Citation57 Citation94 Citation95, entomopathogenic fungi Citation12 Citation85, new anti-malarials (or the use of old ones in sequence or combination Citation31 Citation51 Citation67), vaccines Citation35 Citation36 Citation48 Citation65 and genetically modified (GM) mosquitoes Citation16 Citation41,Citation61–63.
With regard to all of these approaches, there are population level considerations Citation20 Citation86 in addition to the medical and molecular ones. For example, in the case of GM mosquitoes, De Roode and Read Citation20 suggest that ‘the technological challenges of manipulating genes in vivo are trivial compared to the ecological challenges …’. Likewise, Scott et al. Citation86 stress the ‘need to study gene flow, … mosquito population size and structure, mechanisms of population regulation, …,’ etc., while Hancock and Godfray Citation29 emphasize ‘the interplay of mortality … at different stages of the lifecycle …’.
In the present paper, we explore the interacting consequences of seasonality and fitness to GM mosquito introduction. With regard to seasonality, it is well known Citation7 Citation10 Citation68 that over much of the malarious tropics, mosquito densities fluctuate in response to alternating wet and dry seasons (see also, Citation102). With regard to transgene fitness, the overall picture Citation15 Citation16 Citation41 Citation62 Citation86 Citation96 is one of cost, i.e., of reductions in reproduction or survival. From this derives much of the current interest Citation30 Citation42 Citation71 Citation98 Citation101 in ‘driving mechanisms’ to force transgenes into natural populations.
1. Eradication in the abstract
Before turning our attention to GM mosquito introduction, we review alternative control strategies from the vantage of parametric sensitivity. Generally speaking – but see Citation17 – a disease's ability to persist is captured by its basic rate of reproduction, R 0 Citation3 Citation23 Citation50. Essentially, R 0 is the number of secondary infections that result from a single contagion. With R 0>1, there is a persistent, endemic level of infection, whereas with R 0<1, the disease dies out. In such cases, R 0=1 corresponds to a transcritical bifurcation, whereas the ‘no-disease’ and endemic equilibria collide and exchange stability Citation50 Citation82. The practical consequence Citation2–5,Citation22 is that one need not immunize all potential hosts, or, in the case of vector-transmitted infections, kill all of the vectors. Sufficient unto the task is reducing R 0 to a value that is less than 1. In the case of vaccination programmes, this condition is called herd immunity. For application to malaria, see Citation7; for a more general discussion including transient immunity and the evolution of vaccine resistance, see Citation84.
As discussed, for example, in Citation66, R 0 can be estimated from historical notifications, although what is obtained is not necessarily suggestive of a constant fuzzed out by the measurement error. R 0 can also be calculated from mathematical models, in which case the formula depends on the natural history of the disease – more accurately, on the equations used to model its dynamics. For example, in Ross’ original formulation Citation76 Citation77,
Table 1. Determinants of R 0 for Malaria.
Likewise, with the addition Citation59 Citation60 of latency (infected mosquitoes not yet infectious) Citation7,
The parameters in enter Equations (1) in different ways. Hence, sensitivity of R 0 varies from one parameter to the next. Following Citation29 Citation60, and others, we note the following:
| 1. | The mosquito death rate, μ, enters twice, first in proportion to (1/μ), and second, as e−μτ, which is the probability that an infected mosquito survives long enough to infect a human host. It follows that reducing adult mosquito longevity provides the surest approach to reducing R 0. This explains the historical success Citation8 Citation9 Citation64 of residual spraying programmes in which houses, stables and other structures were dusted with DDT once or twice a year. For the same reason, entomopathogenic fungi Citation12 Citation85 and ITNs, which kill mosquitoes in addition to protecting sleeping humans, are attractive control strategies; but see Citation95 for discussion of the downside of ITNs. | ||||
| 2. | The bite rate, A, also enters twice – in proportion to A Footnote2 – reflecting the fact that completion of the parasite's life cycle requires two bites. Thus, a 50% reduction in the number of bites divides R 0 by a factor of 4, etc. Even without killing mosquitoes, control strategies (screens, pesticides, netting) that keep mosquitoes out of peoples’ houses, or at least out of their beds, decrease the bite rate; likewise, covering up in the evening and the use of insect repellents. | ||||
| 3. | The remaining parameters enter once. Vaccines effectively reduce N. Anti-malarials, a principal focus of the current WHO practice [9,99 , increase the recovery rate, r. Interrupting the parasite's life cycle via the introduction of GM mosquitoes reduces the number of mosquitoes, M, in effect; oiling, spraying and draining marshes, introducing larval predators Citation11 Citation83, etc., does so in fact. Reducing local human population density (relocation programmes) reduces N and, to the extent that breeding sites are eliminated, M. | ||||
In sum, control strategies that focus on the death rate of adult mosquitoes and the bite rate are inherently more efficient than ‘non-A/μ’ strategies Citation29 Citation60 Citation92. This is not to deny that interrupting the plasmodium's life cycles at any point would end the scourge. For example, Koenraadt et al. Citation47 attribute the general dearth of malaria in the Kenyan highlands to retarded larval development and survival. Likewise, Baird Citation9 observes that ‘attacking the specific breeding sites of known vectors’ was a major contributor to the successful campaign against malaria during construction of the Panama Canal. Moreover, as Smith and McKenzie Citation92 remind us, ‘Single control measures often affect more than one aspect of malaria transmission, as does integrated malaria control. The important point,’ they continue, ‘long understood but often forgotten, is that the benefits are multiplicative’ [emphasis added]. At the same time, these authors point out that, with the incorporation of mosquito population dynamics, ‘increased mosquito mortality has an effect even larger than was proposed by Macdonald in the 1950s’.
2. Competition between GM and wild-type mosquitoes
In this section, we model competition between wild-type (WT) and transgenic mosquitoes. To do this, we must first of all introduce some form of density dependence. Following Citation29, we suppose that mosquito populations are regulated by density-dependent larval mortality and further that WT and GM larvae are equally affected by crowding. We proceed as follows:
| 1. | The real world being intermediate between assortative mating and panmixia Citation86, we replace genes Citation54 Citation56 Citation87 with strains Citation53 for simplicity. | ||||
| 2. | Likewise, we use a modified version of Ross’ original equations, even though they omit important biological particulars – parasite development time, details of the gonotrophic cycle, acquired host immunity, super-infection, etc. Citation7 Citation29 Citation32 Citation93 | ||||
| 3. | We use adult mosquito density as a proxy variable (see discussion) for the density of larvae and model mosquito population dynamics as | ||||
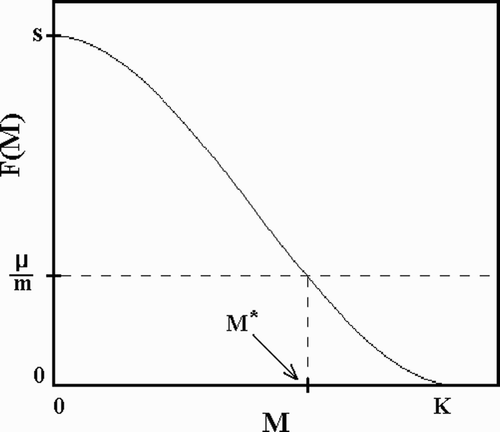
Here m is the maximum per capita birth rate, and F(M, t), captures the (possibly time-dependent) effects of crowding on larval survival.Footnote1 In a constant environment, the equilibrium abundance, M*, of mosquitoes is the solution, M*, to
Here is the magnitude of the seasonal effect, and ω=1 y−1 is the frequency. In constant environments,
, and K(t)=K
0.
Figure 2. Larval survival, F(M, t), according to EquationEquation (4a). M* is the equilibrium density of mosquitoes in a constant environment.
Putting all this together yields
2.1. Costs and benefits
As noted above, most studies of transgenic fitness report a fitness cost to GM mosquitoes. In this regard, a recent paper by Marrelli et al. Citation61 reporting a fitness advantage for hemizygous GM mosquitoes feeding on infected hosts is of interest. Citing Hurd and Cowarker Citation2 Citation38, Marrelli et al. suggest that transgene advantage reflects the fact that ‘even [a] Plasmodium burden of less than five oocysts can significantly reduce the fecundity of anophelines in experimental or natural systems’.Footnote2, Footnote3 Under this assumption, the benefit enjoyed by transgenic mosquitoes is prevalence-dependent,Footnote4 in which case, transgenes can never completely eliminate malaria, absent stochastic extinction. This is because as prevalence declines, so too does transgene advantage, which becomes a disadvantage (inherent transgene cost) before the disease is eradicated.Footnote5 It follows either that a drive mechanism indifferent to transgene fitness, for example, one involving homing endonucleases Citation101, will be required or that genetic engineers will have to come up with a transgene that is inherently superior. However, as discussed in Section 3, it may still be possible to use seasonal variations in prevalence to ‘slingshot’ inferior genotypes into natural populations by appropriate timing of their release.
In the analysis that follows, we assume:
| 1. | GM mosquitoes enjoy a fitness advantage – a consequence of reduced parasite load – relative to WTs feeding on infected hosts. | ||||
| 2. | Absent malaria, GM mosquitoes manifest a fitness deficit relative to WTs, i.e., | ||||
Corresponding to these assumptions, we substitute
2.2. Dimensionless equations
Let
2.3. Reduced system
Before studying Equation (8), we consider the reduced system that is obtained when the number of infected hosts, x, is set to a fixed value. In this case, the derivatives, d w/d t and d g/d t, are independent of y. This allows us to work in the w−g−xspace, with x playing the role of a parameter.Footnote7 Then, the transgenic strain prevails if
| 1. | For fixed prevalence, the corresponding isoclines parallel the −45° line, a consequence of assuming that the two strains are equally susceptible to crowding. Generically, i.e., provided | ||||
| 2. | The transgene isoplane, ġ=0, is independent of prevalence, whereas the WT isoplane, w˙=0, slopes downwards with increasing x. If | ||||
| 3. | If | ||||
Figure 3. Zero growth isoplanes for the two strains with constant prevalence. Competition along the light black dotted line is indeterminate, there being an infinite number of neutrally stable fixed points. Solid circles (nodes) are attractors; half-filled circles, saddles.
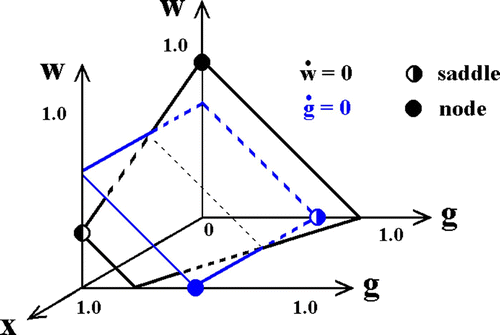
In sum, given
2.4. Full system
We now return to Equation (8). The results that follow were obtained by numerical integration (Adams or Gear's method) of the differential equations and by continuation of equilibria and their bifurcations Citation49 using secant prediction and chord-Newton correction with adaptive step control. As described in Citation44 Citation45, the required Jacobian matrices were computed using concurrent integration of the equations of first variation. Pitchfork (PF), saddle-node (SN) and transcritical bifurcations were identified by the application of standard Citation42 Citation49 criteria.
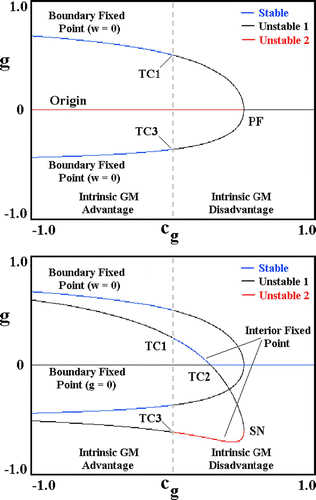
Recall that the reduced system admits to three structurally stable equilibria: the origin, (0, 0), the boundary equilibria, (w, 0) and (0, g), as well as the aforementioned line of structurally unstable equilibria at x=x crit. In the full system, the last is replaced by a unique (for fixed parameters) structurally stable, interior equilibrium (w*, g*). The origin and stabilization of this equilibrium are detailed in . Here we display curves of equilibria and the bifurcations that are obtained when the transgene cost, c g, is varied from −1 (transgene advantage) to +1 (transgene disadvantage), while fixing the WT cost, c w=1. Regarding this figure, we note the following:
| 1. | Top panel. A pair of unstable boundary equilibria, w=0, emerge from the origin, (w, g)=(0, 0), via a PF bifurcation that occurs when the transgene is inherently disadvantageous −c g>0. One of these equilibria corresponds to positive numbers of transgenics; the other, to negative numbers.Footnote9 Both acquire stability via transcritical bifurcations (TC1 and TC3) at c g=0. We conclude that the exclusion of WTs by transgenics requires c g<0, which is to say that the transgene is inherently advantageous. | ||||
| 2. | Bottom panel. A pair of interior equilibria originates via a SN bifurcation, which, like PF bifurcation discussed above, occurs when the transgene is inherently disadvantageous −c g>0. At the bifurcation point, both interior equilibria correspond to negative numbers of transgenics. Both are also unstable, one with a single degree of instability, the other with two. With decreasing c g, the former becomes positive and acquires stability at a value of c g>0 via a transcritical bifurcation (TC2) with the boundary equilibrium, g=0. At this point, the boundary equilibrium loses stability, and transgene exclusion gives way to coexistence. Subsequently, i.e., at c g=0, stability of the interior equilibrium is lost via a second transcritical bifurcation (TC1), at which point coexistence gives way to WT exclusion.Footnote10 | ||||
Figure 4. Curves of equilibria and bifurcations attendant on varying transgene cost, c g. TC1 – TC3 are transcritical bifurcations; PF and SN, pitchfork and saddle-node bifurcations. Top. Origin and stabilization of the boundary equilibria (w=0). Bottom. Origin and destabilization of the interior equilibrium. ‘Unstable 1’ and ‘Unstable 2’ refer to the number of unstable directions in phase space – equivalently, the number of eigenvalues in the right half of the complex plane. Parameters (Equation 8) as follows: a=20; b=1.0; q=2.0; r=4.0; μ=50; s=1.0; m=100, ϵ K =0. Available in colour online.
In sum, as transgene cost declines, populations transition from all WTs to a mixture of WTs and transgenics and finally to one composed entirely of transgenics.
2.5. WT-transgene coexistence
By continuing Citation42
Citation49 the transcritical bifurcations, TC1 and TC2, against WT cost, c
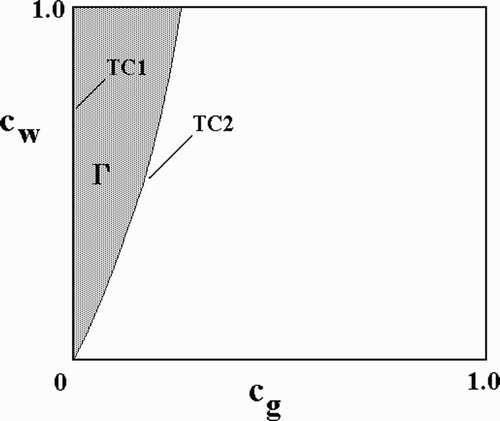
w, we can delineate the region, Γ, in the parameter plane corresponding to coexistence of WT and GM mosquitoes. This is shown in . Note that the curve of TC1 bifurcations is simply to y-axis and that both curves emerge from the origin,
, which is a co-dimension 2 bifurcation corresponding to the identity of the interior and boundary equilibria. Elsewhere, we will document the dependence of Γ on the remaining parameters. The bottom-line result, however, is worth stating here: Any parameter change reducing prevalence, moves the TC2 curve to the left, thereby reducing the maximum transgene cost, c
g, consistent with successful GM introduction.
Figure 5. The region (stippled) of WT–transgene coexistence in the c w−c g parameter plane is bounded by curves of transcritical bifurcations, TC1 and TC2 – see . For points to the right of the TC2 curve, the boundary equilibrium (g, w)=(0, w*) is stable, and the transgene is outcompeted by the WT. For points to the left of the TC1 curve, which is also the y-axis, the transgene prevails. Parameter values as in .
2.6. Stability of the interior equilibrium
In part, the response to transgenic introduction (below) depends on equilibrium stability. In , we show how the eigenvalues of the interior equilibrium change in response to varying transgene cost. Only the values of the two small magnitude eigenvalues are shown. The remaining eigenvalues are real, negative and an order of magnitude greater in absolute value. Hence, to first approximation, the dynamics are played out in two dimensions.
Figure 6. Change of small magnitude eigenvalues of the interior equilibrium in response to varying transgene cost, c g. WT cost c w=1.0. Other parameters as in .
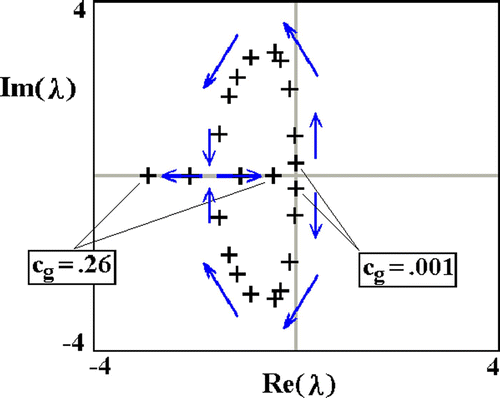
Regarding , we note the following:
| 1. | For c g<0 (points to the left of TC1 in ), the two small amplitude eigenvalues are both real, one being positive, the other, negative (not shown). In this case, the interior equilibrium, which corresponds to negative values of w, is a saddle. | ||||
| 2. | With increasing c g, the small amplitude eigenvalues approach the origin of the complex plane. At c g=0, there is a double zero. This corresponds to TC1, at which point w*=0. | ||||
| 3. | With further increases in c g, now positive, the small amplitude eigenvalues separate, moving away from the real axis, Re(λ), and to the left. Thus, neutral stability gives way to weakly damped oscillations of a large period. | ||||
| 4. | With still further increases in c g, the eigenvalues continue to move left. Initially, the imaginary parts become larger in magnitude, but this trend is subsequently reversed. Eventually, there is a second double eigenvalue – this one to the left of the imaginary axis, Im (λ). At this point, the eigenvalues become real, and the approach to equilibrium, nodal. | ||||
| 5. | Further increases in c g cause the eigenvalues, now real, to separate, with one approaching zero, and the other becoming more negative. Eventually, the larger eigenvalue equals zero, and we are at TC2, at which point g*=0. | ||||
| 6. | Beyond TC2, i.e., for still larger values of c g, the interior equilibrium, now corresponding to negative numbers of transgenics, is a saddle (not shown). | ||||
2.7. Transgenic introduction
The foregoing suggests the following consequences of varying transgene cost to transgene introduction.
| 1. | For small values of c g, GM mosquitoes should dominate, but with the approach to equilibrium being by way of large amplitude, slowly decaying oscillations. | ||||
| 2. | As c g increases, oscillations should become less pronounced and eventually disappear, even as the equilibrial abundance of transgenics declines. | ||||
| 3. | With c g sufficiently large, introductions should fail. | ||||
These expectations are confirmed in for the case of a constant environment. With decreasing transgene cost, c g, the long-term transgene abundance increases, whereas WT abundance declines. This, of course, is to be expected. What is unexpected, absent the preceding analysis, is the emergence of low-frequency, large-amplitude oscillations as c g goes to zero.
Figure 7. Competition between WT and transgenic mosquitoes in a constant environment. Transgenic cost, c g, decreases from left to right and top to bottom. (a) c g=0.5; (b) c g=0.25; (c) c g=0.10; (d) c g=0.05; (e) c g=0.01; (f) c g = −0.001. In all cases, c g=1. Other parameters as in . Note the increasing amplitude and period of oscillations as c g approaches 0. For c g<0, oscillations are abolished, and displacement of WTs (now inherently advantageous) by transgenics is monotonic. Available in colour online.
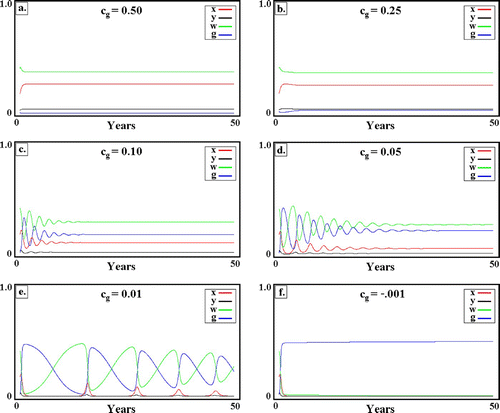
In seasonal environments (not shown), these fluctuations are superposed on the yearly cycle. For the parameter values studied, there was no suggestion of a more complex behaviour Citation82, though this remains a possibility, especially were the model to be extended, for example, by the inclusion of larval densities as an additional state variable.
3. ‘Slingshotting’ transgenics into a population of WT mosquitoes
The production of transgenic mosquitoes being both time-consuming and costly Citation72, and laboratory amplification, a frequent source of inbreeding depression Citation15 Citation16 Citation62 Citation86 Citation96, the question of applied interest is how to maximize the salutary effect of small, infrequent introductions. In seasonal environments, a ‘slingshotting’ technique may be available. Here, one uses the seasonal cycle () much as a space ship uses the gravitational field of a large celestial body to accelerate its velocity. The technique is most effective when seasonality is pronounced and intrinsic transgene cost, small, in which case, post-introduction malaria resurgence can be delayed for significant periods. gives some examples. Here we plot disease prevalence following single small introductions of transgenics at times corresponding to the four phase values indicated in . As expected, WT abundance and post-introduction prevalence decline with decreasing transgene cost. Beyond this is the timing of GM introduction relative to the calendar year, which can result in what we call a phase effect. In this regard, we put time (measured in years) on the unit circle and define the phase, φ, of introduction as
Figure 8. Annual cycle of mosquito abundance, w, and malaria prevalence, x, as modelled by Equation 8. Phase values, ϕ, are defined relative to the calendar year. Here, ϵ K =0.6, and c g=.01. Other parameters as in .
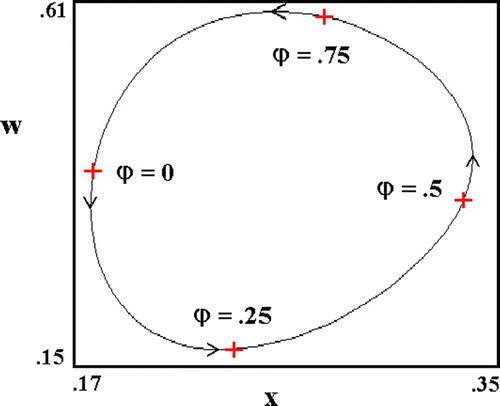
Figure 9. Simulated response to introduction (arrow) of transgenic mosquitoes in seasonal environments. The phase, φ, refers to timing of the introduction relative to the yearly cycle of malaria and mosquitoes as shown in . (a–c) Seasonality fixed at ϵ K =0.6. Phase effect, i.e., time until the first outbreak following GM introduction, increases with decreasing transgene cost, c g. (d–f) Transgene disadvantage fixed at c g=0.01. Phase effect increases with increasing seasonality. Remaining parameters as in . Available in colour online.
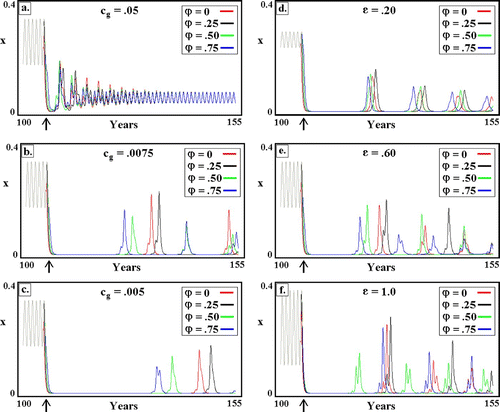
Returning to , we note that panels a–c explore the effect of decreasing transgene cost, c
g, for fixed seasonality, . For c
g=0.05, the timing of GM introduction is of little consequence. For smaller costs, choosing the correct phase can delay post-introduction resurgence by as much as 8–9 years. Likewise for fixed transgene cost (d–f), phase effects become more pronounced with increasing seasonality.
In all cases, the best results were obtained with φ=0.25, i.e., when mosquito abundances are approximately minimal. Thereafter, mosquito populations expand, and introduced GMs can ‘piggyback’ on the natural increase. Conversely, the worst (of the four values shown in ) phase is φ=0.75, which is when mosquito densities are close to maximal and about to decline. Our recommendation for real-world seasonal environments is thus to introduce GM mosquitoes when mosquito populations are about to increase, e.g., after the onset of the rainy season. Lest this appear intuitively obvious, we note that the phase effect is only important when transgene cost is small. In this case, the non-trivial equilibrium is a focus (damped oscillations), and piggy-backing is amplified by the system's inherent tendency to oscillate. By way of contrast, when transgene cost is large, the equilibrium is a node (), and no such amplification is obtained.
4. Discussion
4.1. Competition and larval survival
In the preceding analysis, we used the number of adult, female mosquitoes as a proxy variable for larval density. We also introduced a larval survival function which is incompatible with the more common assumption Citation7 Citation43 Citation52 Citation80 Citation81 of a constant, density-independent rate of anopheline emergence from larval habitats. In short, there are two issues.
4.1.1. Adult mosquito density as a proxy variable for numbers of larvae
The number of larvae, and hence the intensity of larval competition for resources, is a function of the numbers of mosquitoes in the past. One approach to this situation involves the introduction of time delays. Another is to make the number of larvae an independent variable. For example, one might write
4.1.2. Shape of the Survival Function
An alternative to EquationEquation (4b), and one compatible with the more common assumption of constant adult emergence, would be to assume
. Certainly, this is cleaner than EquationEquation (4a)
.Footnote12 But is it more reasonable biologically? Imagine a body of water in which mosquitoes lay eggs. After the eggs hatch, larvae begin to feed and develop. Both their rate of development and their survival depend on the availability of resources – algae, bacteria, fungi, etc. We submit that, except in the most unproductive situations, the abundance of resources will exceed the capacity of a small number of larvae to consume them. So, adding a few more larvae should have minimal effects on growth and survival. However, if we continue to add larvae, there will come a point at which there is an effect. Larval growth rates will slow, and some individuals will die between molts. Ephemeral habitats may dry up before the larvae complete their development or may so deteriorate that many, perhaps all, die. At this point, survival rates will drop significantly. This gives the initial ‘dip’ in the reverse sigmoid. If one continues to add larvae, survival approaches zero at a decreasing rate. This gives the flattening out of the reverse sigmoid.
By way of contrast, assumes that it is the first larval additions that have the most pronounced effect.
There is also the question as to whether or not it is reasonable to assume a maximum larval density beyond which larval survival is zero. Because larval development times increase with decreasing abundance of resources, because small bodies of water can dry up before slowly developing larvae emerge and because successful emergence requires some minimal availability of resources, it seems reasonable to imagine a larval density above which the emergence rate is zero. An alternative view is that earlier emerging larvae in one way or another Citation88 ‘out-muscle’ their younger competitors, so that some larvae emerge, no matter how many eggs are laid. The problem is that infinity is a large number. With an arbitrarily large number of larvae in a finite body of water, the putrid, writhing mass of dying and decaying ‘wrigglers’ would prevent those still alive from gaining access to the air to breathe.
4.2. Extensions
The obvious direction in which to extend the analysis here developed would be to replace strains with genes. After all, it is bits of DNA, not populations that one is trying to substitute. Still, we suggest that before embarking down the genetical path, it would be worth extending EquationEquation (5) by inclusion of at least one larval stage as a state variable. This would repair the aforementioned separation of time scales assumption, which, as noted above, is dubious. In this regard, the observation Citation32 of autonomous cycling in models with explicit larval dynamics is worth noting. This raises the possibility of intrinsic fluctuations, even in the absence of seasonality. More generally, epidemiological systems subject to seasonal forcing can manifest subharmonic resonances of large amplitudes and long periods Citation82. From the viewpoint of ‘slingshotting’, such cycles could be of considerable importance, extending the technique's applicability to non-seasonal environments.
4.3. Boots on the ground
In the real world, small populations are subject to stochastic extinction. Thus, there will be a minimum inoculate size below which transgenics are likely to die out. Correspondingly, successful transgene introductions that reduce prevalence to low levels may result in pathogen extinction and then, by virtue of its selective disadvantage, extinction of the transgene itself. Combining these expectations with small migratory influxes of infected hosts in the case of small transgene cost converts the system studied here into an excitable medium: stimulate and fire, with subsequent slow restoration of excitability. This brings us back to the population-level phenomena discussed in Citation20 Citation86: mosquito population size and structure, gene flow within and between populations, etc. Beyond this is the conflict between transgenic introduction and other control measures. As noted above, any intervention that reduces prevalence reduces the likelihood of getting transgenes into natural populations. While here deduced for a simple, cartoon-like model, this result will likely be obtained for a wide class of models, the principal requirement being the assumption of the cost-benefit structure – inherent transgene cost, prevalence-dependant WT advantage – analysed here.
4.4. Meanwhile
Defeating malaria with genetic engineering would be a great achievement. Still it is worth remembering that ‘substantial malaria control is possible by extending the coverage of existing technologies to impoverished households and communities’ Citation78. Moreover, the successes Citation11 Citation14 Citation83 of Reed and Gorgas in Panama and Cuba remind us that the disciplined application of imperfect techniques can yield favourable results, even in the holoendemic tropics. Of course, the pursuit of high-tech strategies will continue, as well it should. But for now, there is suffering and death on a massive scale, a consequence of both primary infections and their sequellae e.g., Citation13 Citation37 Citation40, to say nothing of interaction with other diseases such as AIDS Citation1 Citation6 Citation40. The accusation Citation8,Citation25–27,Citation91 that the application of the precautionary principle in the case of malaria amounts to an assault by the ‘Haves’ on the ‘Have Nots’, may or may not be justified. But failure to vigorously implement proven control strategies most certainly is not.
Acknowledgements
This paper is dedicated to Jim Cushing, long-time colleague and friend. We thank W.L. Green for counsel on the fine points of differentiability and JBD’s diligent reviewers for considered and helpful comments.
Notes
In terms of , m and μ have units of y−1, while F is dimensionless. The units of EquationEquation (2) are thus mosquitoes/y.
It has been argued Citation2 Citation39 that Plasmodium resistance induced by up-regulating mosquitoes’ immune response in WT mosquitoes does not produce a fitness advantage because immune system activation is costly. By way of contrast, the gene studied by Marrelli et al. inhibits Plasmodium development before such a response is mounted.
The reality of Plasmoduim-induced costs to infected WT mosquitoes has been questioned by Ferguson et al. Citation28 who suggest that significant adverse effects are only observable in unnatural host–parasite combinations.
Less comprehensible is the observation of reduced homozygote fitness as inferred from cage experiments in which transgene frequencies stabilized at p<1. Marrelli et al. suggest several possible explanations, including ‘hitch-hiking’ by deleterious alleles. But the perplexing earlier finding Citation62 is that transgenic mosquitoes fed on non-infected hosts manifest Hardy–Weinberg frequencies when introduced into populations of WTs.
While acknowledging that transgene advantage depends on prevalence, Marrelli et al. dismiss the principal implication of this fact, which is that transgenics can never drive WT mosquitoes to extinction, absent stochastic effects. They write, ‘In the field, where infection prevalence is lower than in laboratory systems and only a relatively small proportion of mosquitoes become infected, gene introgression is predicted to be considerably slower and possibly not of sufficient magnitude to establish the transgene in the population. However, once established, transgenic mosquitoes that interfere with parasite development should make more difficult the reintroduction of the parasite after eradication of malaria from the target area’. [Emphasis added].
Alternatively, wild type mosquito fecundity (EquationEquation 6a) be modelled as
. Preliminary analysis suggests that the resulting equation manifest behaviour similar to those studied here.
Mathematically, this is equivalent to interspecific competition (between w and g) in the presence of extrinsic mortality, x, that affects one species but not the other; see, for example, Citation90.
In greater detail, we require (1) that WTs outcompete transgenics in the absence of malaria, i.e., that c
g
>0 (inherent cost to transgenics), and Equation(2) that, in the presence of sufficient malaria, transgenics outcompete WTs (prevalence-dependent cost to WTs). Since the maximum possible prevalence is x=1, transgenic superiority for this value requires
or
. From these requirements, Condition Equation(10)
follows directly.
Equilibria corresponding to negative numbers of transgenics are, of course, biologically meaningless. Being cognizant of their existence, however, allows one to understand the origination and stabilization of the positive equilibria, which are biologically significant. Moreover, experience [17 suggests that there may be circumstances, for example, perturbations of the model, in which negative equilibria move into the positive quadrant.
The second interior equilibrium also gains a degree of stability at c g=0 via a transcritical bifurcation – this one (TC3) involving the negative w=0 boundary equilibrium. We also note that TC1 and TC3 are ‘at a distance’, by which we mean that equilibria exchange stability but do not collide. By way of contrast, TC2 is generic – collide and exchange.
Thus, if we introduce GM mosquitoes on 1 January 2008, t
0=2008.0, and φ=0; if on 31 January 2008, , and
, etc.
Despite the apparent discontinuity at M=K, our function, F(M), is differentiable and therefore continuous. That is, the left- and right-hand difference quotients both go to zero, as M→K. As a matter of practicality, we note that in none of our calculations did mosquito densities exceed M=K, the point at which the second half of EquationEquation (4a) kicks in.
References
- Abu-Raddad , L. J. , Patnaik , P. and Kublin , J. G. 2006 . Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa . Science , 314 : 1502 – 1605 .
- Ahmed , A. M. and Hurd , H. 2006 . Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis . Microbes Infect. , 8 : 308 – 315 .
- Anderson , R. M. 1982 . “ Directly transmitted viral and bacterial infections in man ” . In Population Dynamics of Infectious Diseases: Theory and Applications , Edited by: Anderson , R. M. 1 – 37 . London : Chapman and Hall .
- Anderson , R. M. and May , R. M. 1979 . Population biology of infectious diseases: I . Nature , 280 : 361 – 367 .
- Anderson , R. M. and May , R. M. 1982 . Directly transmitted infectious diseases: control by vaccination . Science , 215 : 451 – 454 .
- Andrews , K. T. , Michelle , L. , Gatton , M. L. , Skinner-Adams , T. S. , McCarthy , J. S. and Gardin , D. L. 2007 . HIV-malaria interactions: don't forget the drugs . Science , 315 : 1791
- Aron , J. L. and May , R. M. 1982 . “ The population dynamics of malaria ” . In The Population Dynamics of Infectious Diseases: Theory and Applications , Edited by: Anderson , R. 139 – 179 . London : Chapman and Hall .
- Attaran , A. , Roberts , D. R. , Curtis , C. F. and Kilama , W. L. 2000 . Balancing risks on the backs of the poor . Nat. Med. , 6 : 729 – 731 .
- Baird , J. K. 2000 . Resurgent malaria at the millennium: control strategies in crisis . Drugs , 59 : 719 – 743 .
- Baruah , I. , Das , N. G. and Kalita , J. 2007 . Seasonal prevalence of malaria vectors in Sonitpur district of Assam, India . J. Vector. Borne Dis. , 44 : 149 – 153 .
- Bennet , I. E. 1915 . History of the Panama Canal , Washington, DC : Historical Publishing Co .
- Blanford , S. , Chan , B. H.K. , Jenkins , N. , Sim , D. , Turner , R. J. , Read , A. F. and Thomas , M. B. 2005 . Fungal pathogen reduces potential for malaria transmission . Science , 308 : 1638 – 1641 .
- Boivin , M. J. 2002 . Effects of early cerebral malaria on cognitive ability in Senegalese children . J. Dev. Behav. Pediatr. , 23 : 353 – 364 .
- Cameron , I. 1972 . The Impossible Dream: Building the Panama Canal , New York : Morrow .
- Catteruccia , F. , Godfray , H. C.J. and Crisanti , A. 2003 . Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes . Science , 299 : 1225 – 1227 .
- Catteruccia , F. , Nolan , T. , Loukeris , T. G. , Blass , C. , Savakis , C. , Kafatos , F. C. and Crisanti , A. 2000 . Stable germline transformation of the malaria mosquito Anopheles stephensi . Nature , 405 : 959 – 962 .
- Chitnis , N. , Cushing , J. M. and Hyman , J. M. 2006 . Bifurcation analysis of a mathematical model for malaria transmission . IAM J. Appl. Math. , 67 : 24 – 45 .
- Collins , F. H. and Paskewitz , S. M. 1995 . Malaria: current and future prospects for control . Ann. Rev. Entomol. , 40 : 195 – 219 .
- Courtney , O. , Gillingwater , K. , Gomes , P. A.F. , Garcez , L. M. and Davies , C. R. 2007 . Deltamethrin-impregnated bednets reduce human landing rates of sandfly vector Lutzomyia longipalpis in Amazon households . Med. Vet. Entomol. , 21 : 168 – 176 .
- De Roode , J. C. and Read , A. F. 2003 . Evolution and ecology, after the malaria genomes . Trends Ecol. Evol. , 18 : 60 – 61 .
- Desowitz , R. 1993 . The Malaria Capers , New York : W. W. Norton & Co .
- Dietz , K. 1975 . “ Transmission and control of arbovirus diseases ” . In Epidemiology , Edited by: Ludwig , D. and Cook , K. L. 104 – 121 . Philadelphia : SIAM .
- Dietz , K. 1976 . The incidence of infectious diseases under the influence of seasonal perturbations . Lect. Notes. Biomath. , 11 : 1 – 15 .
- Douglas , N. 1956 . Old Calabria , 6 , Brace, NY : Harcourt .
- Driessen , P. 2003 . Eco-imperialism. Green Power. Black Death , Bellevue, Washington : Free Enterprise Press .
- Dyson , D. December 2000 . DDT should not be banned , December , South Africa Readers Digest . (Reprinted by Center for International Development at Harvard University)
- Editorial . 2000 . Caution required with the precautionary principle . Lancet , 356 : 265
- Ferguson , F. R. , Rivero , A. and Reed , A. F. 2002 . Why is the effects of malaria parasites on mosquito survival still unresolved? . Trends Parasitol. , 18 : 256 – 261 .
- Flessa , S. 1999 . Decision support for malaria-control programmes – a system dynamics model . Health Care Manag. Sci. , 2 : 181 – 191 .
- Ghosh , A. K. , Ribolla , P. E.M. and Jacobs-Lorena , M. 2001 . Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library . Proc. Nat. Acad. Sci. USA , 98 : 13278 – 13281 .
- Gogtay , N. J. 2004 . Malaria- hope on the horizon . J. Postgrad. Med. , 50 : 5 – 6 .
- Hancock , P. A. and Godfray , H. C. 2007 . Application of lumped age-class technique to studying the dynamics of malaria-mosquito human interactions . Malaria J. , 6 ( 30 July ) : 98
- Harrison , G. 1978 . Mosquitoes, Malaria and Man: A History of the Hostilities Since 1880 , New York : E. P. Dutton .
- Hay , S. L. , Cox , J. , Rogers , D. J. , Randolph , S. E. , Sternk , D. I. , Shanks , G. D. , Myers , M. F. and Snow , R. W. 2002 . Climate change and the resurgence of malaria in East African highlands . Nature , 415 : 905 – 909 .
- Hoffman , S. L. 2006 . A protective paradox . Nature , 444 : 824 – 827 .
- Hoffman , S. L. , Oster , C. N. , Plowe , C. V. , Woollett , G. R. , Beier , J. C. , Chulay , J. D. , Wirtz , R. A. , Hollingdale , M. R. and Mugambi , M. 1987 . Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications . Science , 237 : 639 – 642 .
- Holding , P. A. and Snow , R. W. 2001 . Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence . Am. J. Trop. Med. Hyg. , 64 ( Supp. 1–2 ) : 68 – 75 .
- Hurd , H. 2003 . Manipulation of medically important insect vectors by their parasites . Ann. Rev. Entomol. , 48 : 141 – 161 .
- Hurd , H. , Taylor , P. J. , Adams , D. , Underhill , A. and Eggleston , P. 2005 . Evaluating the costs of mosquito resistance to malaria parasites . Evolution , 59 : 2560 – 2572 .
- Idro , R. , Otieno , G. , White , S. , Kahindi , A. , Fegan , G. , Ogutu , B. , Mithwani , S. , Maitland , K. , Neville , B. G.R. and Newton , C. R.J.C. 2005 . Decorticate, decerebrate and opisthotonic posturing and seizures in Kenyan children with cerebral malaria . Malaria J. , 4 : 57 – 66 .
- Ito , J. , Ghosh , A. , Moreira , L. A. , Wimmer , E. A. and Jacobs-Lorena , M. 2002 . Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite . Nature , 417 : 452 – 455 .
- Khibnik , A. I. , Kuznetsov , Y. A. , Levitan , V. V. and Nikolaev , E. V. 1990–1992 . Interactive LOCal BIFurcation Analyzer . Unpublished Manual to LOCBIF Version , 2
- Killeen , G. F. and Smith , T. A. 2007 . Exploring the contributions of bed nets, cattle, insecticides and excitorepellency in malaria control: a deterministic model of mosquito host-seeking behaviour and mortality . Trans. Roy. Soc. Trop. Med. , 101 : 867 – 880 .
- King , A. A. 1999 . Hamiltonian limits and subharmonic resonance in models of population fluctuations , Tucson : University of Arizona . Ph.D. thesis
- King , A. A. and Schaffer , W. M. 1999 . The rainbow bridge: Hamiltonian limits and resonance in predator-prey dynamics . J. Math. Biol. , 39 : 439 – 469 .
- Koella , J. C. and Antia , R. 2003 . Epidemiological models for the spread of anti-malarial resistance . Malaria J. , 2 : 11
- Koenraadt , C. J.M. , Paaijmans , K. O. , Schneider , P. , Githeko , A. K. and Takken , W. 2006 . Low larval vector survival explains unstable malaria in the western Kenya highlands . Trop. Med. Int. Health , 11 : 1195 – 1205 .
- Kumar , K. A. , Sano , G. , Boscardinm , S. , Nussenzweig , R. S. , Nussenzweig , M. C. , Zavala , F. and Nussenzweig , V. 2006 . The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites . Nature , 444 : 937 – 940 .
- Kuznetsov , Y. A. 1995 . Elements of Applied Bifurcation Theory , New York : Springer-Verlag .
- Lajmanovich , A. and Yorke , J. A. 1976 . A deterministic model for gonorrhea in a nonhomogeneous population . Math. Biosci. , 28 : 221 – 236 .
- Laufer , M. K. , Thesing , P. C. , Eddington , N. D. , Masonga , R. , Dzinjalamala , F. K. , Takala , S. L. , Taylor , T. E. and Plowe , C. V. 2006 . Return of chloroquine antimalarial efficacy in Malawi . NEJM , 355 : 1959 – 1966 .
- Le Menach , A. , Takala , S. , McKenzie , F. E. , Perisse , A. , Harris , A. , Flahault , A. and Smith , D. L. 2007 . An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets . Malaria J. , 6 ( 25 January ) : 10
- Li , J. 2004 . Simple mathematical models for interacting wild and transgenic mosquito populations . Math. Biosci. , 189 : 39 – 59 .
- Li , J. 2005 . Heterogeneity in modelling of mosquito populations with transgenic mosquitoes . J. Diff. Equa. Appl. , 11 : 443 – 469 .
- Li , J. Seventh Mississippi State – UAB Conference on Differential Equations Computational Simulations . November 1–3 , Birmingham, AL. Continuous-time mathematical modeling of transgenes mosquitoes in preventing malaria transmission ,
- Li , J. 2008 . Differential equations models for interacting wild and transgenic mosquito populations . J. Biol. Dynam.v , 62 : 241 – 258 .
- Lindblade , K. A. , Gimnig , J. E. , Kamau , L. , Hawley , W. A. , Odhiambo , F. , Olang , G. , ter Kuile , F. O. , Vulule , J. M. and Slutsker , L. 2006 . Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes . J. Med. Entomol. , 43 : 428 – 432 .
- Lomborg , B. 2004 . Global Crises. Global Solutions , UK : Cambridge University Press .
- MacDonald , G. 1952 . The analysis of equilibrium in malaria . Trop. Diseases Bull. , 49 : 813 – 828 .
- MacDonald , G. 1957 . The Epidemiology and Control of Malaria , London : Oxford University Press .
- Marrelli , M. T. , Li , C. , Rasgon , J. L. and Jacobs-Lorena , M. 2007 . Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood . Proc. Nat. Acad. Sci. USA. , 104 : 5580 – 5583 .
- Moreira , L. A. , Wang , J. , Collins , F. H. and Jacobs-Lorena , M. 2004 . Fitness of anopheline mosquitoes expressing transgenes that inhibit plasmodium development . Genetics , 166 : 1337 – 1341 .
- Moreira , L. A. , Ito , J. , Ghosh , A. , Devenport , M. , Zieler , H. , Abraham , E. G. , Crisanti , A. , Nolan , T. , Catteruccia , F. and Jacobs-Lorena , M. 2002 . Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes . J. Biol. Chem. , 277 : 40839 – 40843 .
- Mouchetm , J. , Manguin , S. , Sircoulon , J. , Laventure , S. , Faye , O. , Onapa , A. W. , Carnevale , P. , Julvez , J. and Fontenille , D. 1998 . Evolution of malaria in Africa for the past 40 years: impact of climatic and human factors . J. Am. Mosq. Control Assoc. , 14 : 121 – 130 .
- Muelle , A.-K. , Labaied , M. , Stefan , H. I. , Kappe , G. H.I. and Matuschewski , K. 2005 . Genetically modified Plasmodium parasites as a protective experimental malaria vaccine . Nature , 433 : 164 – 167 .
- Nishiura , H. 2007 . Time variations in the transmissibility of pandemic influenza in Prussia, Germany, from 1918–1919 . Theoret. Biol. Med. Model. , 4 : 20 doi:10.1186/1742-4682-4-20
- Nosten , F. , van Vugt , M. , Price , R. , Luxemberger , C. , Thway , K. L. , Brockman , A. , McGready , R. , ter Kuile , F. , Looareesuwan , S. and White , N. J. 2000 . Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study . Lancet , 356 : 297 – 302 .
- Oduro , A. R. , Koram , K. A. , Rogers , W. , Atuguba , F. , Ansah , P. , Anyorigiya , T. , Ansahm , A. , Anto , F. , Mensah , N. , Hodgson , A. and Nkrumah , F. 2007 . Severe falciparum malaria in young children of the Kassena-Nankana district of northern Ghana . Malaria J. , 6 ( 27 July ) : 96
- Póvoa , M. M. , Conn , J. E. , Schlichting , C. D. , Amaral , J. C.O.F. , Nazaré , M. , Segura , O. , Da Silva , A. N.M. , Dos Santos , C. C.B. , Lacerda , R. N.L. , De Sousa , R. T.L. , Galiza , D. , Santa Rosa , E. P. and Wirtz , R. A. 2003 . Malaria vectors, epidemiology, and the reemergence of Anopheles darlingi in Belém, Pará, Brazil . J. Med. Entomol. , 40 : 379 – 386 .
- Ranson , H. , Claudianos , C. , Ortelli , F. , Abgrall , C. , Hemingway , J. , Maria , V. , Sharakhova , M. V. , Unger , M. , Collins , F. H. and Feyereisen , R. 2002 . Evolution of supergene families associated with insecticide resistance . Science , 298 : 179 – 181 .
- Rasgon , J. L. and Scott , T. W. 2004 . Impact of population age structure on Wolbachi transgene driver efficacy: ecologically complex factors and release of genetically modified mosquitoes . Insect Biochem. Mol. Biol. , 34 : 707 – 713 .
- Riehle , M. A. , Srinivasan , P. , Moreiram , C. K. and Jacobs-Lorena , M. 2003 . Towards genetic manipulation of wild mosquito populations to combat malaria: advances and challenges . J. Exp. Biol. , 206 : 3809 – 3816 .
- Roberts , D. R. , Manguin , S. and Mouchet , J. 2000 . DDT house spraying and re-emerging malaria . Lancet , 356 : 330 – 332 .
- Roberts , D. R. , Laughlin , L. L. , Paul Hsheih , P. and Legters , L. J. 1997 . DDT, global strategies and a malaria control crisis in South America . Emerg. Infect. Dis. , 3 : 295 – 302 .
- Romi , R. , Sabatinelli , G. and Majori , G. 2001 . Could malaria reappear in Italy? . Emerg. Infect. Dis. , 7 : 915 – 919 .
- Ross , R. 1911 . The Prevention of Malaria , London : John Murray .
- Ross , R. 1916 . An application of the theory of probabilities to the study of à priori pathometry. I . Proc. Roy. Soc. London Ser. A , 92 : 204 – 230 .
- Sachs , J. D. 2002 . A new global effort to control malaria . Science , 298 : 122 – 124 .
- Sacker , R. J. and von Bremen , H. F. 2007 . Stability in a periodic model for genetically altered mosquitos , preprint http://www.csupomona.edu/∼nabramzon/WINTER2007/PHY410/Hubertus%20Von%20Bremen%202.pdf
- Saul , A. 1990 . Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching . Malaria J. , 2 ( 19 September ) : 32
- Saul , A. J. , Graves , P. M. and Kay , B. H. 2003 . A cyclical feeding model for pathogen transmission and its application to determine vector capacity from vector infection rates . J. Appl. Ecol. , 27 : 123 – 133 .
- Schaffer , W. M. and Bronnikova , T. V. 2007 . Parametric dependence in model epidemics . J. Biol. Dynam. , 1 : 183 – 195 .
- Schelsenger , A. M. and Israel , F. L. 1999 . “ Building the Panama Canal ” . In Chronicles from National Geographic , Philadelphia : Chelsea House Publ .
- Scherer , A. and McLean , A. 2002 . Mathematical models of vaccination . Brit. Med. Bull. , 62 : 187 – 199 .
- Scholte , E.-J. , Ng'habi , K. , Kihonda , J. , Takken , W. , Paaijmans , K. , Abdulla , S. , Killeen , G. F. and Knols , B. G.J. 2005 . An entomopathogenic fungus for control of adult African malaria mosquitoes . Science , 308 : 1641 – 1642 .
- Scott , T. W. , Takken , W. , Knols , B. G.J. and Boëte , C. 2002 . The ecology of genetically modified mosquitoes . Science , 298 : 117 – 119 .
- Selgrade , J. F. and Roberds , J. H. 2007 . Global attractors for a discrete selection model with periodic immigration . J. Diff. Equa. Appl. , 13 : 275 – 287 .
- Service , M. W. 1993 . Mosquito Ecology. Field Sampling Methods , London : Elsevier .
- Sidhu , A. B.S. , Verdier-Pinard , D. and Fidock , D. A. 2002 . Chloroquine resistance in plasmodium falciparum malaria parasites conferred by pfcrt mutations . Science , 298 : 210 – 213 .
- Slobodkin , L. B. 1961 . Growth and Regulation of Animal Populations , New York : Holt, Rinehart and Winston .
- Smith , A. G. 2000 . How toxic is DDT? . Lancet , 356 : 267 – 268 .
- Smith , D. L. and McKenzie , F. E. 2004 . Statics and dynamics of malaria infection in Anopheles mosquitoes . Malaria J. , 3 ( 4 June ) : 13
- Smith , T. , Maire , N. , Dietz , K. , Killeen , G. F. , Vounatsou , P. , Molineaux , L. and Tanner , M. 2006 . Relationship between the entomologic inoculation rate and the force of infection for Plasmodioum falcuparum malaria . Am. J. Trop. Med. Hyg. , 75 ( Supp. 2 ) : 11 – 18 .
- Soremekun , S. , Maxwell , C. , Zuwakuu , M. , Chen , C. , Michael , E. and Curtis , C. 2004 . Measuring the efficacy of insecticide treated bednets: the use of DNA fingerprinting to increase the accuracy of personal protection estimates in Tanzania . Trop. Med. Int. Health , 9 : 664 – 672 .
- Taken , W. 2002 . Do insecticide-treated bednets have an effect on malaria vectors? . Trop. Med. Int. Health , 7 : 1022 – 1030 .
- Takken , T. and Scott , T. W. 2003 . Ecological Aspects for Application of Genetically Modified Mosquitoes , Netherlands : Wageningen .
- Tumwiine , J. , Mugisha , J. Y.T. and Luboobi , L. S. 2007 . On oscillatory pattern of malaria dynamics in a population with temporary immunity . Comp. Math. Meth. Med. , 8 : 191 – 203 .
- Turelli , M. and Hoffmann , A. A. 1999 . Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into an arthropod population . Insect Mol. Biol. , 8 : 243 – 255 .
- Wellems , T. E. 2002 . Plasmodium chloroquine resistance and the search for a replacement antimalarial drug . Science , 298 : 124 – 126 .
- WHO . 2006 . Malaria vector control and personal protection , Geneva, , Switzerland : World Health Organization Tech. Rep. 936 .
- Windbichler , N. , Papathanos , P. A. , Catteruccia , F. , Ranson , H. , Burt , A. and Crisanti , A. 2007 . Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos . Nucleic Acids Res. , 35 : 5922 – 5933 .
- Wiwanitkit , V. 2006 . Correlation between rainfall and the prevalence of malaria in Thailand . J. Infect. , 52 : 227 – 230 .