Abstract
The larch budmoth (LBM) population in the Swiss Alps is well known for its periodic outbreaks and regular oscillations over several centuries. The ecological mechanisms that drive these oscillations, however, have not been unambiguously identified, although a number of hypotheses have been proposed. In this article, we investigate several LBM resulting from these different ecological hypotheses. We first study a leaf quality-moth population model and then two moth–parasitoid models. Existence and stability of equilibria are investigated and sufficient conditions for which populations can persist are derived. We then provide conclusions based on our analysis.
1. Introduction
Outbreaking forest insects provide fascinating examples of complex population dynamics. The population of larch budmoth (LBM), Zeiraphera diniana, in the Swiss Alps is well known for its periodic outbreaks and regular oscillations Citation2. As in most outbreaking forest insects, identifying causal mechanisms for LBM outbreaks has proven difficult. It is becoming clear, however, that exogenous factors cannot generate consistent periodic cycles and that density-dependent effects from biotic interactions are the most likely mechanisms Citation5.
A number of biotic mechanisms have been proposed. There is a lack of empirical support for maternal effects, and although viral infections were observed to cause substantial mortality during two intensively studied LBM outbreaks, subsequent outbreaks collapsed without being accompanied by an increase in viral mortality Citation2. Two non-mutually exclusive hypotheses for the cause of LBM cycles are currently en vogue: Equation(1) plant quality effects and Equation(2)
parasitoid–host interactions. Plant quality and parasitism both have the necessary interaction effects to induce cycles Citation12. For example, it usually takes several years for the leaf quality to recover after a serious LBM outbreak, and field and laboratory studies show that poor plant quality has a strong effect on LBM survival and reproduction Citation2, the consequence of which could result in regular cycles. As for parasitoid effects, previous analyses for direct density-dependent effects suggested that parasitoids had little role in creating population cycles because parasitism rates at peaks in LBM population size were relatively low Citation2. However, as Turchin et al. Citation12 pointed out, population cycles are the result of second-order lags in density-dependent effects, and thus rejection of the parasitoid hypothesis was inappropriate.
The goal of the present study is to provide mathematical analysis on the models proposed by Turchin et al. Citation12. We first investigate a leaf quality-LBM model and then two LBM-parasitoid models. Because LBM has an annual life cycle with non-overlapping generations, the models are systems of difference equations. We investigate the existence of simple stationary solutions of the systems and their stabilities. Bifurcation analysis for each of the models will also be performed. Criterion for population persistence will be derived. We will compare and contrast these analytical results. In the following section, we study a leaf quality-LBM model. Section 3 investigates parasitoid-LBM models. The final section provides some biological conclusions.
2. A plant quality-LBM model
Let N t denote the LBM density at time t, t=0, 1, …, where the moth population density is measured in terms of the number of third instar larvae per kilogram of larch branches. Since third instar larvae have approximately the same dimensions, the density is proportional to the biomass. On the other hand, population densities are usually used in host–parasitoid interactions. For these reasons, the LBM density is used in the leaf quality LBM model instead of biomass.
The leaf quality (e.g., raw fibre and protein content) at time t is denoted by Q t . Good leaf quality means low fibre and high protein content. The needle length is a good index of plant quality since it is well correlated with raw fibre and protein content of needles Citation7 Citation10. Moreover, data collected in Citation4 also indicated that needle length has a strong effect on larval survival and pupal biomass, where pupal biomass is closely related to adult fecundity. Therefore, the index of plant quality Q is measured in terms of average needle length L from field studies using a linear conversion
The dynamics of leaf quality in the absence of the moth population is modelled by a simple linear recursive equation , where 1−α is the recovery rate of plant quality. The plant quality decays to 0 at a rate α if the plant cannot renew itself. It is also assumed that the effect of leaf quality is on budmoth's intrinsic growth rate but not on its carrying capacity, and the effect of plant quality on budmoth is modelled by a simple expression with saturation
, where δ>0 is the half-saturation constant for the plant quality. The uptake of the moth population upon the plant is modelled using a Michaelis–Menton form
, where γ>0 is the half-saturation constant for the moth population uptake.
Under the above biological consideration, the interaction between plant quality and LBM population can be described by the following system of two first-order non-linear difference equations:
We first rescale the system by letting and
. Then system Equation(1)
can be rewritten as
Lemma 2.1
Solutions of system
Equation(2)
remain non-negative for t>0 and are bounded.
System Equation(2) has a trivial stationary solution E
0=(0, 1) for all parameter values where LBM population is extinct. The Jacobian matrix of system Equation(2)
evaluated at E
0 has the form
Theorem 2.2
If
then equilibrium E
0=(0, 1) is globally asymptotically stable for system
Equation(2)
.
Proof
We claim that if
. This is clear if λ<1. If λ=1, then x
t+1<x
t
for t≥0 implies
. If ◯>0, then from the first equation of Equation(2)
we would have a contradiction, namely
Since 1 is the maximum plant quality which the larch trees can maintain, can be interpreted as the maximal growth rate of the moth population. The moth population can attain this maximal growth rate only when the tree quality is at its optimal condition. Consequently, the moth population becomes extinct if this maximal growth rate is less than 1.
Suppose now . Then E
0 is unstable with stable manifold lying on the non-negative Q-axis. The x, Q-components of a coexisting equilibrium where both populations can persist must satisfy
The Jacobian matrix of system Equation(2) evaluated at Ē has the form
We next prove that both populations can persist if . System Equation(2)
is said to be uniformly persistent if there exists a constant A>0 such that
and
for all solutions (x
t
, Q
t
) of system Equation(2)
with x
0>0 and Q
0>0. We shall apply the techniques introduced by Hofbauer and So Citation8.
Theorem 2.3
Let
. Then system
Equation(2)
has a unique interior steady state
and system
Equation(2)
is uniformly persistent. Moreover, Ē
is locally asymptotically stable if
Equation(5)
holds.
Proof
By the above discussion, we only need to prove the uniform persistence of system Equation(2). Let
. Then
is forward invariant for system Equation(2)
and Equation(2)
has a global attractor X. It is clear that the only invariant set in Y is {E
0}. We proceed to verify that {E
0} is isolated in X and the stable set of E
0 is contained in Y.
Since , there exists ϵ>0 such that
In order to study the system further, we numerically simulate system Equation(2). We choose δ=2, m=15, α=0.5, and use λ as a bifurcation parameter. The choice of using λ as the bifurcation parameter is motivated by the fact that fecundity of female adult LBM can vary significantly depending on environment factors such as temperature, moisture, etc.
When λ is less than 3=δ+1, the moth population crashes as demonstrated analytically in Theorem 2.2. When λ is between 3 and 10, then simulations revealed that there is only steady-state dynamics. All solutions randomly chosen converge to the coexisting equilibrium. The bifurcation diagrams presented here start with λ=10 and end with λ=60. It is clear that a period-doubling bifurcation occurs when λ is somewhere between 22 and 25 and the moth population density of the coexisting steady state in increases as we increase λ before hitting the first bifurcation value. The opposite situation is observed for the plant quality in .
Figure 1. Bifurcation diagrams for system Equation(2) with λ as the bifurcation parameter. Other parameter values are δ=2, m=15, and α=0.5. The vertical axis represents moth population density (a) and leaf quality (b).
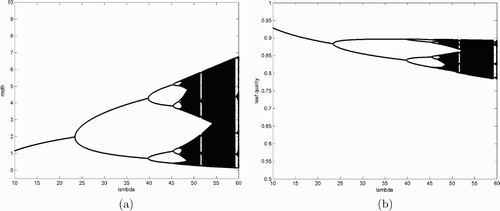
These numerical results confirm our earlier analysis. It can be seen that the system clearly has a positive period two solution when λ is just beyond 25, right after the period-doubling bifurcation. and are the bifurcation diagrams of system Equation(2) for the moth population density and plant quality, respectively. We see from these diagrams that period-doubling bifurcations occur continuously and the system eventually becomes chaotic when λ is further increased. Notice that similar dynamical behaviour of system Equation(2)
is observed when we change parameter values of δ, α, and m. In particular, bifurcation diagrams similar to those of for the budmoth population and leaf quality are obtained when we vary parameter values of δ, α, and m.
3. Moth–parasitoid models
It is well known that parasitoids play an important role in population dynamics of forest insects Citation11. For this reason, LBM parasitoids were intensively studied from the beginning of the systematic research on LBM oscillations. There are two main groups of parasitoids that are important in affecting LBM population, eulophid species, and ichneumonids. Eulophids attack primarily the third instar of LBM, while the ichneumonid attack mainly the fifth instar. In this section, we present and investigate two models proposed by Turchin et al. Citation12.
3.1. A simple moth–parasitoid model
Similar to the previous section, we let N t be the LBM population density at time t. The parasitoid population density at time t is denoted by P t , which is the number of adult parasitoids per kilogram of larch branches. Following that of Beddington et al. Citation3, the first model is based on the Nicholson–Bailey model Citation9 with parasitoid interference functional response, aP/(1+awP), as suggested by the preliminary data analysis on the moth–parasitoid interaction, where a is the parasitoid searching rate and w is the parasitoid wasted time. The model is given below:
To simplify the system, we define new state variables , y
t
=aP
t
, and let c=ab/β. Then system Equation(6)
becomes
Observe that each of the non-negative coordinate axes is forward invariant for system Equation(7). Moreover, system Equation(7)
reduces to the classical Ricker equation
Lemma 3.1
Solutions of system
Equation(7)
remain non-negative for t>0 and are bounded.
Since system Equation(7) is a predator–prey model, steady state E
0=(0, 0) always exists where both populations are extinct. The Jacobian matrix of system Equation(7)
evaluated at E
0 has a simple form
. It follows that E
0 is locally asymptotically stable if λ<1 and unstable if λ>1. Since
and
for t≥0, it can be easily shown that E
0 is globally asymptotically stable if λ<1. The proof of the following theorem is omitted.
Theorem 3.2
Steady state E
0=(0, 0) is globally asymptotically stable for system
Equation(7)
in
if λ<1.
Observe that λ is the inherent net growth rate of the moth population. It is the growth rate of the population when population size is very small before intraspecific competition making any effect. The population cannot survive if the inherent growth rate λ is less than 1. If λ>1, then E
0 is unstable with stable manifold lying on the non-negative y-axis and there exists another boundary steady state where only the budmoth population can survive. The linearization of system Equation(7)
with respect to E
1 yields the Jacobian matrix
Therefore, E
1 is locally asymptotically stable if
It can be shown that E
1 is globally asymptotically stable for system Equation(7) whenever it is locally asymptotically stable.
Theorem 3.3
Let λ>1. Then system
Equation(7)
has two boundary equilibria E
0=(0, 0) and
, where E
0
is unstable and E
1
is globally asymptotically stable in
if inequalities
Equation(10)
and
Equation(11)
hold.
Proof
We only need to prove global attractiveness of E
1 in when EquationEquations (10)
and Equation(11)
are satisfied. Let (x
t
, y
t
) be an arbitrary solution of system Equation(7)
with x
0>0. Since lnλ is globally asymptotically stable for EquationEquation (8)
in (0, ∞) by EquationEquation (10)
and
holds for t>0,
. Therefore, for any ϵ>0, we can find t
0>0 such that
for t≥t
0. Since
, we can choose ϵ>0 such that
. It follows from the second equation of Equation(7)
that
for t≥t
0. This shows that
, and as a result we have
. Consequently, E
1 is globally attracting in
and the proof is complete. ▪
Since each parasitized LBM can reproduce c number of parasitoids, clnλ can be interpreted as the reproductive number of the parasitoid when the moth population is stabilized at the lnλ level. The parasitoid population cannot invade the moth population if this reproductive number is less than one.
We proceed to discuss the existence of coexisting equilibria. Let (x, y) be an interior steady state of system Equation(7). Then
Let and
for
. Then g(0)=0,
, and g is increasing and concave up on (0, 1/w). Also
,
,
for
, and
has a unique solution in
, i.e., h(x) is a one-humped function on
. Using these, it can be shown that system Equation(7)
has a unique interior steady state when
.
Theorem 3.4
Let λ>1. Then system
Equation(7)
has a unique interior equilibrium
if and only if
. Moreover, system
Equation(7)
is uniformly persistent if
and
.
Proof
Suppose . We claim that EquationEquation (14)
has no solutions satisfying Equation(15)
. For simplicity, we let
. Since cr<1, it is clear that
for 0<x<1/w. Moreover,
holds for 0<x<r. Therefore,
for
and EquationEquation (14)
has no solutions satisfying Equation(15)
. Consequently, system Equation(7)
has no interior steady state if
.
Suppose now . It can be easily shown that EquationEquation (14)
has a unique solution satisfying Equation(15)
. Indeed, by our assumption of cr>1, we see that y=h(x) lies above y=g(x) for x>0 and x small. Assume r≤1/w. The case when r>1/w can be argued similarly. Notice h(r)=0 and g(r)>0 imply that h(x)=g(x) has at least one solution in (0, r). If there were more than one solution in (0, r), let 0<x
1<x
2<r be the first two solutions. Then we must have
and
. Since
, we obtain
by the above two inequalities and arrive at a contradiction because h is concave down on (0, r). Therefore, Equation(14)
has a unique solution satisfying Equation(15)
and we conclude that system Equation(7)
has a unique interior equilibrium
if and only if
.
To prove the uniform persistence of system Equation(7), observe that since
, EquationEquation (8)
has only simple dynamics, namely the positive steady state lnλ is globally asymptotically stable in (0, ∞). The proof is similar to the proof of Theorem 2.3. We shall apply Theorem 4.1 of Citation8. Let Y be the set of non-negative x- and y-axes. It is clear that system Equation(7)
has a global attractor X and the only invariant subset in Y is {E
0, E
1}. We need to verify that {E
i
} is isolated in X and the stable set of E
i
lies in Y for i=0, 1. It is straightforward to show that {E
0} is isolated in X using λ>1. Indeed, if {E
0} is not isolated in X, then for any ϵ>0 we can find a maximal invariant set M
0 in
such that {E
0} is a proper subset of M
0. Since λ>1, we can choose ϵ>0 such that
and let
. Then
and there exists y
s
,
, such that
. Let
. It follows that
which contradicts the choice of x
0. Similarly, if {E
1} is not isolated in X, then for any ϵ>0 given we can find a maximal invariant subset M
1 in
with
. Notice
. We choose ϵ so that
and
. Notice there exists x
u
,
, such that
. Let
. Then
which is impossible.
It remains to verify that the stable set of E
i
lies in Y. Suppose there exist x
0>0 and y
0>0 such that . Then for any ϵ>0 there exists t
0>0 such that
and
for t≥t
0 We choose ϵ>0 such that
. It follows that x
t+1>x
t
for t≥t
0, and we would have
, which is impossible. The proof for the stable set of E
1 lying in Y is similar. Therefore, system Equation(7)
is uniformly persistent by Theorem 4.1 of Citation8. ▪
Notice in the above theorem we only showed that both populations can persist when and
. It is suspected that populations can coexist even when lnλ is greater than 2 but not too large. We use a numerical example to demonstrate that the parasitoid population may become extinct when lnλ is large. The parameter values used in these simulations are c=10, w=1, and λ ranges from 30 to 60. It can be seen from that the parasitoid population crashes when λ is just less than 55. This is somewhat biologically unexpected. One would expect that the large inherent net reproductive rate of the moth population will enhance the growth of parasitoid since it relies on the moth population for its own reproduction. is the corresponding bifurcation diagram for the moth population. From this we see that the LBM population survives, whereas the parasitoid population goes extinct when λ is large. This unexpected phenomenon is probably due to the frequent fluctuation of the LBM, which drives the parasitoid to extinction.
Figure 2. Bifurcation diagrams for system Equation(7) using λ as the bifurcation parameter with c=10 and w=1. (a) budmoth population; (b) parasitoid population.
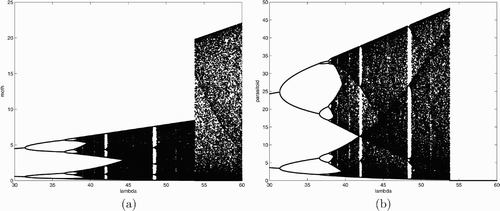
Suppose now so that E
1 is unstable and system Equation(7)
has a unique coexisting equilibrium
. Note from Equation(12)
we have
, i.e., the LBM population density in the coexisting steady state is always less than the LBM population density of the non-trivial boundary steady state
for which the parasitoid population is extinct. The stability of E* can be determined by the corresponding Jacobian matrix
Recall that in we use w=1 for the simulations. Although we are only able to prove analytically that only a period-doubling bifurcation can occur when E* loses its stability for w≥1/2, bifurcation analysis seems to suggest that this is the case for all 0<w≤1. Moreover, it is showed that a +1 bifurcation cannot occur for all 0<w≤1 when E* loses its stability. Although the figures are not presented here, similar bifurcation diagrams as those in are obtained if we vary parameters w and c.
3.2. A moth–parasitoid model with host self-regulation
In this subsection we investigate a moth–parasitoid model that is also based on the Nicholson–Bailey model. Similar to system Equation(6), N
t
and P
t
are denoted as the LBM and parasitoid densities at time t, respectively. In addition to the parasitoid mutual interference incorporated in system Equation(6)
, the model also takes into account of the host self-regulation and saturation. The probability of an individual host being parasitized per unit time is now given by
, where h>0 is the handling time of the parasitoid and parameters a and w have the same biological meanings as in the previous model Equation(6)
. The new model takes the following form:
Lemma 3.5
Solutions of system
Equation(17)
remain non-negative and are bounded.
Similar to the previous moth–parasitoid model Equation(7), system Equation(17)
always has the trivial steady state E
0=(0, 0) where both populations are extinct. Notice E
0 is locally asymptotically stable if λ<1, and it can be easily shown that it is globally asymptotically stable whenever λ<1. Therefore, global extinction of the two populations occurs when the inherent net growth rate, λ, of the moth population is less than one. The proof of the following theorem is straightforward and is omitted.
Lemma 3.6
Steady state E
0=(0, 0) is globally asymptotically stable for system
Equation(17)
if λ<1.
Assume λ>1. Then E
0 is a saddle point, with the stable manifold lying on the non-negative $y$-axis, and there exists another boundary steady state where only the LBM population can persist. The Jacobian matrix of Equation(17)
evaluated at E
1 has the form
Lemma 3.7
Let λ>1. Then system
Equation(17)
has another steady state
and E
1
is globally asymptotically stable in
if
and
.
Let (x, y) denote an interior steady state of system Equation(17) and set
. Then
We next consider the case when . Set d(x)=0, we have
Theorem 3.8
Let λ>1. Then system
Equation(17)
has a unique interior steady state if and only if
Moreover, system
Equation(17)
is uniformly persistent if
and
We now use simulations to study system Equation(17). Fix w=1, c=10 as in , and s=0.5, and let λ be a bifurcation parameter with values ranging from 10 to 60. Since
Figure 3. This figure provides bifurcation diagrams for system Equation(17) when w=1, c=10, and s=0.5, (a) and (b) moth and parasitoid population densities, respectively.
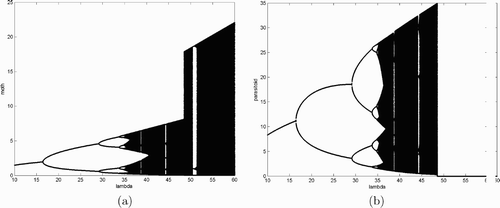
4. Conclusions
In this article, we studied one LBM-leaf quality model and two LBM-parasitoid models, proposed by Turchin et al. Citation12. From the mathematical analysis and bifurcation diagrams presented in Sections 2 and 3, it seems to suggest that the dynamics of leaf quality LBM and LBM parasitoid systems are topologically conjugate Citation6 to each other under some restrictions on the parameter values. In other words, although the systems are derived from very different biological phenomena and assumptions, their dynamics seem to be topologically equivalent to each other. Therefore, it is very difficult to arrive at conclusions based on mathematical analysis performed on these models as whether leaf quality or parasitoid is the main factor that is responsible for the LBM population cycling. However, each of these models does confirm our biological intuition. Namely, there exist population thresholds in terms of model parameters above which populations can persist and below which populations go extinct. Furthermore, the statistical study provided by Turchin et al. Citation12 does suggest that the parasitism is probably the more important factor for LBM cycling.
Acknowledgements
The authors thank the referees for their suggestions.
Additional information
Notes on contributors
Sophia R.-J. Jang
†Present address: Department of Mathematics and Statistics, Texas Tech University, Lubbock, TX USAReferences
- Allen , L. 2006 . An Introduction to Mathematical Biology , Upper Saddle River, NJ : Prentice Hall .
- Baltensweiler , W. and Fischlin , A. 1988 . “ The larch budmoth in the Alps ” . In Dynamics of Forest Insect Populations: Patterns, Causes, Implications , Edited by: Berryman , A. 331 – 351 . New York : Plenum .
- Beddington , J. R. , Free , C. A. and Lawton , J. H. 1975 . Dynamic complexity in predator-prey models framed in difference equations . Nature , 255 : 58 – 65 .
- Benz , G. 1974 . Negative Ruckkopelung durch Raum- und Nahrungskonkurrenz sowie zyklische Veranderung der Nahrungsgrundlage als Regelsprinzip in der Popultionsdynamik des Grauen Larchenwicklers, Zeiraphera diniana (Guenee) (Lepidoptera, Tortricidae). Zeitschrift fuer angewandte . Entomologie , 76 : 196 – 228 .
- Berryman , A. A. 1996 . What causes population cycles of forest Lepidoptera? . Trends Ecol. Evol. , 11 : 28 – 32 .
- Devaney , R. 1989 . An Introduction to Chaotic Dynamical Systems , Redwood City, CA : Addison-Wesley .
- A. Fischlin, Analyse eines wald-insekten-systems: der subalpine Larchen-arvenwald und der graue Larchenwickler Zeiraphera diniana Gn (Lepidoptera, Tortricidae), Dissertation Number 6977, ETH, Zurich, Switzerland, 1982
- Hofbauer , J. and So , J. 1989 . Uniform persistence and repellors for maps . Proc. Am. Math. Soc. , 107 : 1137 – 1142 .
- Nicholson , A. J. and Bailey , V. A. 1935 . The balance of animal populations . Proc. Zool. Soc. Lond. , : 551 – 598 .
- F.X. Omlin, Zur populationsdynamischenWirkung der durch Raupenfrass und Dungung veranderten Nahrungsbasis auf den Grauen Larchenwickler Zeiraphera diniana Gn. (Lep: Tortricidae), Dissertation Nr. 6064, ETH Zurich, Switzerland, 1977
- Turchin , P. 2003 . Complex Population Dynamics , New Jersey : Princeton University Press .
- Turchin , P. , Wood , S. , Ellner , S. , Kendall , B. , Murdoch , W. , Fischlin , A. , MccAuley , E. and Briggs , C. 2003 . Dynamical effects of plant quality and parasitism on population cycling of larch budmoth . Ecology , 84 : 1207 – 1214 .