Abstract
A deterministic model with spatial consideration for a class of human disease-transmitting vectors is presented and analysed. The model takes the form of a nonlinear system of delayed ordinary differential equations in a compartmental framework. Using the model, existence conditions of a non-trivial steady-state vector population are obtained when more than one breeding site and human habitat site are available. Model analysis confirms the existence of a non-trivial steady state, uniquely determined by a threshold parameter, , whose value depends on the distribution and distance of breeding site j to human habitats. Results are based on the existence of a globally and asymptotically stable non-trivial steady-state human population. The explicit form of the Hopf bifurcation, initially reported by Ngwa [On the population dynamics of the malaria vector, Bull. Math. Biol. 68 (2006), pp. 2161–2189], is also obtained and used to show that the vector population oscillates with time. The modelling exercise points to the possibility of spatial–temporal patterns and oscillatory behaviour without external seasonal forcing.
1. Introduction
Vector-borne diseases (e.g. malaria, dengue, fever, yellow fever, lyme disease, trypanosomiases, and leishmania), amongst all the human infectious diseases, continue to remain a public health concern and a severe burden on economies, causing high human mortality in the world. These diseases have not only posed problems to national economies, but have also caused poverty and low living standards, especially in countries in the tropical and subtropical regions of the worlds. For example, the vector-borne disease, malaria, caused by the plasmodium parasite and transmitted from one human to another by the female anopheles vector mosquito, continues to plague the world especially the developing nations. By the WHO World malaria report Citation22, the parasite, and hence malaria, caused an average of nearly 900,000 thousand deaths in 2006, of which 85% were of children under the age of five. Also, dengue fever, yellow fever, trypanosomiases, and leishmania are all highly prevalent tropical and subtropical diseases. Some vector-borne diseases, such as malaria, dengue, and yellow fever, that used to be common in some developed nations of the world have been successfully put under control Citation3. However, these diseases are still a threat to developing nations and hence a potential threat to many regions of the world. Given recent trends in climate change Citation2, global warming Citation6 Citation7, and increased movement between different nations, disease-transmitting vectors may be able to (re)-colonize and survive in zones not formerly possible. Therefore, understanding the population dynamics of vectors that transmit diseases is an important area of scientific inquiry.
The main agents necessary for a successful transmission of a vector-borne disease are: the transmitting vector,Footnote1 the infectious agent,Footnote2 and the host.Footnote3 For the success of these diseases, the infectious agents adapt their life cycle so that part of it is harboured in the host and the other part in the vector, with the vector being the vehicle that transports the disease agent from one host to another. Hence, it is essential to study and understand the mechanism by which these transmitting vectors operate from a mathematical modelling perspective. Here, our focus will be on these vectors, with the main objective being to understand the dynamics of the transmitting vehicles – the vectors, by taking into consideration their behavioural aspects, their interaction with hosts, and their breeding patterns and habitats. Our special interest will be on insect-transmitting vectors though the ideas can easily be applied to any blood-sucking vector.
The geographical and local distribution of different kinds of disease-transmitting vectors in different regions is determined by a complex set of factors – climatic and geographic factors, or may even be associated with hosts endangered by the vectors Citation15. However, the basic transmission pattern for most vector-borne diseases are similar. The vectors interact with the host in some way, for the most part, in search of blood or other factors that can enhance its successful existence over different generations. During this interaction and search, the vector may either be successful or may fail and seek to try again, or may be killed. Upon a successful interaction, the vector may be infected by an infectious agent or on the other hand infect the host with an infectious agent. In our analysis, we will consider that a vector actively seeks the host Citation8 and hence seeks the interaction with a host. In addition, the vector lives, rests, and breeds in a habitat (vector-breeding site) and the host also lives and reproduces in its habitat with both habitats interacting. In trying to model the disease-transmitting vectors, we will focus on blood-feeding vectors with distinct stages of development, which include an egg laying, larval development, and adult eclosion vector stage, harboured in different breeding sites and habitats. In our modelling, these metamorphic stages of development will be accounted for. In addition, the model will take into consideration the human populations in their habitats that are endangered by the disease-transmitting vectors (not necessarily the disease) and the interactions between the two habitats.
Mathematical models to study such vector dynamics and in particular mosquito dynamics have been rare. Most models have focused on the population dynamics of disease agents, e.g. in the case of malaria see Citation11 Citation13 Citation14 Citation17 Citation19, or on the dynamics of the disease agent within a host Citation9 or within a vector Citation18 Citation20. Ngwa Citation12 introduced a deterministic delayed differential equation model of the population dynamics of the malaria vector when only a single host habitat and single vector (mosquito) habitat or breeding site is concerned. His model incorporated vector deaths in stages prior to the adult vector eclosion stage. With his model, he showed that when a non-zero steady-state vector population density exists, it can be stable but can also be driven to instability via a Hopf bifurcation to periodic solutions.
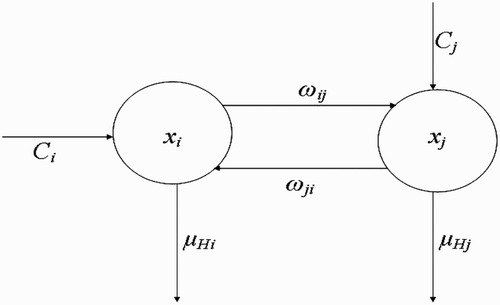
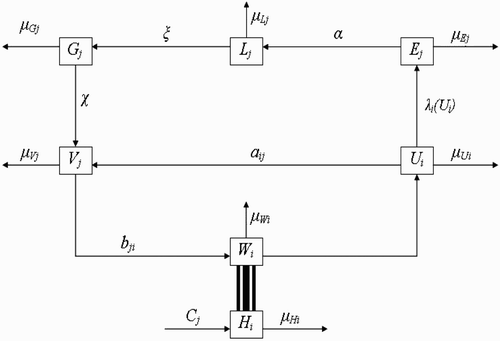
Here, we extend the deterministic model by Ngwa Citation12, to include more than one vector-breeding site and also more than one host habitat. The goal will be to understand how variation in the number of human habitats or variation in the number of breeding sites enhances or affects the interaction between the vector habitat or breeding sites and the host habitat dynamics and how this ultimately affects the dynamics and existence of the vector populations. Secondly, the solutions derived from the mathematical model will be obtained and studied to ascertain that oscillatory dynamics known to exist within disease vector populations are captured. The compartmental framework on which we base our model is displayed in .
Figure 1. Schematic framework showing the inter-relationship between the different classes of vector populations and also the life cycle of vectors at breeding site.
The rest of the paper is organized as follows: In Section 2, we define the notations used, describe the methodology used, outline the essential assumptions, and briefly derive the mathematical model. In Section 3, the analysis of the interactive model is presented. In addition, we analyse the existence and stability of the steady-state vector and host population densities. In Section 4, we simplify our model for the vector population densities by studying a simple case (one-human habitat–one-vector-breeding site). For this simple case, we establish the stability of the non-zero steady state with help of Hopf bifurcation methods. We round up with a conclusion and discussion in Section 5.
2. Derivation of the model
The basic model divides the entire vector population into three compartmental classes representing physiological status. These classes are: the class of fed and reproducing vectors returning from human habitats to vector-breeding sites represented by the variable U; the class of unfed and resting vectors present at vector-breeding sites represented by the variable V; and the class of unfed vectors questing (or foraging) for food (blood meal) in human habitats represented by the variable W. Each class of vectors is again subdivided into subclasses representing spatial locations. If we assume that there are M human habitats , and N vector-breeding sites
, then the classes of vectors U, V, and W are subdivided into subclasses U
i
, V
j
, and W
i
, i=1, …, M, j=1, …, N, respectively, where U
i
’s represent fed vectors returning from location
, W
i
’s represent unfed vectors foraging for food at location
, and V
j
’s are unfed and resting vectors resting at the vector-breeding site
. With these considerations, at time t we have
For the analysis, it is assumed that vectors of type V
j
are located at breeding sites , j=1, …, N, in which they grow by undergoing four stages of metamorphosis (
). They experience natural death at rate
. In the interactive model, we take into consideration only adult female vectors. Thus, all vectors present at the breeding site
are either young emerging adult vectors or vectors that have just returned from the human habitat. Also, vectors located at
, can make visits to the human habitat
, in search of a blood meal. When a vector leaves the breeding site and arrives at human habitat
(to forage for blood meal), it immediately becomes a vector of type W
i
, and the decision by such a vector to visit a particular human habitat,
, is influenced by how far the human habitat is from the vector-breeding site, as well as the number of resource agents (humans) present at the human habitat. At habitat
, vectors W
i
interact with human according to standard mass action principle with a contact rate τ
i
. This interaction is successful with probability p∈[0, 1] and a blood meal is taken, or else, it is unsuccessful with probability (1−p) and the vector is assumed killed. Vectors of type W
i
experience natural death with rate denoted by
. It is assumed that all vector-breeding sites are equally safe and that there is a constant alternative blood source for the vectors (may be from animals). However, we assume that the anthropophilicFootnote4 vectors, which form the basis of our mathematical study, have such a strong preference for human blood that they would fail to live in the absence of humans. Vectors which have successfully obtained a blood meal at human habitat
join the class U
i
of fed vectors returning to breeding site
,
. We assume that the decision by such a vector to go to a particular breeding site depends on how far the breeding site is from the human habitat, and perhaps on the degree of safety and availability of other vectors at that site. Vectors of type U
i
experience a natural death at rate
. In this paper, we do not take into consideration intervisitation of breeding sites by vectors.
Next, humans reside at human habitat , i=1, …, M, and for each i, the human population density at time t is labelled H
i
(t). We assume that there is a net constant migration, C
i
, of humans in habitat
and that the humans experience a per capita natural death rate
. The term C
i
incorporates births, immigration, and emigration. Humans from habitat
migrate to other human habitat
at rate
. In human habitat
, the humans are visited by vectors and there is an interaction between the two species based on standard mass action contact. It is assumed at this stage that the humans do not suffer any deaths that can be directly associated with their contact and interaction with the vectors.Footnote5 However, every vector that interacts with a human and fails to obtain a blood meal is assumed killed. For a two-dimensional consideration, let d
2:
be the usual metric on ℝ2. In other words,
It can be shown Citation12 that the net rate of adult eclosion at breeding site j resulting from vectors coming from human habitat sites i, for i=1, …, M, is
For simplicity, we assume that and set
, with all parameters positive.
2.1. Dynamics of human population
Let the density of human population at habitat be H
i
(t). Let also C
i
incorporate births, emigration, and immigration. Suppose that these resource agents, H
i
, experience a constant natural death rate
and migrate to human habitat
at rate w
ij
(see the flow diagram in which describes this situation). Then, the rate of change of H
i
(t) with respect to time t could be written as follows:
2.2. Dynamics of vector population with spatial characteristics
Vectors at the breeding site (V
j
): From EquationEquation (8) and the assumptions
and
, we have that
Figure 3. Schematic framework that models the flow of vectors V j (t) from breeding site y j to human habitat x i .
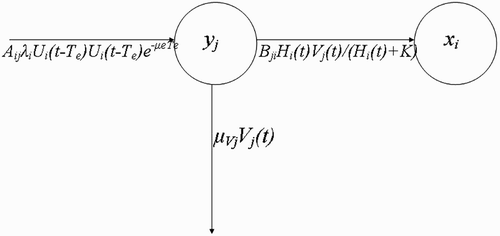
Vectors visiting resource agents (questing vectors) (W
i
): Vectors from a particular breeding site, , are attracted to human habitat,
(see the flow diagram in ), at the rate
Figure 4. Changes in the population density of questing vectors W i with respect to time t, resulting from their interaction with humans at the human habitat x i .
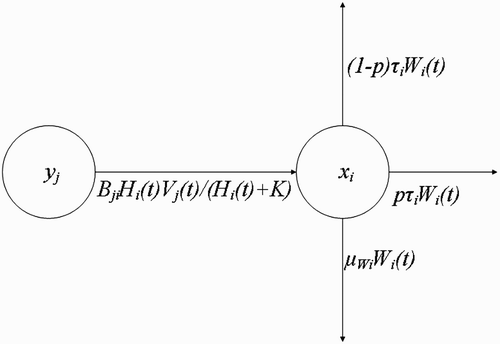
Then at the human habitat , i=1, …, M, the rate of change of the population density of the questing vectors with respect to time is
Vectors returning from resource agent sites (U
i
): Let U
i
be the vectors which succeed in their quest for blood at the human habitat . They can move to any of the breeding sites
, j=1, …, N, within their flight range, at rate A
ij
. Suppose also that the fed vectors experience a natural constant death rate
as they return to the breeding sites. Then using the flow diagram (), the rate of change of U
i
(t) with respect to time t can be written as
Figure 5. Dynamic interplay between fed vectors U i at human habitat x i returning to breeding site y j .
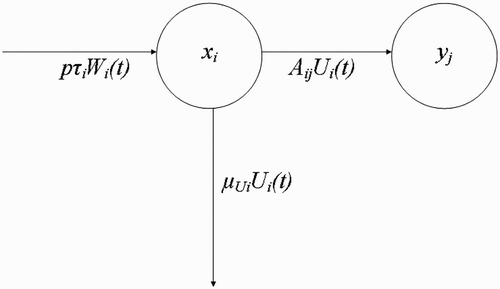
Next, we make the following assumption.
Assumption 2.1
for all integers k and l not belonging to the sets
and
, respectively,
.
Then without loss of generality, setting
To complete the formulation, it is expedient to give an explicit functional form to the function λ: and also to supply the initial data for our model. We begin with the functional form.
Definition 2.1
A function
is a suitable birth rate function for the vectors of type U if it satisfies the following three assumptions:
-
A1:
-
A2: λ(U) is continuously differentiable with
-
A3: There exists a positive number, called the vectorial basic reproduction number (denoted by
such that
Assumptions A1 and A2 ensure the existence of for x>0, with
, while Assumption A3 ensures the existence of a threshold parameter, ℛ0, with the property that, when
, a positive non-trivial equilibrium, given by
The choice of the functional form of λ(U) is based on the fact that in ecology nonlinearity in the dynamics of the population of a single species can arise due to competition, usually for resources, between members of the population. When there is competition for a common resource between members of the same species, two types of competition can be identified, namely contest competition (competition for a resource that is partitioned unequally so that some competitors obtain all they need and others less than they need (i.e. there are winners and losers)) and scramble competition (competition for a resource that is inadequate for the needs of all, but is partitioned equally among contestants, so no competitor obtains the amount it needs) Citation5. It seems reasonable to assume a contest type competition for mosquito dynamics based on the fact that there is abundance of humans for blood meal; however, some of the vectors are killed (losers) in their quest for a blood meal. In this paper, the following form of birth rate function, which satisfies Assumptions A1–A3, will be used:
Next we define initial data for t∈[−T, 0] as follows:
3. Analysis of the interactive model
In this section, we examine the spatially explicit model and show that there exists a steady-state spatial distribution of vector populations over the entire region of study (N>0, M>0 arbitrary) and derive conditions under which such population densities could exist. Before this, we briefly analyse the dynamics of the human population.
3.1. Existence of a steady-state human population density
Here, we examine the existence and stability of steady-state solutions for the density of human populations. In this section, we shall assume that the death rate of humans, , are identical for all habitats. In addition, we assume that the migration of humans between any two human habitats, w
ij
, is also identical so that
and w
ij
=w, where w and μ are constants. This means that the human habitats are identical. To examine and analyse the stability of the steady state of the human population, we state and prove a lemma and theorem that will be essential.
Lemma 3.1
Let
be an n×n real symmetric matrix depending on positive parameters x, y. If in addition, for each
the matrix
has only two distinct elements such that
Proof
Let n≥2 be an integer. We easily verify that
Now,
We now use Lemma 3.1 to prove the following theorem.
Theorem 3.2
The linear system of equations
Proof
We examine EquationEquation (20) when the time derivatives are set to zero. Let
be the steady-state solution. Then on setting w
ij
=w and
, as assumed, we obtain that
satisfies the system
We next substitute EquationEquation (25) into EquationEquation (24)
to obtain EquationEquation (21)
. To establish the stability of this steady state, let
Remark 3.1
The extension of the results of Theorem 3.2 to cover the general cases, where are different, is solved by observing that EquationEquation (20)
can be written in matrix form as
3.2. Existence of a steady-state vector population density
We examine in this section the spatially explicit model for the distributions of vectors. We show that there exists a spatial distribution of vector population densities over the entire region of study and derive conditions under which such steady population densities could exist. Our focus is now on the following three equations (taken from system Equation(14)):
The steady states are obtained by setting the time derivatives in EquationEquations (33)–Equation(35)
to zero and solving for the 2M+N quantities
, and
We then establish that
Definition 3.1
Let
S
v
and
S
h
be the sets of all vector-breeding and human habitats sites, respectively, and
. Let
, d>0 the maximum flight range of vectors from location
. Define
Definition 3.2
Let
,
’s the flight range domains of vectors at
’s, i=1, …, r, respectively. We say that the
’s are disjoint if
Definition 3.3
Let
’s the flight range domains of vectors at
’s, i=1, …, r, respectively. We say that the
’s are one-point-intersected if
Definition 3.4
-
(i) Let
. We define the distance from a site
to the set A as
-
(ii) Let
,
’s the flight range domains of vectors at
’s
respectively. We say that the
’s full-intersect if there exist at least two sites
s.t.
We set.
Definition 3.5
Let
and
i=1, …, r
1, j=1, …, r
2,
Suppose
and
s.t.
and
Define
Remark 3.2
We similarly define the concepts of disjoint, one-point-intersection and full-intersection flight range domains when ’s,
, are taken in S
h
.
We state and prove the following proposition using the case study approach that is later generalized.
Proposition 3.1
The system
Equation(44)
has exactly a two-solution set in which
. The trivial solution,
which always exist, and a non-trivial solution, with
whose existence is uniquely determined by a threshold parameter ℛ
in the sense that
-
(a) if
then the system has no positive non-zero real solutions;
-
(b) if
then the system has exactly one real positive non-zero solution.
Proof
In all the proofs, we suppose that , since the solution
is trivial. Without loss of generality, we rewrite EquationEquation (44)
replacing
by V
j
, ∀ j:
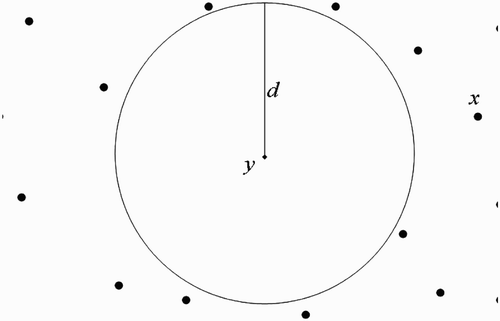
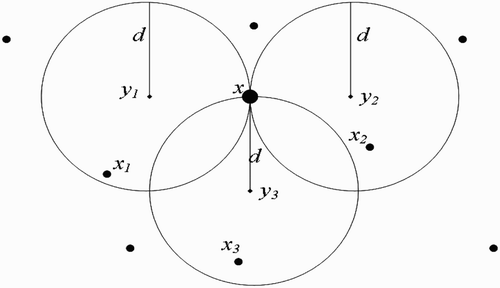
(a) We first consider the situation where human habitats are not found within flight range domain of vectors at breeding site ().
In this case, we deduce from Equation(5), Equation(6)
, and Equation(13)
that
Assumption 3.1
Within their flight range domain, vectors located at breeding site
always have a unit of human habitats,
centred at
where they find blood meal to live and prosper.
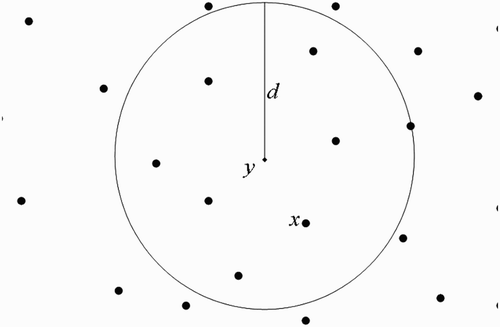
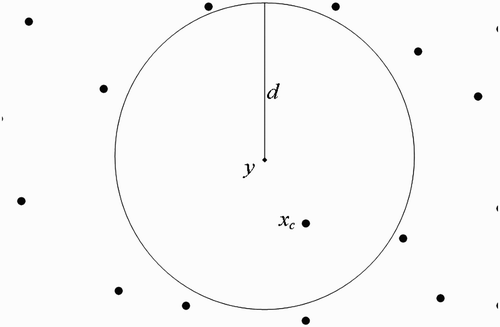
(b) We now suppose that human habitats belong to the flight range domain of vectors located in breeding site ().
Taking into consideration Assumption 3.1 and using , we shall use the centre of the unit U
y
to represent all the human habitats within the flight range of vectors located at
(we therefore think as if we have a system with one breeding site and one human habitat found within the flight range of vectors located at the breeding site), set
Case 2: N=2 In this case, EquationEquation (45) becomes
-
(a) Disjoint flight range domains ():
In this case,
, therefore vectors in
do not visit sites in
, and vice versa. Then by EquationEquation (13)
, we obtain
Substituting EquationEquation (51)into EquationEquations (42)
and Equation(43)
, we get
Substituting EquationEquation (52)into EquationEquation (50)
gives
which has as solution set when, the singleton
We observe that for j=1, 2,This implies the following definition -
(b) One-point-intersection flight range domains ():
We consider here vectors present in human habitats
, and intersection of the flight range domains
and
. At this site
, vectors decide (according to our assumptions), after a successful interaction with humans, to return either to the breeding site
, or to the breeding site
, with equal chance. In EquationEquation (13)
where we define the rates at which vectors move from human habitat
to vector-breeding site
, and from breeding site
to human habitat
, respectively, we see that a ij and b ji depend essentially on the distance from
to
. With this, we obtain that at habitat x
Substituting EquationEquation (56)into EquationEquations (39)
, Equation(42)
, and Equation(43)
, we have
This means that at habitat x, all parameters are constant and positive (this is easily verified). Substitute EquationEquation (57)into EquationEquation (45)
to obtain the following system of equations:
Equate the two equations in EquationEquation (58)to obtain
Substitute this equality in any one of the equations in EquationEquation (58)to have the following solution set
For j=1, 2, we see thatTherefore, we choose -
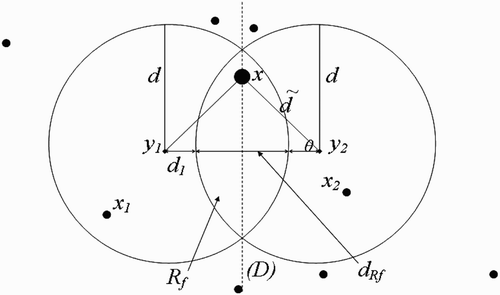
(c) Full-intersection flight range domains ():
Let
, and
and
be the flight range domains of vectors present at
and
, respectively. Let us define the following:
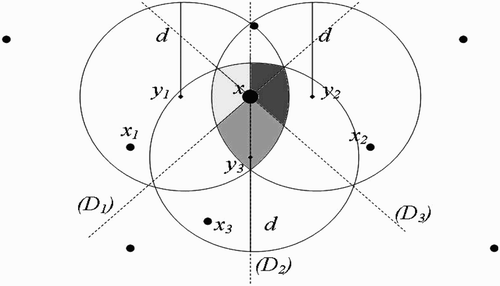
Figure 9. Disjoint flight range domains with the respective centres representing all the belonging human habitats.
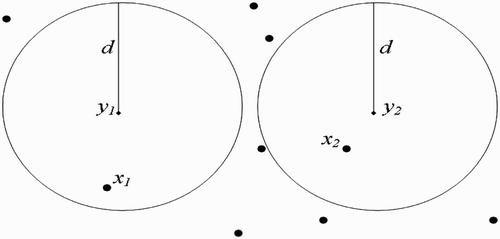
Figure 10. One-point-intersected flight range domains with the respective centres representing all the belonging human habitats.
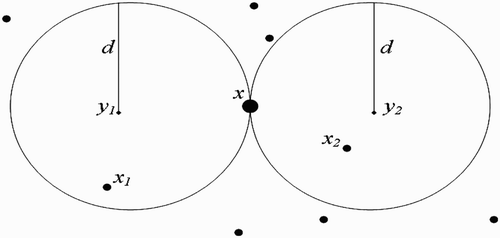
Figure 11. Full-intersected flight range domains with the respective centres representing all the belonging human habitats.
We make the following remark:
Remark 3.3
must belong to the line (D), which is equidistant to both
and
. For, if x does not belong to (D), then vectors present in x will choose to return to the nearest breeding site, that is
or
. Thus, cosθ cannot be equal to zero.
By considering the terms defined in EquationEquation (61), we proceed as in the case of one-point-flight range domains above, with [dtilde] in the place of d, ã
ij
and [btilde]
ji
in places of a
ij
and b
ji
, respectively. We therefore obtain similar results, that is
Case 3: N=3 In this case, EquationEquation (45) becomes
-
(a) Disjoint flight range domains:
For similar reasons as in previous cases,
This implies thatEquationEquation (64)then becomes (using EquationEquation (66)
)
The non-zero solution of EquationEquation (67)is the singleton set
Again, for j=1, 2, 3,Therefore, we set -
(b) One-point-intersection flight range domains ():
this implies thatUsing EquationEquation (71)into EquationEquation (64)
, we obtain
Equate all the equations in EquationEquation (72)to have
Substituting the last equality in any one of the equations in EquationEquation (72), we have the following non-zero solution set
From this, for j=1, 2, 3,Therefore, we define -
(c) Full-intersection flight range domains ():
We begin the following definition of a full point.
Definition 3.6
Let
j=3, …, N, be breeding sites. Let Δ
ij
be the lines equidistant from
and
∀ i, j. Then there are
of these lines, with
We consider in the current situation only human habitat present at the F-point of
,
, and
, for it is the only human habitat within flight range of vectors that is equidistant from all breeding sites.
As in EquationEquation (61), we define the following:
General case: N≥2
-
(a) Disjoint flight range domains: As in previous cases,
This implies thatUsing EquationEquation (81)into EquationEquation (45)
, we obtain the system of N equations
which has as non-zero solution set the singletonThis result tells us that within their respective flight range domains, vectors live and prosper whenever there is available nutrient from humans in human habitats. Observe that in the present situation, the number N of vector-breeding sites does not affect the value of the respective. From EquationEquation (83)
,
Therefore, we define -
(b) One-point-intersection flight range domains: As in previous cases, at the F-point,
This implies thatUsing EquationEquation (86)into EquationEquation (45)
, we obtain the system of N equations
Equating all equations in system Equation(87), the following relation is clear:
We use this equality into any one of the equations of the system and obtain the equationWe therefore solve this equation for the unknown V 1 and have the solutionHenceis the non-zero solution set of EquationEquation (87).
so that we define -
(c) Full-intersection flight range domains: We use the definitions in EquationEquation (77)
of case N=3, and similar reasoning. The results in this case therefore are similar to those of case N=3. That is,
Since this situation, with the definitions in EquationEquation (77), is similar to the one-point-intersection flight range domains case, the conclusion is also similar to that of the quoted case. Then, we define
where, and [Qtilde] are defined as in case N=3.
We now state the following corollary which results from Proposition 3.1.
Corollary 3.1
Define
4. Example: the one-vector-breeding site–one-human habitat model with no delay
Using M=1, N=1, , and L
1=L (as defined in EquationEquation (15)
) in the model Equation(14)
, we obtain the simplified system which is studied at any time t
1
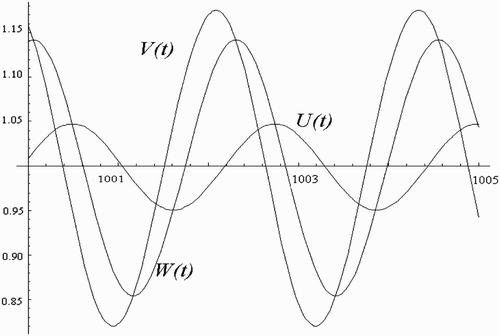
We use a step-by-step procedure, as explained in Citation4, to completely determine the nature of the periodic solutions arising from the Hopf bifurcation. Tedious but straightforward computations yield the approximation solutions
5. Conclusion and discussion
A mathematical model, originally introduced by Ngwa Citation12 to study the population dynamics of the malaria vector, was extended to include spatial component and dispersal in which vectors, from different breeding sites, can visit host habitats within their flight range domain and interact with them. During the interaction, the vectors can be successful, fail and seek to try again, or fail and be killed. In our modelling, the vectors were assumed to be located at N breeding sites spatially distributed among M human habitats. Vector deaths prior to the adult vector stage and a delay factor T were taken into consideration in the modelling. Analysis of the model equations under a given set of defined assumptions and using a Verhulst–Pearl birth function for the vectors led to the following results:
-
(1) There is always a unique non-zero steady-state distribution of humans over the number of human habitats, which is globally and asymptotically stable for all reasonable parameter values.
-
(2) For the vector population, a trivial steady-state solution always exists. In addition, there is a non-trivial steady-state vector distribution whose existence is uniquely determined by a threshold parameter
. When the threshold parameter
, there exists exactly one real positive non-trivial steady state solution, and if
the only steady state is the trivial steady state. The non-trivial steady states were obtained for different distributions of the vector-breeding sites.
-
(3) Furthermore, for the one-human habitat, one-vector-breeding site scenario with no time delay, Ngwa Citation12 partially established the presence of a Hopf bifurcation in a particular parameter space. Here, we use a step-by-step procedure, as explained in Citation4, to completely determine the nature of the periodic solutions arising from the Hopf bifurcation.
The modelling techniques used can be applied to analyse the dynamics of many different vectors. In addition, the results obtained can be very useful in designing effective control strategies aimed at fighting and eradicating vector-borne diseases. Two major ways in which disease vectors can be controlled are chemical or biological. Biological control of vectors can be realized by the introduction of a predator (i.e. another organism which feeds on the vectors) at the vector breeding site. For example, in the case of swamp insect vectors, control can be implemented by the introduction of a species of fish which feeds on the larvae of the vectors. Usually, the effectiveness of this kind of control is long term, since the predator needs some time to establish itself in the environment. On the other hand, chemical control can be realized through direct application of a chemical and toxic substances in the vectors themselves or in their breeding sites, in order to interrupt their reproduction cycle. For example, pesticides like DDT and toxic bed nets are used to control mosquitoes and midges (see Citation16 for more on human disease vectors and their control measures). Therefore, understanding vector dynamics is crucial in the understanding how both biological and chemical control can impact vector-borne disease control. For example, continuous application of control measures (use of insecticides, bed nets, specialized candles, and mosquito traps Citation10) when the vector population is at its minimum amplitude may result in a decrease in the growth of the vector population and possible extinction in extreme cases. On the other hand, the application of control measures when the population is at its peak may not be an efficient strategy.
The model presented is the first to the best of our knowledge, and just the first step in completely understanding the population dynamics of vectors when there is more than one breeding site interacting with the host habitat. This is very important especially when applied to the Anopheles mosquito, the vector that transmits the malaria-causing agent – plasmodium. In order to control malaria, multiple interventions are needed Citation18 Citation19 which involve a combination of: vector control Citation12; reduction of contact between humans and vector Citation17; control of the infectious agent, Plasmodium, both within the human host Citation19 and the vector Citation18; and education (of the people that are most affected) Citation17 and control of the vector-breeding sites. Hence, our paper seeks to further the understanding of the vector population dynamics which could assist in the planning and implementation of new malaria intervention and vector control strategies.
Many extensions exist, some of which are currently under investigation, and include the following:
-
(1) Analysing the stability of the steady states obtained.
-
(2) Using a shorter method of establishing the existence of steady states for any number of vector-breeding sites and human habitats, and obtaining an explicit form of the steady states and analysing their stability.
-
(3) Incorporating the fact that many of the vector-breeding sites are dynamic. In most endemic regions such as Cameroon, breeding sites are created due to muddy ponds and washed out due to heavy rains or dried up due to high heat. Hence, most of the breeding sites very close to human habitats are temporal and created at some rate and destroyed at some rate [21 . Therefore, incorporating the lifespan of breeding sites and the dynamics of their formation and destruction into our model will prove very crucial in understanding mosquito dynamics and will be important in control measures at eradicating vector-borne diseases that are transmitted by vectors that live in breeding sites and rely on host habitats for their main sustenance and survival.
-
(4) Incorporate predation at the vector-breeding sites and see what the impact to the mosquito dynamics will be.
-
(5) Include disease dynamics in the model in which questing vectors that feed successfully can in the act transmit an infectious agent that can cause a disease or be infected with an infectious agent that can again be transmitted at a later feeding.
Acknowledgements
The first author (S. Nourridine) and the third author (G.A. Ngwa) wish to acknowledge support of a grant from the Faculty of Science, University of Buea, Cameroon, under the Faculty of Science Research Grant Scheme 2008/2009. The second author (M.I. Teboh-Ewungkem) wishes to acknowledge support of the NSF Grant Award No. OISE-0855380 that made it possible for all three authors to meet and to work at the University of Buea. All three authors will like to thank the reviewers for their time in reading this paper.
Notes
These are usually blood-sucking athropods.
It could be a virus, protozoa, bacteria, fungi, or helminth.
By host we mean the organism infected by the infectious agent and which interacts with the vector.
These are vectors with a preference for human blood. If the vectors have a preference to animal blood, they are said to be zoophilic.
It is understood that once the infectious disease is involved and accounted for in the model, e.g. when either the vector or human is infected, then this assumption must be relaxed.
Human's blood preference factor of the vectors (H=human density).
Animal's blood preference factor of the vectors (A=animal density).
References
- Brännström , Å. and Sumpter , D. J.T. 2005 . The role of competition and clustering in population dynamics . Proc. R. Soc. B. , 272 : 2065 – 2072 .
- Githeko , A. K. , Lindsay , S. W. , Confalonieri , U. E. and Patz , A. J. 2000 . Climate change and vector-borne diseases: A regional analysis . Bull. World Health Organ. , 78 ( 9 ) : 1136 – 1147 .
- Gubler , D. J. , Reiter , P. , Ebi , K. L. , Yap , W. , Nasci , R. and Patz , A. J. 2001 . Climate variability and change in the United States: Potential impacts on vector - and rodent-borne diseases . Environ. Health Perspect. , 109 ( 2 ) : 223 – 233 .
- Hassard , B. D. , Kazarinoff , N. D. and Wan , Y.-H. 1981 . Theory and Applications of Hopf Bifurcation , Vol. 41 , Cambridge : Cambridge University Press . London Mathematical Society Lecture Note Series, London
- Jang , S. R.J. 2005 . Contest and scramble competition with a dynamic resource . Nonlinear Anal. , 63 : 109 – 118 .
- Martens , W. J.M. , Jetten , T. H. , Rotmans , J. and Niessen , L. W. 1995 . Climate change and vector-borne diseases: A globally modelling perspective . Global Environ. Change , 5 : 195 – 209 .
- Martens , P. , Kovats , R. S. , Nijhof , S. , de Vries , P. , Livermore , M. T.J. , Bradley , D. J. , Cox , J. and McMichael , A. J. 1999 . Climate change and future populations at risk malaria . Global Environ. Change A , 9 : S89 – S107 .
- McCall , P. J. and Kelly , D. W. 2002 . Learning and memory in disease vectors . Trends Parasitol. , 18 ( 10 ) : 429 – 433 .
- McKenzie , F. E. and Bossert , W. H. 1997 . The dynamics of Plasmodium falciparum blood-stage infection . J. Theor. Biol. , 188 ( 1 ) : 127 – 140 .
- Mosquito Solutions . Dragonfly Mosquito System(R); available at http://www.cdc.mosquitosolutions.com (accessed May 2009)
- Ngwa , G. A. 2004 . Modelling the dynamics of endemic malaria in growing populations . Discrete Contin. Dyn. Syst. Ser. B , 4 ( 4 ) : 1173 – 1202 .
- Ngwa , G. A. 2006 . On the population dynamics of the malaria vector . Bull. Math. Biol. , 68 : 2161 – 2189 .
- Ngwa , G. A. and Shu , W. S. 2000 . A mathematical model for endemic malaria with variable human and mosquito populations . Math. Comput. Model. , 32 ( 7–8 ) : 747 – 763 .
- Ngwa , G. A. , Ngonghala , C. N. and Sama Wilson , N. B. 2001 . A model for endemic malaria with delay and variable populations . J. Cameroon Acad. Sci. , 1 ( 3 ) : 168 – 186 .
- Schmidt , G. D. and Roberts , L. S. 1989 . Foundations of Parasitology , 4 , Times Mirror/Mosby College Publishing .
- J.D. Smyth, Animal Parasitology, Low Price Edition, Vol. 19(1), Cambridge University Press, Cambridge, 1996
- Teboh-Ewungkem , M. I. 2009 . “ Malaria control: The role of local communities as seen through a mathematical model in a changing population-Cameroon ” . In Advances in Disease Epidemiology , Edited by: Tchuenche , J. M. and Mukandavire , Z. 103 – 140 . New York : Nova Science Publishers .
- Teboh-Ewungkem , M. I. and Yuster , T. 2010 . A within-vector mathematical model of Plasmodium falciparum and implications of incomplete fertilization on optimal gametocyte sex ratio . J. Theor. Biol. , 264 ( 2 ) : 273 – 286 .
- Teboh-Ewungkem , M. I. , Podder , C. N. and Gumel , A. B. 2010 . Mathematical study of the role of gametocytes and an imperfect vaccine on malaria transmission dynamics . Bull. Math. Biol. , 72 ( 1 ) : 63 – 93 .
- Teboh-Ewungkem , M. I. , Yuster , T. and Newman , N. H. 2010 . “ A mathematical model of the within-vector dynamics of the Plasmodium falciparum protozoan parasite ” . In Infectious Disease Modelling Research Progress , Edited by: Tchuenche , J. M. and Chiyaka , C. 177 – 199 . New York : Nova Science Publishers .
- Wanji , S. , Mafo , S. S. , Tendongfor , N. , Tanga , M. C. , Tchuente , F. , Bilong Bilong , C. F. and Njine , T. 2009 . Spatial distribution, environmental and physicochemical characterization of Anopheles breeding sites in the Mount Cameroon region . J. Vector Borne Dis. , 46 : 75 – 80 .
- WHO . World malaria report, 2008. Available at http://www.int/malaria/publications/atoz/9789241563697/en/index.html (accessed May 2010)