Abstract
Spatial segregation among life-cycle stages has been observed in many stage-structured species, including species of the flour beetle Tribolium. We investigate density-dependent dispersal of life-cycle stages as a possible mechanism responsible for this separation. We explore this hypothesis using stage-structured, integrodifference equation (IDE) models that incorporate density-dependent dispersal kernels. We first investigate mechanisms that can lead to spatial patterns in juvenile–adult IDE models. We show, via numerical simulation, that density-dependent dispersal can lead to the spatial segregation of life-cycle stages in the sense that each stage peaks in a different spatial location. We then construct a three-stage spatial model to describe the population dynamics of Tribolium castaneum and Tribolium confusum and assess density-dependent dispersal mechanisms that are able to explain spatial patterns that have been experimentally observed in these species.
1. Introduction
The spatial separation of similar species is a commonly observed phenomenon, allowing coexistence in situations where competitive exclusion might otherwise drive one of the species to extinction Citation7 Citation10 Citation22. These differences in spatial distributions may be due to niche partitioning as a result of differing food or shelter preferences as well as differing predation risks.
Spatial segregation has also been observed to occur between different stages of development in cannibalistic species Citation11–15,Citation20 Citation23. This non-uniform spatial structure may be promoted by interactions among age classes or life-cycle stages, as spatial heterogeneities can provide vulnerable stages an opportunity to escape from the cannibalistic stages and minimize predation risk. The utilization of available refuges in the presence of cannibals has been demonstrated experimentally in Saduria entomon Citation13, Thermosphaeroma thermophilum Citation23 and Gammarus pulex Citation15. Leonardsson Citation14 gives evidence that small isopods avoid larger conspecifics, and that the severity of this avoidance behaviour increases with the density of the larger isopods.
Cannibalism may play an important role in spatial structure as well as in density regulation. Many cannibalistic species exhibit age- or stage-dependent habitat selection, potentially evolutionary adaptations to reduce predation mortality. A study of the cave-dwelling mysids Hemimysis speluncola Citation20 found juveniles near the cave entrance while adults inhabited the innermost parts of the cave. Juveniles of the amphipod species Pallasea quadrispinosa Citation11 tend to occupy shallow waters while adults are mainly found in deeper, cooler waters. Juveniles of several species of gammaridean amphipods Citation12 undergo a change in phototactic response upon reaching an age when they become less susceptible to cannibalism.
Ghent Citation9 experimentally documented differences in the spatial distribution of life-cycle stages in the cannibalistic flour beetles Tribolium castaneum and Tribolium confusum. He studied the depth distribution of these beetles in cylindrical vials and discovered that the adult- and larval-stage densities peaked at different depths. Ghent's experiment is described further in Section 3.2.
The above examples show interactions between age classes or life-cycle stages can promote differences in their spatial distributions. We suggest density-dependent interstage interactions alone can be the sufficient cause for the formation of these spatial patterns, and that the spatial segregation of life-cycle stages may be the result of density-dependent dispersal rather than stage-specific resource niches.
In this paper, we introduce a two-dimensional stage-structured density-dependent integrodifference equation (IDE) model of the form developed in Citation21 and investigate mechanisms that can lead to spatial patterns. We show, via numerical simulation, that under certain conditions, density-dependent dispersal can lead to the segregation of life-cycle stages in the sense that juveniles and adult numbers peak in different spatial locations. We then develop a spatial extension of the well-studied larva–pupa–adult (LPA) model for Tribolium Citation2 dynamics and study its spatial attractors. Specifically, we look for conditions promoting the spatial separation of larvae and adults. We compare the results to Ghent's experimental findings.
2. A juvenile–adult model
2.1 Model construction
In this section, we study a juvenile–adult IDE model with density-dependent dispersal kernels on a closed domain. General stage structured models of this type were developed and analysed in Citation21. In these models, a discrete time matrix model is used to describe the non-spatial population dynamics and a (possibly density-dependent) dispersal kernel is used to dictate the rules for movement in space, although not all individuals are required to disperse. Here we explore if and when density-dependent dispersal can lead to spatial patterns in a two-stage model, specifically to patterns in which juveniles and adults are spatially segregated.
To describe the non-spatial dynamics of our populations of interest, we consider a juvenile–adult difference equation model:
The dispersal of an individual may be affected by other individuals as well as by innate spatial preferences. We now incorporate density-dependent dispersal by assuming that each stage tries to avoid the other. That is, the presence of adults at a given spatial location decreases the probability of a juvenile moving there, and visa versa. Specifically, we modify the density-independent dispersal kernels as follows:
We note that although the general density-dependent IDE theory developed in Citation21 allows for density to affect an individual's decision to disperse as well as distance dispersed, here we assume that all individuals disperse every time step. We also note that with kernels Equation(2) a chance of mortality accompanies dispersal, since the integral over space is always less than or equal to one. Biologically, this means individuals can be lost, but not gained, during dispersal.
Our juvenile–adult IDE model, with population dynamics given by EquationEquation (1) and the density-dependent dispersal kernels Equation(2)
, is
2.2 Model results
In this section, we look at some model simulations for specific population dynamics and dispersal kernels. We consider the following juvenile–adult difference equation model:
We take the density-independent dispersal kernels to be
We incorporate density dependence into kernels Equation(5) in the same way as kernels Equation(2)
:
Figure 1. Representative attractors of juvenile–adult IDE model Equation(7). b=25 μ
L
=μ
A
=0.2 for all simulations. (a) Temporal equilibrium: D
J
=D
A
=0. (b) Temporal equilibrium, spatial segregation of juveniles and adults: D
J
=0.5, D
A
=0. (c) Two-cycle, temporal segregation of juveniles and adults: D
J
=0, D
A
=0.5. (d) Four-cycle, temporal segregation of juveniles and adults: D
J
=D
A
=0.5.
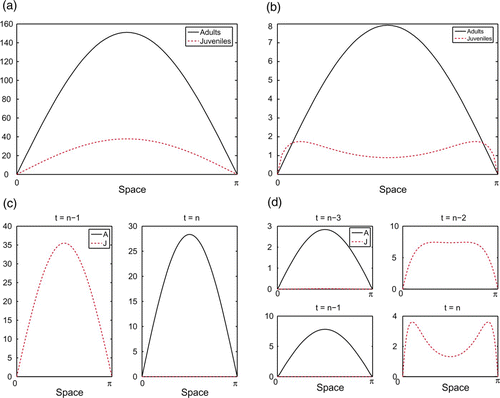
If we increase D J from 0 to 0.5 so juveniles ‘avoid’ adults (keeping all other parameters the same), we still have a temporal equilibrium. However, we now see spatial segregation of juveniles and adults in the sense that the stages peak in different spatial locations (). Adult numbers are still greatest in the centre of the domain, but juveniles now accumulate towards the sides of the domain, exhibiting a bi-modal distribution with two peaks and a dip between them in the centre of the domain.
If we return D J to 0 and instead increase D A to 0.5 so adults ‘avoid’ juveniles (all other parameters remain the same), we see temporal segregation rather than spatial segregation. We no longer have an equilibrium in time, but instead a temporal two-cycle. At any given time, one stage dominates at all spatial locations. The other stage is present, but only in very small numbers. The dominant stage alternates temporally between juveniles and adults, each peaking in the centre of the domain. An illustration of this appears in .
Next we look at the case where D J =D A =0.5, keeping all population dynamics parameters the same. The result is a temporal four-cycle, again showing what we call temporal segregation. Juveniles, when dominant, exhibit a bi-modal distribution. This is shown in .
shows the properties of the model attractor for various regions in the D
J
, D
A
parameter plane for increasing values of b. For , no spatial segregation is possible for any values of D
J
or D
A
. This is consistent with the mathematical results obtained in Citation21, namely that near the primary bifurcation point
, non-extinction equilibrium distributions J
t
(x) and A
t
(x) resemble the eigenvector, which here has components that are multiples of sin(x).
Figure 2. Shown is a section of the D
J
−D
A
parameter plane, 0≤D
J
≤1 and 0≤D
A
≤1, for increasing values of b. (a) For b=2.5, all combinations of D
J
and D
A
in the range shown result in equilibrium dynamics for model Equation(7). Spatial segregation is possible for large enough D
J
and small enough D
A
. (b) For b=10 we can see two-cycles and temporal segregation in addition to the model Equation(7)
attractors seen for b=2.5. (c) For b=25, we can see four-cycles and four-cycles with temporal segregation in addition to the model Equation(7)
attractors seen for b=10.
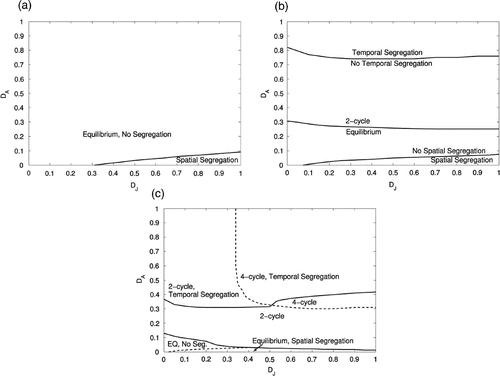
As b is increased, we see through simulation that more patterns become possible. For b=2.5, there is only a small region of parameter space where we see spatial segregation of adults and juveniles (). D J must be sufficiently large, and D A must be sufficiently small. All attractors are temporal equilibria.
Increasing b to 10, we can achieve temporal two-cycles as well as equilibria. If D A is large enough, we see temporal segregation in the sense defined earlier (). If b is increased to 25, we add temporal four-cycles to the list of potential attractors ().
2.3 Conclusions
We have shown in this section (through simulation) that low-dimensional stage-structured IDE models can result in both spatial and temporal segregation of life-cycle stages. Specifically, our study of this model suggests the following conclusions. Increasing D J (the sensitivity of juveniles to adult densities) tends to increase the degree of spatial segregation, while increasing D A (the sensitivity of adults to juvenile densities) tends to increase the degree of temporal segregation between the two life-cycle stages. Increasing the birth rate leads to larger D J −D A parameter regions where juvenile–adult spatial segregation occurs, and an increase in the number of possible types of model behaviour.
3. Application to Tribolium
In this section, we apply the modelling methodology in Section 2, and the illustrated mechanisms that can cause the spatial segregation of life-cycle stages, to an experimental system in which such spatial patterns were observed to occur, namely Ghent's experiments for species of Tribolium Citation9. To do this, we base the spatial model on a matrix population model that is appropriate for the organisms involved, and we utilize dispersal kernels appropriate for the experimental setup. The population model is a three life-cycle stage model called the LPA model, which has been shown to be an accurate model of experimental cultures of Tribolium Citation1–6.
3.1 Density-dependent dispersal
There is substantial evidence that density plays a role in the dispersal of the mobile stages of both T. castaneum and T. confusum Citation17 Citation24. Naylor Citation18 documented an interstage density-dependent avoidance response in T. confusum. When presented with a choice between unoccupied flour and flour occupied by medium-sized larvae, adult females tended to choose the unoccupied flour. Furthermore, the number of adults found in vials of flour decreased with increasing larval density. The tendency of T. confusum to move to locations of unoccupied flour over occupied flour was also observed by Naylor Citation16.
Further evidence of the avoidance of larvae by adults comes from an experiment conducted by Prus Citation19 to look at emigration ability and surface numbers of adult beetles of different strains and sexes. All cultures were composed of a single sex of adults, except for one replicate where several males accidentally were mixed in with a culture of females. This resulted in the appearance of larvae, and a notable increase in the surface numbers and emigration ability of this replicate Citation19. Later investigations confirmed the effect of the presence of larvae in increasing adult surface numbers. In adult-only cultures, the intensity of emigration was found to depend on the relation of the current density of beetles to the maximum possible density Citation25.
3.2 Depth distribution of life-cycle stages
In 1966, Ghent Citation9 studied the depth distribution of the life-cycle stages of the cannibalistic species and
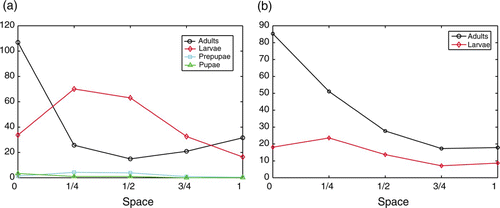
in vials of flour. For each species, he placed 200 adults on the surface of a cylindrical fractionable-shell vial filled with flour. The vial was divided into five rings, each being 10 mm in high. Eight grams of flour filled this vial to 41–42 mm. So each of the bottom four rings were filled and the last contained the surface layer made up of the uppermost 1–2 mm of flour. Ghent placed this vial in an incubator for 26 days and counted the number of individuals in each stage in each of the four quarters of flour and on the surface. For the two species of flour beetles studied, 26 days is enough time to produce eggs, larvae and pupae, but not new adults. Ghent found adults of both species to be most abundant near the surface of the flour, while larval densities peaked slightly below the surface in the upper quarter of flour. shows the observed depth distributions for various life stages of T. castaneum and T. confusum.
Figure 3. Data from Ghent Citation9. 0 represents the surface of the vial and 1 represents the bottom. (a) Depth distribution of larvae, pre-pupae, pupae and adults in T. castaneum. (b) Depth distribution of larvae and adults in T. confusum.
3.3 LPA model
One of the most heavily analysed and well-validated models in mathematical ecology is the LPA model, a three-dimensional system of nonlinear difference equations designed to describe the population dynamics of T. castaneum and T. confusum Citation2:
3.4 Spatial LPA model
The LPA model is one of the most successful models in mathematical biology in the sense that it has been parameterized and well-validated with laboratory data, and laboratory experiments have verified its predicted dynamics, attractors and bifurcations (including a route to chaos) Citation5 Citation6. Many modifications of the LPA model have also been successful over the last decade in investigations of numerous phenomena, such as competition between flour beetle species Citation8 and population dynamics in a periodically fluctuating habitat Citation1.
Here, we construct a spatial extension of the LPA model in order to determine if the spatial patterns in T. castaneum and T. confusum described in Section 3.2 can be explained by density-dependent interstage interactions. The spatial patterns observed by Ghent Citation9 in a cylindrical vial of flour can be looked at as one-dimensional in space, where the spatial dimension is depth. Due to gravity, there is a gradient of increasing flour density from the surface to bottom of the bottle Citation9. Individuals can live on the surface but cannot pass above it. We model this environment as a one-dimensional domain with mixed boundary conditions, where the bottom of the bottle is a hostile boundary and the surface is a no-flux boundary. In order to construct the spatial LPA model, we need to make assumptions regarding the dispersal behaviour of the two mobile life-stages – larvae and adults (pupae are immobile and have zero probability of dispersing). We assume that adults prefer areas with lower larval densities than their current location, and larvae prefer areas with lower adult densities than their current location. Thus, the fraction of larvae that move from one location to another depends on the difference in adult densities, after population dynamics occur, between the two locations. Adult dispersal depends similarly on density of larvae. We assume both larvae and adults view the environment in the same way, preferring flour near the surface over the more dense flour near the bottom and so take our spatial domain Ω to be the finite interval [0, π/2] representing depth in a cylindrical vial, with 0 corresponding to the surface and π/2 corresponding to the bottom of the vial. The kernels for larval and adult spatial redistribution are given by the following, respectively:
3.5 Spatial LPA model results
shows simulations of model Equation(11) for various parameter values in the dispersal kernel Equation(9)
. Parameters for population dynamics were chosen so as to fall in the maximum likelihood 95% confidence intervals calculated from control cultures of the Desharnais experiment for LPA parameters Citation3. The LPA model equilibrates for this set of parameter values.
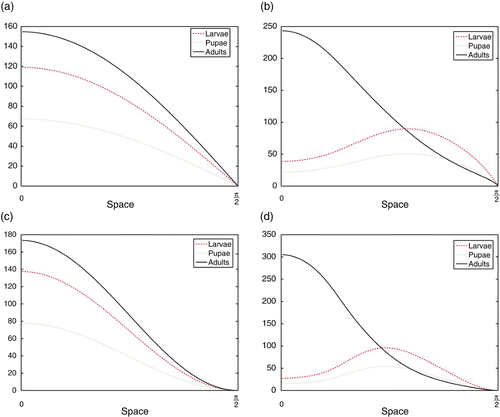
Figure 4. Simulations of the spatial LPA model Equation(11) for various parameter values in the dispersal kernels Equation(9)
. All attractors shown are temporal equilibria. LPA parameter values given by b=10, c
el=0.01, c
ea=0.0175, μ
L
=0.435, c
pa=0.016, μ
A
=0.075. (a) m=1, D
LA
=D
AL
=0. (b) m=1, D
LA
=D
AL
=0.01. (c) m=2, D
LA
=D
AL
=0. (d) m=2, D
LA
=D
AL
=0.01.
When D
LA
=D
AL
=0 in the kernel, there is no spatial segregation between stages in simulations of model Equation(11) for any set of LPA parameter values. Spatial distributions of all stages reflect that of the kernel, cos
m
(x), reaching a maximum at the no-flux boundary 0 and reaching their minimum (of zero) at the hostile boundary π/2. This can be seen in , where m=1, and , where m=2.
If the adults and larvae have equal dispersal sensitivities of , the larvae are ‘pushed’ towards the bottom of the vial along with the pupae, while adult density peaks on the surface. This is shown in for m=1 and for m=2. Both of these results are qualitatively similar to Ghent's observations shown in . As the parameter m is increased, the bottom of the habitat becomes increasingly undesirable to both stages. The adults reach their maximum density at the surface for all values of m, but the peak of the larval distribution moves towards the surface as m increases.
In general, spatial segregation of larvae and adults is observed for a range of D LA >0, even if D AL =0. The effect is more severe when combined with values of D AL >0, but this is not necessary. However, the reverse is not true. It is necessary that larvae move in response to adult densities; if D LA =0 and only adults disperse in response to larval densities, we do not see the segregation. Adults will not be pushed to the bottom of the habitat for any values of D LA and D AL . Furthermore, if D LA and/or D AL become too large, we begin to see temporal patterns as well.
We did not observe multiple attractors of the spatial LPA model for the range of parameter values considered. Initial conditions of the form where C
L
, C
P
, C
A
and m are real positive constants lead to the attractors in . The boundary conditions can also be satisfied by piecewise continuous step functions of the form
where C
L
, C
P
, C
A
are real positive constants and
is equal to 1 on
and 0 on
. This set of initial conditions also leads to the attractors in .
4. Discussion
Making use of IDE models with density-dependent kernels, we have shown that density-dependent dispersal can lead to the segregation of life-cycle stages in stage-structured populations (such as flour beetles). These results are not intuitively obvious. In the case of a juvenile–adult species, even if juveniles and adults avoid each other, juveniles will eventually become adults and adults will give rise to new juveniles, seemingly leading to spatial mixing between stages.
We referenced spatial habitat segregation as a potential adaptation to avoid cannibalism or predation. In Tribolium, larvae are not being directly cannibalized by the adults but density-dependent dispersal, and the avoidance of adults, may still be a beneficial adaptation. T. castaneum and T. confusum pupae are cannibalized by adults, so if larvae pupate in a location inhabited by a large number of adults, they may not survive the pupal stage.
Based on our studies, we also hypothesize about mechanisms that promote adult dispersal. Eggs of both species are cannibalized by larvae, and the adults may want to lay their eggs in flour where they have the greatest chance of hatching, and where there is the greatest food supply once their young become larvae. This is our rationale for adults avoiding larvae in the dispersal kernels for the spatial LPA model.
The toy models analysed in Section 2 show that density-dependent dispersal can result in both spatial segregation of life-cycle stages as well as temporal segregation (if mortality is associated with dispersal). We stress that complex population dynamics are not necessary for the formation of these spatial patterns and that our models do not result in spatial segregation in the absence of density-dependent dispersal (as long as all mobile stages ‘view’ the environment the same way).
As an application that offers some validation of our modelling methodology, we aimed to explain the uneven depth distribution of larval and adult flour beetles of the species T. castaneum and T. confusum observed by Ghent Citation9. Due to gravity, flour is more dense at the bottom of a vial of flour than at the top, and most likely less desirable an environment to beetles than the less dense flour near the surface. We modelled this biological system assuming no-flux boundary conditions at the surface and hostile boundary conditions at the bottom of the bottle, as well as the density-dependent dispersal of adults and larva (assuming adults avoid larvae and visa versa). Even though larvae and adults have the same spatial preferences, the adults end up with higher densities in the preferred flour. Larvae and pupae appear to be ‘pushed’ towards the bottom of the bottle. These model-predicted results agree qualitatively with what Ghent observed ().
We do not expect highly accurate quantitative agreements between model results and Ghent's data for multiple reasons. First, parameter values for flour beetle populations can vary from strain to strain, and parameter values for the strain used by Ghent are not available. Second, the vials used by Ghent held 8 g of flour, while the typical bottles used in experiments and parametrization of the LPA model held 20 g of flour Citation3 Citation4. Also, Ghent's data were collected after 26 days in the vial, not long enough for new adults to emerge. Our results are asymptotic model attractors.
As discussed in Section 3.1, there is literature to support the density-dependent dispersal of flour beetles, but the exact mechanisms and rates of dispersal are unknown. Our results do show that density-dependent interactions and the resulting dispersal kernels are possible explanations for the observed spatial distributions. A main message of this case study is that density-dependent dispersal can produce a spatial pattern very similar to that experimentally observed by Ghent and is likely to have been a factor in its development.
Acknowledgements
This work was done as part of S.L. Robertson's PhD thesis, Spatial patterns in stage-structured populations with density-dependent dispersal, Interdisciplinary Program in Applied Mathematics, University of Arizona, 2009.
Additional information
Notes on contributors
Suzanne L. Robertson
Present address: Mathematical Biosciences Institute, The Ohio State University, Columbus, OH 43210, USA.References
- Costantino , R. F. , Cushing , J. M. , Dennis , B. , Desharnais , R. A. and Henson , S. M. 1998 . Resonant population cycles in temporally fluctuating habitats . Bull. Math. Biol. , 60 : 247 – 275 .
- Cushing , J. M. 2004 . The LPA model . Fields Inst. Commun. , 42 : 29 – 55 .
- Cushing , J. M. , Costantino , R. F. , Dennis , B. , Desharnais , R. A. and Henson , S. M. 2003 . Chaos in Ecology: Experimental Nonlinear Dynamics , Vol. 1 , New York : Academic Press (Elsevier Science) . Theoretical Ecology Series
- Dennis , B. , Desharnais , R. A. , Cushing , J. M. and Costantino , R. F. 1997 . Nonlinear demographic dynamics: Mathematical models, statistical methods, and biological experiments . Ecol. Monogr. , 65 : 261 – 281 .
- Dennis , B. , Desharnais , R. A. , Cushing , J. M. and Costantino , R. F. 1997 . Transitions in population dynamics: Equilibria to periodic cycles to aperiodic cycles . J. Anim. Ecol. , 66 : 704 – 729 .
- Dennis , B. , Desharnais , R. A. , Cushing , J. M. , Henson , S. M. and Costantino , R. F. 2001 . Estimating chaos and complex dynamics in an insect population . Ecol. Monogr. , 71 : 277 – 303 .
- Edington , J. M. and Edington , M. A. 1972 . Spatial patterns and habitat partition in the breeding birds of an upland wood . J. Anim. Ecol. , 41 : 331 – 357 .
- Edmunds , J. , Cushing , J. M. , Costantino , R. F. , Henson , S. M. , Dennis , B. and Dasharnais , R. A. 2003 . Park's Tribolium competition experiments: A non-equilibrium species coexistence hypothesis . J. Anim. Ecol. , 72 : 703 – 712 .
- Ghent , A. W. 1966 . Studies of behavior of the Tribolium flour beetles. II. Distributions in depth of T. castaneum and T. confusum in fractionable shell vials . Ecology , 47 : 355 – 367 .
- Hardin , G. 1960 . The competitive exclusion principle . Science , 131 : 1292 – 1297 .
- Hill , C. 1988 . Life cycle and spatial distribution of the amphipod Pallasea quadrispinosa in a lake in northern Sweden . Holartic Ecol. , 11 : 298 – 304 .
- Hunte , W. and Myers , R. A. 1984 . Phototaxis and cannibalism in gammaridean amphipods . Mar. Biol. , 81 : 75 – 79 .
- Jormalainen , V. and Shuster , S. M. 1997 . Microhabitat segregation and cannibalism in an endangered freshwater isopod, Thermosphaeroma thermophilum . Oecologia , 111 : 271 – 279 .
- Leonardsson , K. 1991 . Effects of cannibalism and alternative prey on population dynamics of Saduria entomon (Isopoda) . Ecology , 72 : 1273 – 1285 .
- McGrath , K. E. , Peeters , E. T.H.M. , Beijer , J. A.J. and Scheffer , M. 2007 . Habitat-mediated cannibalism and microhabitat restriction in the stream invertebrate Gammarus pulex . Hydrobiologia , 589 : 155 – 164 .
- Naylor , A. F. 1959 . An experimental analysis of dispersal in the flour beetle, Tribolium confusum . Ecology , 40 : 453 – 465 .
- Naylor , A. F. 1961 . Dispersal in the red flour beetle Tribolium castaneum (tenebrionidae) . Ecology , 42 : 231 – 237 .
- Naylor , A. F. 1965 . Dispersal responses of female flour beetles, Tribolium confusum, to presence of larvae . Ecology , 46 : 341 – 343 .
- Prus , T. 1966 . Emigrational ability and surface numbers of adult beetles in 12 strains of Tribolium confusum duval and T. castaneum herbst . Ekologia Polska Seria A , 14 : 548 – 588 .
- Ribes , M. , Coma , R. , Zabala , M. and Gili , J. 1996 . Small scale spatial heterogeneity and seasonal variation in a population of a cave-dwelling Mediterannean mysid . J. Plankton Res. , 18 : 659 – 671 .
- Robertson , S. L. 2009 . Spatial patterns in stage-structured populations with density dependent dispersal . Ph.D. thesis, University of Arizona
- Shigesada , N. , Kawasaki , K. and Teramoto , E. Spatial segregation of interacting species . J. Theor. Biol. , 1979 ( 1 ) 83 – 99 .
- Sparrevik , E. and Leonardsson , K. 1995 . Effects of large Saduria entomon (isopoda) on spatial distribution of their small S. entomon and Monoporeia affinis (amphipoda) prey . Oecologia , 101 : 177 – 184 .
- Ziegler , J. R. 1976 . Evolution of the migration response: Emigration by Tribolium and the influence of age . Evolution , 30 : 579 – 592 .
- Zyromska-Rudzka , H. 1966 . Abundance and emigrations of Tribolium in a laboratory model . Ekologia Polska Seria A , 14 : 491 – 518 .