Abstract
The boreal toad Bufo boreas boreas, once common in the western USA, is listed as an endangered species in Colorado and New Mexico, and protected in Wyoming. Populations have dramatically declined due to the presence of the fungal pathogen Batrachochytrium dendrobatidis (Bd). A gender- and stage-structured model for the boreal toad is formulated which depends on its life cycle and breeding strategies. In addition, an epizootic model for the spread of Bd is formulated. Analysis of these models provides two thresholds. The first threshold, the basic reproduction number for the population, ℛ0, determines whether the population persists and the second threshold, the basic reproduction number for the fungal disease, ℛF, determines whether the disease persists. If ℛ0>1 and ℛF<1, then the population becomes disease-free. However, if both thresholds are greater than one, the population levels are severely reduced by the fungal pathogen.
1. Introduction
A recently identified fungal pathogen known as Batrachochytrium dendrobatidis (Bd) has been associated with amphibian declines worldwide. This fungal pathogen causes a disease known as chytridiomycosis in susceptible amphibians which results in very high mortality Citation8 Citation12 Citation22 Citation29 Citation35 Citation36. The decline of the boreal toad (Bufo boreas boreas) in the western USA has been attributed to multiple factors, including the presence of this fungal pathogen Citation8 Citation9 Citation33. The boreal toad is currently listed as an endangered species by Colorado and New Mexico, and is a protected species in Wyoming (http://wildlife.state.co.us/Research/Aquatic/BorealToad/). Environmental factors such as low water availability and freezing temperatures may have also impacted this decline Citation8. The boreal toad is found at elevations ranging from sea level to 12,000 feet. The higher elevations in the mountains of Colorado (7000–12,000 feet) and cooler temperatures are favourable conditions for the growth of Bd Citation6 Citation8 Citation11 Citation14 Citation21.
Our goal in this investigation is to develop models that accurately incorporate the developmental stages and reproductive behaviour of the boreal toad. The models will be useful to further study populations in areas where they are threatened or endangered. A variety of mathematical models have been developed to study the impact of the environment or disease on small or declining populations of amphibians Citation3 Citation7 Citation15 Citation16 Citation24 Citation25 Citation30 Citation31. We will summarize these models and discuss how they relate to our modelling efforts.
A Leslie matrix model was developed for the boreal toad to study the sensitivity of model parameters and to understand the contribution of specific life stages to persistence of wild populations in near-equilibrium conditions Citation24. This model did not include Bd infection nor the within-year life cycle stages. In Citation15 Citation16, stage-structured models were formulated with two or three developmental stages in a generic amphibian life cycle, larvae (tadpoles), juveniles (non-reproductive), and adults (LJA or JA model). Free-living fungi were included as another variable F. Density-dependent survival was assumed from egg to larval stage. To distinguish whether the stage was infected with Bd, each stage was identified as susceptible, infectious, or recovered, denoted as S, I, or R. Complete vertical transmission was assumed because infected adults contaminate ponds, where eggs and larvae mature and become infected. For the models in Citation15 Citation16, conditions were derived on model parameters for complete population extinction. In Citation3, a simplified model with susceptible and infectious adult amphibians was analysed. A threshold was derived for the adult-fungi model so that below the threshold the population becomes disease-free. A simple metapopulation model was formulated with different movement patterns for susceptible and infectious adult stages.
A more complete analysis of Citation15 was performed by Salceanu and Smith Citation30 Citation31. In Citation30, for the three-stage LJA–SIR model, criteria for persistence of the population were established as well as global stability results for host extinction and for the disease-free equilibrium. Salceanu and Smith Citation31 extended their analysis to the two-stage JA–SI model by considering <100% vertical transmission.
In Citation7, a hybrid model was applied to the mountain yellow-legged frogs (Rana mucosa), a system of difference equations for larvae and adult stages and a system of differential equations for disease transmission and reproduction of infected individuals during summer months. Environmental stochasticity was included through Monte Carlo simulations of randomly chosen combinations of parameters. The model was shown to be consistent with data. In Citation25, a system of partial differential equation models with delay was applied to tadpole development for the European common toad Bufo bufo, a toad infected by Bd. Long-term survival of Bd in encysted form was assumed Citation13. In numerical simulations, it was shown that a free-living saprobic stage of Bd is able either to cause extinction of the toad population or to establish an enzootic level of infection but with the host population severely reduced in numbers. None of the preceding models include gender-structure and none apply to B. b. boreas, except for the model of McDonald and Tse Citation24.
Discrete-time population models for seasonally breeding populations with some of the same features as the models in this investigation were studied by Ackleh and Jang Citation1 and Ackleh et al. Citation2. Stability and persistence were studied in these latter investigations.
In the following sections, a set of basic model assumptions is described that is unique to the life cycle of the boreal toad. Then, an annual model is formulated, a system of difference equations for the female population. Based on the model assumptions, a threshold ℛ0 is derived, the basic reproduction number for the population. A globally stable positive equilibrium exists if and population extinction occurs if
. Under a weaker set of assumptions, it is shown that the population is permanent, if
. The population model is extended to an epizootic model with Bd infection. A second threshold, the basic reproduction number for the fungal disease,
, is derived to show that if
and
, then the disease-free equilibrium is stable. The effects of environmental change, modelled as a change in the timing of the onset of reproduction or in the breeding frequency, are considered in Section 4. Some numerical results, presented in Section 5, illustrate the possible effects on the boreal toad population due to Bd infection in breeding and non-breeding sites. The conclusion summarizes the results and suggests further research.
2. Population model
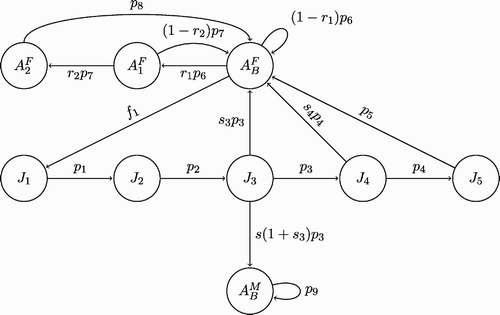
Our mathematical model is based on the life history characteristics of boreal toads taken from the literature Citation8 Citation9 Citation27 Citation32 Citation34. The year is divided into five periods which describe breeding, tadpole development, metamorphosis, dispersal, and hibernation. In addition, the developmental stages of the toad are divided into egg E; tadpole T; juvenile, J (non-reproductive); and adult A stages. The five periods that occur each year are described in . We include the approximate timing during the year that corresponds to boreal toad populations in the Southern Rocky Mountains. However, these times vary with latitude, elevation, and weather conditions.
Table 1. Life cycle stages of the boreal toad during a 1 year period.
Breeding begins after snow and ice melts Citation8. Males arrive at the breeding pond first, awaiting the arrival of females. After breeding, a female toad may lay, on average, 6600 in one to five clutches Citation8 or up to 12,500 eggs in two strings in shallow water Citation34 or even as high as 16,000 in a single clutch Citation32, depending on the situation of the habitat. Given a sufficient number of males, the total number of eggs depends on the number of breeding females. If males are scarce, then the number of eggs will also depend on both breeding males and females. Females are generally not reproductively mature until 4, 5, or even 6 years of age and they do not necessarily breed every year Citation8 Citation27. After breeding, eggs develop into tadpoles, which generally takes about two weeks. The number of tadpoles that develop from eggs depends upon the temperature, a parameter we neglect in our preliminary stage-structured model. Metamorphosis is the transition from tadpoles to toadlets or first-year juveniles. A density-dependent relationship is assumed at metamorphosis, where the number of first-year juveniles that develop from tadpoles depends on the density of tadpoles. This assumption is based on the supposition that predation and competition among tadpoles for food and space limits the number of first-year juveniles. At this time, all stages are updated; that is, tadpoles become first-year juveniles, first-year juveniles become second-year juveniles, and so on. After mating, females leave the breeding site and disperse into the surrounding areas (surviving primarily by feeding on insects) and males stay at the breeding site longer than females Citation27. Dispersal and metamorphosis occur simultaneously. For modelling purposes, we assume that dispersal takes place after metamorphosis. After dispersal, toads hibernate and activities cease; this is the last period of the life cycle.
2.1. Model assumptions
We make some assumptions based on the previous discussion to formulate a population model. Additional assumptions are made in Section 3.1 to formulate an epizootic model, when Bd infection is included in the model. Our population model is based on the following characteristics concerning breeding age, breeding frequency, and life history of the boreal toad.
-
(H1) Males generally do not breed until age four, and females generally begin breeding at either 4, 5, or 6 years of age Citation8,Citation26.
-
(H2) After females become reproductive, they may breed every year, every 2 years, or every 3 years depending on their body sizes and environmental conditions [27 . For modelling purposes, we assume the proportion of females that breed every year is (1−r 1), the proportion that breed every second year is r 1(1−r 2), and the remaining proportion of females that breed every third year is r 1 r 2.
-
(H3) The gender is determined at the age of four.
-
(H4) The developmental stages include an egg stage E; a tadpole stage T; three juvenile stages, where gender is not yet determined, J 1, J 2, J 3; two female juvenile stages J 4, J 5; three female adult stages, one breeding and two non-breeding stages,
,
and
; and one breeding male adult stage,
.
We make five more model assumptions about births, density-dependent survival, and sex ratio.
-
(H5) The birth function,
, is a function of breeding females, where we have assumed that there is a sufficient number of males. Unless noted otherwise, we shall assume the birth function B is a linear function of
,
, where c is the clutch size [10 .
-
(H6) At metamorphosis, the proportion of first-year juveniles that develop from tadpoles is ρ(T), a strictly decreasing differentiable function of T, the density of tadpoles, and ρ(T)T is a non-decreasing function of T for
such that
-
(H7) The sex ratio is constant for a given population. The ratio s, s>0, is the proportion of males to females.
-
(H8) The survival probability of eggs to the tadpole stage is a constant d, 0<d<1.
-
(H9) The survival probability of toads in stage i to stage i+1 during period 5 is a constant satisfying 0<p i5<1, i=1, …, 5 and survival probability of stage i during period j is a constant satisfying
, i=1, …, 9,\; j=1, …, 4.
Assumption (H6) is relaxed in Section 2.4. A family of functions satisfying assumption (H6) is
2.2. Difference equations
A system of difference equations is formulated for the population dynamics of boreal toads during the five periods based on assumptions (H1)–(H9). The five periods are important for modelling of Bd infection, which depend on the time spent in the water. The model can be simplified to an annual model with time beginning in period 1 (). First, we consider this annual population model, and then we consider the epizootic model. Population counts are made prior to period 1, before dispersal. Since, at this stage, there are neither eggs nor tadpoles, those stages are not included in the annual model and only the juvenile stages and adult stages are included. The time spent (fraction of a year) for each of the five periods, beginning with the dispersal period, is denoted as t j , j=1, …, 5; the interval t 0 to t 5 denotes 1 year.
For period l, l=1, …, 4, the model has the form
The parameters and d are probabilities of survival and c is clutch size, defined in (H9), (H8), and (H5), respectively.
For period 5, the model has the form
The derivation of the annual model consists of repeated back substitution of the variables at the intermediate times into the preceding system of equations. The annual model is
Note that the first eight equations in system Equation(3) do not depend on the breeding males,
. This is due to the fact we have assumed
. Thus, to study system Equation(3)
, it is not necessary to include the breeding males. If J
3(t) is known, the solution to the breeding males is a solution to a linear, constant coefficient non-homogeneous difference equation. Hence, we reduce the system of difference Equationequations (3)
to a system of eight equations, written in matrix form as follows:
2.3. Equilibria and stability
Let
Next, we verify the global stability of the extinction equilibrium when and of the positive equilibrium when
.
Theorem 2.1
Assume model
Equation(5)
satisfies assumptions (H1)–(H9). If
, then the extinction equilibrium of system
Equation(5)
is globally asymptotically stable and if
, then the extinction equilibrium is unstable and the positive equilibrium of system
Equation(5)
is globally asymptotically stable.
Proof
The existence of the equilibria has already been verified. To prove the global asymptotic stability of the extinction equilibrium, observe that the Jacobian matrix J
0 of system Equation(5), evaluated at the zero equilibrium, is given by
Now suppose that so the positive equilibrium exists. Then, the Jacobian matrix evaluated at the zero equilibrium, J
0, has a unique positive strictly dominant eigenvalue greater than unity since h
Equation(1)
<0; the zero equilibrium is unstable. In order to prove the global asymptotic stability of the positive equilibrium, we first prove the local stability. The Jacobian matrix of system Equation(5)
, evaluated at the positive equilibrium F¯, is given by
In order to prove the global stability, we write system Equation(5) in an equivalent form, as a scalar nonlinear difference equation in
and use the fact that
is a globally (locally) stable equilibrium of this difference equation if and only if the vector F¯ is a globally (locally) stable equilibrium of EquationEquation (5)
. We apply a result of Grove and Ladas [Citation18, Theorem 1.10, p. 15] to prove the global asymptotic stability of the positive equilibrium. This theorem is stated as Theorem A1 in the appendix for reference purposes.
System Equation(3) can be expressed as a scalar nonlinear, fifth order, difference equation in
,
2.4. Permanence
It is worthwhile to look at the permanence of the corresponding system which determines whether the population persists independent of the stability of the equilibrium. Permanence ensures that the population neither vanishes nor blows up. We prove a theorem about permanence of system Equation(5) imposing some weaker assumptions on the density-dependent function ρ. Assumption (H6) is replaced with the following assumption:
-
(H6)′ The functions
and
are non-negative, continuous and bounded above for
.
We apply a theorem of Kon et al. [Citation20, Theorem 3, p. 519] to show permanence which is given in the appendix as Theorem A2 for reference purposes. The theorem requires that system Equation(5) be dissipative. First, we state formal definitions of dissipative and permanence.
Let
In order to prove that a system is dissipative, it suffices to prove that each stage is uniformly bounded over time. That is, there exists [Mtilde] such that for every X(0) there exists a time t(X(0)), where , i=1, …, n, for all
. Then the set
serves as the required compact set.
Theorem 2.2
Assume the population model
Equation(5)
satisfies assumptions (H1)–(H5), (H6)′, (H7)–(H9). Then system
Equation(5)
is permanent if
.
Proof
To apply Theorem A2 in the appendix to model Equation(5) requires that the vector C(F(t))F(t) be continuous, the system be dissipative, matrix C(0) be irreducible, and
be forward invariant. The continuity of C(F(t))F(t), the forward invariance of
, and the irreducibility of C(0) are obvious. The only non-trivial part is to show that the system is dissipative. Suppose that
for
. Let ε>0 be given. It follows from systems Equation(3)
and Equation(9)
that, for all t,
Thus, each stage is uniformly bounded independent of the initial conditions so that system Equation(5) is dissipative. The condition
guarantees the existence of an eigenvalue whose magnitude is greater than one (see proof of Theorem 2.1). Hence from Theorem A2 in the appendix, it follows that the system is permanent. ▪
This definition of permanence allows some stages to be zero during certain years. For example, there may be no juveniles in a particular stage in one year but in subsequent years, this stage must have some individuals for the population to be permanent. This is a weaker form of permanence which is sometimes called p-permanence Citation19. A stronger form is c-permanence in which every stage is positive. For c-permanence, the expression in EquationEquation (11) is replaced by
Corollary 2.3
Assume the population model
Equation(5)
satisfies assumptions (H1)–(H5), (H6)′, (H7)–(H9). Then system
Equation(5)
is c-permanent if
.
The proof follows directly from Theorem A3 Citation19, given in the appendix.
3. Epizootic model
In this section, we include infection in the boreal toad population by the fungus Batrachochytrium dendrobatidis, more commonly referred to as chytrid fungus or simply Bd. The disease in amphibians is known as chytridiomycosis. The infective stage of Bd is the zoospore. Bd zoospores grow in the epidermis of adult amphibians or in the keratinized mouth parts of tadpoles, where they develop asexually within a sporangium Citation19. Mature zoospores emerge from discharge tubules of the sporangium. The skin of infected amphibians generally sloughs or peels off. We model the susceptible (healthy) and infected juvenile and adult amphibians based on the population model developed and analysed in the previous sections. We assume that amphibians do not recover. This new model is known as the epizootic model.
Throughout this section, both males and females are considered with the birth function . We use the decomposition of the year into the five periods shown in . This division of the year is required for the epizootic model because the disease is spread by different methods during these periods. Boreal toads are susceptible to chytridiomycosis, resulting in a high mortality rate; infected toads die in about 2 weeks (experimentally infected toadlets died an average of two weeks after exposure Citation9). We use, in the superscripts, the letter ‘S’ to denote susceptible toads and the letter ‘I’ to denote infected and infectious toads. Thus, our population vector is
3.1. Model assumptions
We assume that infection via fungal zoospores depends on the density of free-living chytrid F B or F N or on the density of infected amphibians (which produce zoospores that are released into breeding ponds or non-breeding sites). We use a Poisson distribution to model infection,
Some additional assumptions relate the life cycle of the boreal toad and the mode of infection by Bd for each of the five periods as described in .
-
(H10) In the dispersal period 1, the disease does not spread between amphibians and there are no new infections.
-
(H11) During hibernation, period 2, infection of all the stages may occur via contacts with fungal zoospores F N that may be present in non-breeding sites.
-
(H12) In period 3, breeding males and females may become infected through contacts with fungal zoospores F B in contaminated breeding ponds.
-
(H13) During tadpole development, if breeding males remain at the breeding ponds, then adult males may become infected through contact with fungal zoospores F B in contaminated breeding ponds.
-
(H14) During metamorphosis, first-stage juveniles may become infected through contacts with fungal zoospores F B or from infected males (fungal zoospores from infected males) in contaminated breeding ponds.
-
(H15) Juvenile and adult toads infected during period j do not survive to the next period j+1.
3.2. Difference equations
Define the following three vectors, representing various infectious stages,
The last term in each of the scalar products denotes the survival of fungi, ,
and
, rather than reproduction. We assume that free-living fungi does not reproduce (requires keratin from amphibians), hence
,
and
represent only survival (not reproduction) of free-living fungi, which means these parameters are less than one. More specifically,
-
(H16)
.
The five periods that contribute to the annual epizootic model are described. All parameters are non-negative, unless noted otherwise.
In the dispersal period 1, there are no new infections, only survival of susceptible individuals. All amphibians that became infected during the previous period die (assumption (H15)). Thus, there are only susceptible amphibians,
During period 2, hibernation, all stages can be infected if the site for hibernation is contaminated with chytrid F
N, assumption (H11). Frequency-dependent transmission is not assumed during hibernation because toads generally hibernate in groups; hence, density-dependent transmission may be more realistic. The parameters α
i
, i=1, …, 7, come from the Poisson distribution Equation(14), where
. The expression
accounts for the proportion of amphibians that does not become infected from the free-living fungi in non-breeding ponds, and
is the proportion that does become infected. The model for period 2 is
During period 3, only the breeding males and females can become infected if the breeding site is Bd-infected, F
B, assumption (H12). Non-breeding sites may increase their Bd infection if amphibians were infected during hibernation, . The expressions
, i=6, 9 account for the proportion of breeding amphibians that does not become infected from the free-living fungi in the breeding ponds, and
is the proportion that does become infected. The model for period 3 is
During period 3, it is not known whether frequency-dependent transmission or density-dependent transmission occurs. In the simulations, we check the numerical results for both types. For comparison purposes, at the positive disease-free equilibria (DFE), we assume the transmission parameters are equal. Thus, for frequency-dependent transmission, we replace the parameters β
i
by , where
and
denote the equilibrium stages of the population model Equation(5)
. In addition, the
are replaced by
After breeding, in period 4, tadpoles are produced but at this stage, we do not include infection of tadpoles. Tadpoles are only infected in their mouth parts. Free-living Bd may increase if breeding males and females are infected, . In addition, breeding males that remain at the pond can become infected during this stage (assumption (H13), where the probability of no infection is
and the probability of infection is
. The model for period 4 is
During period 5, metamorphosis from the tadpole stage to first-stage juvenile may result in infection of first-stage juveniles from Bd-infected ponds (F
B), assumption (H14). The probability of infection is , and the probability of no infection is
. Production of free-living fungi in breeding sites is
. The model for period 5 is
Combining the models for each of the five periods, an annual model, , can be expressed as follows:
System Equation(15) can be written in matrix form:
In the epizootic model Equation(15), we assumed density-dependent transmission. However, as noted previously, during the breeding stages, periods 3 and 4, frequency-dependent transmission may also be a reasonable assumption. Frequency-dependent transmission implies that transmission depends on the population proportion rather than the population density as in density-dependent transmission. In the breeding stages, periods 3 and 4, it is not clear whether the transmission should be frequency-dependent or density-dependent. Thus, we compare the dynamics of density-dependent and frequency-dependent transmission in the numerical examples in Section 5. The following analysis applies to the epizootic model with density-dependent transmission.
3.3. Equilibria and stability
To find the DFE for the epizootic model with density-dependent transmission, we set the infectious stages to zero. Then we are left with the population model Equation(5). There are two DFE (the extinction equilibrium and the positive equilibrium) defined by EquationEquation (6)
. To test the stability of these DFE, we calculate the Jacobian matrix of Equation(16)
and evaluate at each of the DFE. The Jacobian matrix has the following form:
In the case of the extinction equilibrium, matrix
The equilibrium values of are either equal to zero (extinction DFE, i=0) or equal to their values at the positive DFE, i=1. Define
The disease persists only if the fungus survives either in breeding sites (m
1>1) or in non-breeding sites (m
2>1). Disease persistence depends only on the fungus because infected toads do not survive to the next period. Thus, can be considered the basic reproduction number of the disease. We show if
and
, then the positive DFE is locally asymptotically stable.
Matrix U does not affect stability because M is a block triangular matrix. Matrices J
0 and J
+ are the matrices studied in system Equation(3). We can apply the results from Theorem 2.1 to the matrices M
01 and M
11: if
, the spectral radius of M
01 is less than one, and if
, the spectral radius of matrix M
01 is greater than one but the spectral radius of M
11 is less than one. The local stability of the positive DFE depends on the eigenvalues of the matrix M
12.
Theorem 3.1
Assume model
Equation(15)
with density-dependent transmission satisfies assumptions (H1)–(H16). Then, there exists two DFE of system
Equation(15)
, an extinction DFE and a positive DFE. If
, then the extinction equilibrium is globally asymptotically stable. If
, then the extinction equilibrium is unstable and furthermore, if
, where m
1
and m
2
are defined as above and evaluated at the positive equilibrium, then the positive DFE is locally asymptotically stable.
Proof
The existence of the two DFE can be easily verified by substituting zero for infected stages together with F
B and F
N in system Equation(15) and then solving the resulting system. Therefore, we prove only the stability results in detail. Suppose
. Using the fact that
for θ>0, we derive the following inequality from system Equation(15)
:
The Jacobian matrix of system Equation(15), evaluated at the positive DFE, is
4. Effects of environmental change on reproduction
The models discussed thus far are based on the life history characteristics of the boreal toads B. b. boreas in the western USA. The life cycle in various regions depends on many factors including latitude, temperature, and water availability. For example, the female boreal toad found in Southern Rocky Mountains in Colorado is generally not reproductive until the age of six Citation8 and does not breed every year. However, in the Oregon Cascade Range, females are reproductive as early as 4 years of age but the breeding frequency varies, from 1 to 4 years Citation27. Environmental changes that result in increased temperatures, earlier springs and shorter winters, may affect the reproductive pattern of boreal toads. We investigate the effect of changes in reproductive patterns on the threshold value ℛ0. Increases or decreases in ℛ0 ultimately result in either a greater or smaller chance of survival for the boreal toad, respectively.
Suppose environmental change affects the onset of breeding in females and the breeding frequency. That is, suppose the parameters r
i
, i=1, 2 and s
i
, i=3, 4 are affected by changes in temperature or other factors. For example, if r
1=0, then females breed every year and if r
1=1 and r
2=0, then females breed every 2 years. In addition, if s
3=0, then females do not become reproductive until age five. The parameters for Bd growth also vary with temperature. For example, Bd does not grow under laboratory settings at temperatures above 30 °C Citation28. Here, we only consider environmental changes as they might affect the population through the model Equation(5) and not the impact on Bd. We investigate the impact of the parameters r
i
, i=1, 2 and s
i
, i=3, 4 on ℛ0. All other survival and reproduction parameters are the same as in the previous sections: the probability that a stage i toad survives to the next stage during period k=1 (or in the same stage during periods k=2, …, 5) is p
ik
for i=1, …, 9.
Example 4.1
Suppose and r
1=0 in the population model Equation(5)
. A female becomes reproductive at age four and breeds every year. In this case, the new threshold is
Example 4.2
Suppose s
3=0=s
4 and r
1=0 in the population model Equation(5). A female becomes reproductive at age six and breeds every year. The threshold value for this model is
Example 4.3
Suppose s
3=0=s
4, r
1=1 and r
2=0 in model Equation(5). A female becomes reproductive at age six and breeds every 2 years. The threshold value for this model is
Example 4.4
As a final example, suppose s 3=0=s 4, r 1=1, and r 2=1. A female becomes reproductive at age six and breeds every three years. In this case,
In the next section, some numerical values are assigned to the parameters to illustrate some possible quantitative changes in breeding onset and frequency.
5. Numerical examples
Several numerical examples are given to illustrate some of the models’ dynamics. Only a few of the large number of parameters are available in the literature. The scarcity of data prompted us to use hypothetical but reasonable values for many of the parameters. First, we consider the population model described in Section 2. Samollow Citation32 estimated clutch size as 16,000. Carey et al.
Citation8 estimated average clutch sizes of 6600 with one to five clutches per female for populations in the Southern Rocky Mountains. Stebbins Citation34 gave estimates of up to 12,500 eggs in two strings in shallow water. We use c=8000 for total number of eggs per reproductive female. The survival probabilities ) are taken from McDonald and Tse Citation24 and adjusted to our model. Juvenile toad survival is p
i
=0.422, and adult reproductive toad survival is p
i
=0.8. These probabilities are based on an equilibrium distribution for females with half of the females breeding every year beginning in year 6. In our model, we also need to account for males and for early breeding of females. Since censuses are taken directly after breeding in McDonald and Tse Citation24, our value of p
1 does not have the same meaning. Thus, we let p
i
=0.422 for i=1, 2 and the female survival probabilities for years 3 and 4 which correspond to
and
. In addition, p
i
=0.8 for i=5, …, 9 (). In our model, the egg and tadpole survival is set to dp
0=0.02, but an additional mortality is assumed due to the density-dependent factor ρ. The sex ratio at the breeding pond can vary depending on many factors. Olson Citation27, whose data is based on Bufo boreas in the Oregon Cascade range, observed that the sex ratio (male:female) at the breeding ponds generally fluctuated between 2 and 3 for a period of 8 years. This was probably due to the fact that the non-breeding adult females who breed every 2 or 3 years are not present at breeding ponds. We assume that the sex ratio for all amphibians is s=1. In addition, we assume a Beverton–Holt type function for density-dependent survival, ρ with α=1 in EquationEquation (2)
, equal to
Table 2. Parameter values for the population model Equation(5) .
.
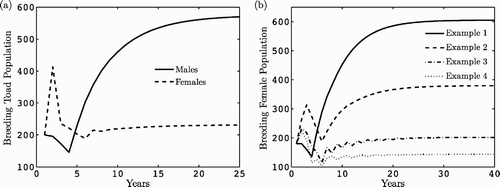
(a) shows the dynamics over time of the breeding females and males ( and
) in the population model Equation(5)
for the parameter values given in . Note that the breeding pond sex ratio is approximately 2.5 (
), which is consistent with the sex ratio observed by Olson Citation27 at three sites in the Oregon Cascade range. are graphs of the breeding females (
) for the parameter values in and based on the assumptions for Examples 4.1–4.4. It is clear that survival is greatest for the parameters in Example 4.1 and lowest for Example 4.4.
Figure 2. Dynamics over time of (a) breeding female and male populations for the general model Equation(5) where ℛ0=12.13 and (b) breeding females for the parameter values in Examples 4.1–4.4 where ℛ1=30.06, ℛ2=19.24, ℛ3=10.69, and ℛ4=7.88.
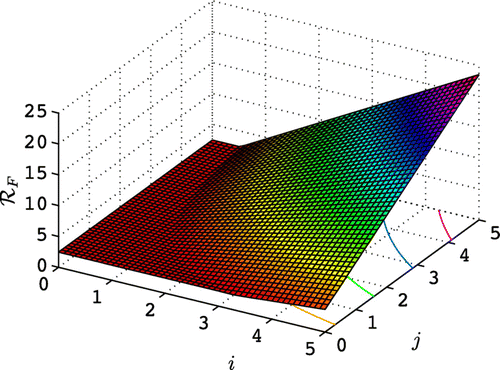
The threshold value ℛ0 as a function of breeding onset s
i
, i=3, 4 and breeding frequency, r
i
, i=1, 2 is graphed in . The threshold ℛ0 increases rapidly with s
3 but levels off at a maximum value as , that is, when
but
is constant, so that breeding onset for females is at age four. Also ℛ0 is smallest when r
1=1=r
2, when female breeding frequency is every third year.
Figure 3. Threshold value ℛ0 as a function of (a) breeding onset, parameters s 3 and s 4, and (b) breeding frequency r 1 and r 2. All other parameter values are given in .
Parameter values used in the simulations for the epizootic model Equation(15) are given in , in addition to those in . The parameters p
ji
satisfy
, j=1, …, 9, so that they agree with the values in the population model, see EquationEquation (4)
. These parameter values are hypothetical and chosen for illustration purposes.
Table 3. Parameter values for the epizootic model Equation(15) .
.
shows the dynamics of the breeding males and females in the population model Equation(5) and in the epizootic model Equation(15)
with density-dependent transmission. In this case,
. In , breeding males and females are graphed in the epizootic model with frequency-dependent transmission. The equilibrium vectors
Figure 4. Dynamics over time of breeding female and male populations for the population model Equation(5) and the epizootic model. In (a), density-dependent transmission is assumed and in (b), frequency-dependent transmission. The parameter values are given in and .
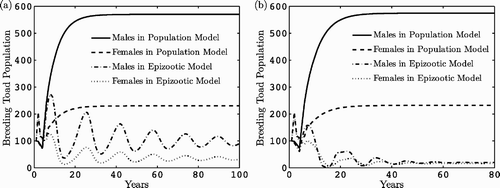
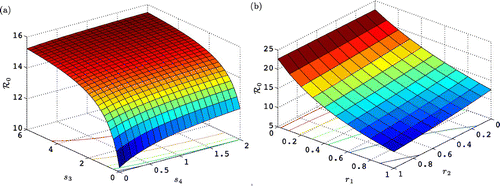
The graphs give an indication of the possible impact of disease on the population. The larger the value of βN, the greater potential for survival of Bd in non-breeding sites leading to a reduction in the toad population. On the other hand, the larger the values of δB and γB, the greater potential for survival of Bd in breeding sites, which also leads to reduction in the toad population. The impact of the two parameter vectors δB and γB on the basic reproduction number in the epizootic model (Theorem 3.1) can be seen in for the parameter values in and . The term m
1 depends on Bd in breeding sites and m
2 depends on Bd in non-breeding sites. The parameters γB and δB affect Bd in breeding sites, the term m
1. For small values of γB and δB,
, infection is maintained in non-breeding sites. For larger values of γB and δB, then
increases and infection is primarily dependent on Bd in breeding sites.
6. Conclusion
New population and epizootic models were formulated for the boreal toad based on life history and breeding strategies. For the autonomous population and epizootic models, two threshold parameters, ℛ0 and , were defined for population survival or for disease survival. If
, then population extinction occurs. If
and
, then the population survives without Bd infection. But if
and
, then Bd infection can become established and cause severe reduction in toad populations. These two thresholds identify important relationships among parameters that affect boreal toad survival. The parameter ℛ0 was used to compare the effects of female breeding onset and breeding frequency on toad survival (Examples 4.1–4.4). In addition, increasing the value of parameters related to growth of Bd at breeding sites was shown to significantly increase the value of
(). In the epizootic model formulation, frequency-dependent versus density-dependent transmission of Bd at breeding sites needs to be carefully considered. Frequency-dependent transmission of Bd was shown to have a more drastic reduction on toad populations than density-dependent transmission ().
Numerical simulations can be used to investigate more thoroughly the effects of environmental changes and Bd infection on various life stages of the boreal toad in these new population and epizootic models. In addition, the basic modelling framework can be extended to investigate the effects of temporally varying parameters, environmental and demographic variability, and spatial effects on toad survival.
Acknowledgements
Financial support was provided by the National Science Foundation DMS-0718302. We thank Keith Emmert, for the help with the initial formulation of this model. In addition, we thank two anonymous referees for their helpful suggestions.
References
- Ackleh , A. S. and Jang , S. R.-J. 2007 . A discrete two-stage population model: Continuous versus seasonal reproduction . J. Differ. Equ. Appl. , 13 : 261 – 274 .
- Ackleh , A. S. , Dib , Y. M. and Jang , S. R.-J. 2007 . A three-stage discrete-time population model: Continuous versus seasonal reproduction . J. Biol. Dyn. , 1 : 291 – 304 .
- Allen , L. J.S. and van den Driessche , P. 2008 . The basic reproduction number in some discrete-time epidemic models . J. Differ. Equ. Appl. , 14 : 1127 – 1147 .
- Berger , L. , Speare , R. , Daszak , P. , Green , D. E. , Cunningham , A. A. , Goggin , C. L. , Slocombe , R. , Ragan , M. A. , Hyatt , A. D. , McDonald , K. R. , Hines , H. B. , Lips , K. R. , Marantelli , G. and Parkes , H. 1998 . Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America . Proc. Nat. Acad. Sci USA , 95 : 9031 – 9036 .
- Beverton , R. J.H. and Holt , S. J. 1957 . On the Dynamics of Exploited Fish Populations , London : Chapman and Hall .
- Bosch , J. , Martínez-Solano , I. and García-París , M. 2001 . Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricians) in protected areas of central Spain . Biol. Conserv. , 97 : 331 – 337 .
- Briggs , C. J. , Vredenburg , V. T. , Knapp , R. A. and Rachowicz , L. J. 2005 . Investigating the population-level effects of chytridiomycosis: An emerging infectious disease of amphibians . Ecology , 86 ( 12 ) : 3149 – 3159 .
- Carey , C. , Corn , P. S. , Jones , M. S. , Livo , L. J. , Muths , E. and Loeffler , C. W. 2005 . “ Factors limiting the recovery of boreal toads Bufo b. boreas ” . In Amphibian Declines: The Conservation Status of United States Species , 222 – 236 . Berkeley, CA : U. Calif. Press .
- Carey , C. , Bruzgul , J. E. , Livo , L. J. , Walling , M. L. , Kuehl , K. A. , Dixon , B. F. , Pessier , A. P. , Alford , R. A. and Rogers , K. B. 2006 . Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis) . EcoHealth , 3 : 5 – 21 .
- Caswell , H. 2001 . Matrix Population Models , 2 , Sunderland, MA : Sinauer Assoc., Inc. Pub .
- Collins , J. P. , Brunner , J. L. , Miera , V. , Parris , M. J. , Schock , D. M. and Storfer , A. 2003 . “ Ecology and evolution of infectious disease ” . In Amphibian Conservation , Edited by: Semlitsch , R. D. 137 – 151 . Washington : Smithsonian Institution .
- Daszak , P. , Cunningham , A. A. and Hyatt , A. D. 2003 . Infectious disease and amphibian population declines . Divers. Distrib , 9 : 141 – 150 .
- Di Rosa , I. , Simoncelli , F. , Fagotti , A and Pascolini , R. 2007 . The proximate cause of frog declines? . Nature , 447 : E4 – E5 .
- Drew , A. J. , Allen , E. J. and Allen , L. J.S. 2006 . An investigation of climatic and geographic factors on the presence of chytrid fungus on amphibian populations in Australia . Dis. Aquat. Organ , 68 : 245 – 250 .
- Emmert , K. E. and Allen , L. J.S. 2004 . Population persistence and extinction in a discrete-time, stage-structured epidemic model . J. Differ. Equ. Appl. , 10 : 1177 – 1199 .
- Emmert , K. E. and Allen , L. J.S. 2006 . Population extinction in deterministic and stochastic discrete-time epidemic models with periodic coefficients with applications to amphibians . Nat. Res. Model , 19 ( 2 ) : 117 – 164 .
- Gantmacher , F. R. 1964 . The Theory of Matrices , Vol. 2 , New York : Chelsea Publications Company .
- Grove , E. A. and Ladas , G. 2005 . Periodicities in Nonlinear Difference Equations. Advances in Discrete Mathematics and Applications , Vol. 4 , CRC Press .
- Kon , R. 2005 . Nonexistence of synchronous orbits and class coexistence in matrix population models . SIAM J. Appl. Math. , 66 ( 2 ) : 616 – 626 .
- Kon , R. , Saito , Y. and Takeuchi , Y. 2004 . Permanence of single-species stage-structured models . J. Math. Biol. , 48 : 515 – 528 .
- Kriger , K. , Pereoglou , M. F. and Hero , J.-M. 2007 . Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia . Conserv. Biol. , 21 : 1280 – 1290 .
- Lips , K. R. , Brem , F. , Brenes , R. , Reeve , J. D. , Alford , R. A. , Voyles , J. , Carey , C. , Livo , L. , Pessier , A. P. and Collins , J. P. 2006 . Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community . Proc. Nat. Acad. Sci. USA , 103 : 3165 – 3170 .
- Meserve , B. E. 1982 . Fundamental Concepts of Algebra , New York : Dover Publications .
- D. McDonald and T. Tse, Matrix life-cycle model for boreal toad, in Boreal Toad (Bufo boreas boreas) Technical Conservation Assessment Appendix A, prepared by D. Keinath and M. McGee, USDA Forest Service, Rocky Mountain Region Species Conservation Project, 2005, pp. 62–69
- Mitchell , K. M. , Churcher , T. S. , Garner , W. J. and Fisher , M. C. 2007 . Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction . Proc. R. Soc. Lond. B , 275 : 329 – 334 .
- Muths , E. and Nanjappa , P. 2005 . “ Bufo boreas Baird and Girard, 1852 (b), Western Toad ” . In Amphibian declines: the conservation status of United States species , Edited by: Lannoo , M. 392 – 396 . Berkeley, CA : U. Calif. Press .
- Olson , D. H. Ecological susceptibility of amphibian to population declines . Symposium on Biodiversity of Northwestern California. Report 29 . Edited by: Harris , R. R. and Erman , D. C. pp. 55 – 62 . Berkeley, CA : Wildland Resource Center, University of California . Tech. Coord.
- Piotrowski , J. S. , Annis , S. L. and Longcore , J. E. 2004 . Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians . Mycologia , 96 : 9 – 15 .
- Rohrs , J. R. , Raffel , T. R. , Romansic , J. M. , McCallum , H. and Hudson , P. J. 2008 . Evaluating the links between climate, disease spread, and amphibian declines . Proc. Nat. Acad. Sci. USA , 105 : 17436 – 17441 .
- Salceanu , P. L. and Smith , H. L. 2009 . Persistence in a discrete-time, stage-structured fungal disease model . J. Biol. Dyn. , 3 : 271 – 285 .
- Salceanu , P. L. and Smith , H. L. 2010 . Persistence in a discrete-time, stage-structured epidemic model . J. Differ. Equ. Appl. , 73 : 73 – 103 .
- Samollow , P. B. 1980 . Selective mortality and reproduction in a natural population of Bufo boreas . Evolution , 34 : 18 – 39 .
- Schmetterling , D. A. and Young , M. K. 2008 . Summer movements of boreal toads (Bufo boreas boreas) in two Western Montana basins . J. Herpetol. , 42 : 111 – 123 .
- Stebbins , R. C. 1954 . Amphibians and Reptiles of Western North America , New York : McGraw-Hill .
- Stuart , N. S. , Chanson , J. S. , Cox , N. A. , Young , B. E. , Rodrigues , A. S.L. , Foschman , D. L. and Waller , R. W. 2004 . Status and trends of amphibian declines and extinctions worldwide . Science , 306 : 1783 – 1786 .
- Wake , D. B. and Vredenburg , V. T. 2008 . Are we in the midst of the sixth mass extinction? A view from the world of amphibians . Proc. Nat. Acad. Sci. USA , 105 : 11466 – 11473 .
Appendix. Theorems
The following theorem is used to verify the global stability results in Theorem 2.1 for the population model Equation(5).
Theorem A.1
[Citation18 Theorem 1.10, p. 15] Let [xtilde]∈I be an equilibrium of the difference equation
-
f is non-decreasing in each of its arguments, and
-
f satisfies
for all
.
The following theorem is used to verify permanence in Theorem 2.2 for the population model Equation(5).
Theorem A.2
[Citation20, Theorem 3, p. 519] Suppose the dynamical system
Equation(10)
is continuous and dissipative. Assume the matrix
is irreducible and
is forward invariant (i.e.
for all
. Then, system
Equation(10)
is permanent if
has an eigenvalue whose magnitude is greater than unity.
The following theorem is used to verify c-permanence in Corollary 2.3 for the population model Equation(5).
Theorem A.3
[Citation19, Theorem 4.3, p. 621] Suppose dynamical system
Equation(10)
is continuous, dissipative,
for X(t)≥0
and
for X(t)>0. Also suppose that A(X(t)) is irreducible for all
,
holds for all X(t)∈ bd
and system
Equation(10)
is p-permanent. Then system
Equation(10)
is c-permanent if and only if A(0) is primitive.