Abstract
We study a discrete juvenile–adult model which describes the dynamics of a population that reproduces and disperses between two patches constantly or seasonally. When breeding and dispersal rates are constant, the model has a unique interior equilibrium that is globally attractive, provided the net reproductive number is greater than one. If net reproductive number is less than one, then the extinction equilibrium is globally asymptotically stable. When breeding and dispersal rates are periodic of period 2, the extinction equilibrium is globally asymptotically stable if the net reproductive number is less than one. If the net reproductive number is greater than one, then there exists a unique globally attractive periodic solution. We then use bifurcation analysis to compare constant and seasonal breeding strategies, to explore the effects of different birth and dispersal periodicities and to understand the influence of strong nonlinearities on the dynamics of the model.
1. Introduction
The process of migration and dispersal has been studied in ecology for many years. Not only has the migration and dispersal of animals, such as birds, insects, and fish, been a popular topic to study, but also the migration and dispersal of plants (seed dispersal) and tissue, blood, and cancer cells are being studied Citation7 Citation10 Citation11 Citation12 Citation15 Citation20 Citation24. In the last few decades, ecologists and mathematicians have been interested in the dispersal of amphibian populations (Citation4 Citation5 Citation13 Citation16 Citation17 Citation18 Citation22 Citation23 just to name a few).
In the 1960s, Dole studied the summer dispersal of a population of adult leopard frogs, Rana pipiens, in Cheboygan County, Michigan. He was interested in the movements of the adult leopard frogs and the factors controlling them Citation13. Gibbs examined amphibian movements relative to roads, forest edges, and stream beds by using drift fences and pitfall traps to intercept dispersing amphibians in a forest tract in southern Connecticut. He investigated several amphibian species that were captured, including the pickerel frog, Rana palustris, and the wood frog, Rana sylvatica. Gibbs suggested that the relative permeability of forest-road edges was much reduced compared with the forest interior and with edges between forest and open land Citation16. From 1995 to 1998, Pilliod et al. researched the habitat use and movement patterns of 736 marked and 87 radio-tagged Columbia spotted frogs, Rana luteiventris, in a mountain basin in central Idaho. Their work showed that many of the Columbia spotted frogs used spatially separated, specific habitat patches for breeding, foraging, and hibernating. They also witnessed that individuals could disperse across hundreds to thousands of metres annually among these complementary resources Citation22. Kirkland et al. studied general discrete population models where the populations reproduce and disperse in a landscape consisting of a finite number of patches. They wanted to better understand the evolution of dispersal in spatially heterogeneous landscapes by studying the population dynamics for both conditional and unconditional dispersal strategies Citation19.
We were mainly motivated in this paper by two urban populations of green tree frogs, Hyla cinerea, that are being studied in a joint project by the University of Louisiana at Lafayette and the United States Geological Survey National Wetlands Research Center (USGS NWRC). One population is located at the National Wetlands Research Center/Estuarine Habitat and the Coastal Fisheries Center research complex (referred to as NWRC/EHCFC complex) and the other is located at the Louisiana Immersive Technologies Enterprise Center (referred to as LITE Center). Each location contains man-made ponds with the fringing landscape simulating different wetland types. These populations are relatively isolated as each complex is bordered by buildings, sidewalks, and four lane roads. The distance between the NWRC/EHCFC complex and the LITE Center is approximately half a mile. Dispersal between the ponds is one main topic being studied. For example, in 2008, out of the total number of frogs recaptured at both sites, approximately 1.2% were frogs that had dispersed from one location to the other Citation21.
In this paper, we develop a juvenile–adult discrete model that describes the dynamics of one species dispersing between two locations, patches 1 and 2. In Section 2, we first analyse the case where the breeding and dispersal rates are constant. Then we study the model by assuming that all the breeding and dispersal rates are seasonal (periodic of period 2). In Section 3, we use bifurcation analysis to compare the population levels resulting from seasonal birth and dispersal rates and constant birth and dispersal rates. We also examine the effects on the population dynamics when considering different periods for the birth and dispersal rates. Finally, we give conclusions in Section 4.
2. Two-stage discrete model
We develop a juvenile–adult discrete model that describes the dynamics of one species dispersing between two locations, patches 1 and 2. We assume that the juveniles and adults only compete with themselves and not with each other. We also assume that the juveniles always remain at their location of birth and only the adults disperse. This is typical of amphibians where the tadpoles remain in the body of water they were born in while the adults move around. Let J
i
(t) and A
i
(t) denote the number of juveniles and adults, respectively, at time t at patch i for i=1, 2. Let represent the survivorship of the juveniles and
represent the survivorship of the adults at time t at patch i for i=1, 2. We will denote the time-dependent birth rates at the two locations as b
1(t) and b
2(t). Thus, we obtain the following model:
-
(Σ1) For k=J i , A i , i=1, 2,
,
,
, and S k (0)=a k (0<a k <1).
Clearly, (Σ 1) is satisfied by the Beverton–Holt dynamics given by , for k=J
i
, A
i
and also the Ricker dynamics given by
, for k=J
i
, A
i
.
Model Equation(1) will be analysed in two cases. In the first case, we assume b
i
(t)=b
i
,
and
are positive constants, while in the second case, we assume b
i
(t), m
12(t) and m
21(t) are periodic with period 2.
2.1 Constant birth and dispersion
Consider system Equation(1) with continuous breeding. For the remainder of this section, we assume b
i
(t)=b
i
,
and
are positive constant, for i=1, 2. Thus, system Equation(1)
can be written as
Now, in the following theorem, we provide a stability result for system Equation(1).
Theorem 2.1
Let b
i
(t)=b
i
, m
12(t)=m
12, and
be positive constants, for
i=1,2 and t=0, 1, 2…. Assume (Σ 1) holds. If
then E
0
is globally asymptotically stable.
Proof
We will use techniques similar to Citation2
Citation19. All vector and matrix inequalities hold componentwise. Assume . Define the map
to be the right side of system Equation(1)
. Since B(E
0) is non-negative, irreducible, and primitive it has a positive, simple, and strictly dominant eigenvalue λ. Since
, by [Theorem 1.1.3]Citation8, we know λ<1. Thus, E
0 is locally asymptotically stable. Let v
T>0 be the corresponding left eigenvector of λ. That is,
In the following theorem, we show the existence of a unique non-trivial interior steady state for system Equation(1) which is globally attractive. In addition to (Σ 1), we need to assume that
and
also satisfy
-
(Σ2) For k=J i , A i , i=1, 2,
and
.
Note that (Σ 2) is still satisfied by the Beverton–Holt dynamics given by , for k=J
i
, A
i
. Under (Σ 2), system Equation(1)
is monotone. This facilitates the proof of the global attractivity of the interior equilibrium Citation2
Citation19, which is given in the next theorem.
Theorem 2.2
Let b
i
(t)=b
i
, m
12(t)=m
12, and
be positive constants, for i=1, 2 and t=0, 1, 2…. Assume (Σ 1) and (Σ 2) hold. If
then there exists a unique non-trivial interior steady state for system
Equation(1)
which is globally attractive.
Proof
Assume . Then from the proof of Theorem 2.1, the dominant eigenvalue of B(E
0), λ, is greater than 1. Define the map
to be the right side of system Equation(1)
. Now we will show that system Equation(1)
has a unique interior fixed point X¯. First we will show existence. Let u>0 be the corresponding right eigenvector of λ. That is,
2.2 Seasonal breeding and dispersion
In this section, we assume that breeding and dispersal movements of the population are seasonal. For example, there are times when the environment is suitable for frogs to hunt, mate, breed, and disperse. And to escape harsh weather, many amphibians including frogs hibernate in winter and estivate when the weather is too hot or dry. During these periods each year, the frogs do not eat, move, or breed. All their life processes drop to a very low level. Hence, we assume in model Equation(1) that the dispersal rates m
12(t), m
21(t) and the birth rates b
i
(t), i=1, 2 are periodic with period 2. Specifically, we let
for i=1, 2, and we take
, where
. In other words, if the time unit is half a year and a full cycle consists of one year, the population gives birth and disperses only half the year, while in the other half there is no dispersal or birth.
We will find the net reproductive number for the seasonal breeding case by using similar techniques used in the continuous breeding case. From Citation6
Citation14, since system Equation(1)
is periodic with period 2, we define the projection matrix over a full cycle [Bcirc](X) to be the product of the following matrices, where the short notation S
k
represents S
k
(k), for k=J
i
, A
i
, and i=1, 2.
The following theorem shows the global stability of the extinction equilibrium E 0.
Theorem 2.3
For t=0, 1, 2, …, and for i=1, 2, let
and
where
. Assume (Σ 1) holds. If
then
is globally asymptotically stable.
Proof
Let and (Σ 1) hold. Define the map
to be the right side of EquationEquation (1)
. Since
, by [Theorem 1.1.3, p. 10]Citation8, we have that the dominant positive eigenvalue, λˆ, of the non-negative, irreducible, and primitive matrix [Bcirc](E
0) is also less than one. So E
0 is locally asymptotically stable. From (Σ 1), system Equation(1)
has the property
In the following theorem, we show the existence of a unique non-trivial two-cycle for system Equation(1) which is globally attractive in
. To prove global attractivity, we again require that the assumptions in (Σ 2) hold in order for the system to be monotone.
Theorem 2.4
For t=0, 1, 2, …, and for i=1, 2, let
and
where
. Assume (Σ 1) and (Σ 2) hold. If
then there exists a unique non-trivial two-cycle for system
Equation(1)
which is globally attractive in
.
Proof
Suppose (Σ 1) and (Σ 2) hold and that . We will use techniques akin to Citation1. We see that
Let for i≥0. Then we have the following system
Then by a similar argument as in the proof of Theorem 2.3, we can show that system Equation(6) has a unique interior fixed point
, which is globally attractive. Thus, we have that
It follows that
3. Bifurcation analysis
In Section 3.1, we address the question of whether a seasonal breeding strategy is advantageous or deleterious over a constant breeding strategy. In Section 3.2, using bifurcation analysis, we study our model with birth and dispersal rates that are periodic with periods 3 and 6. We compare these results with the period 2 case studied in Section 2. Then, in Section 3.3, we replace the Beverton–Holt non-linearities with Ricker-type non-linearities to study the effects of strong non-linearities on the dynamics of the population.
3.1 Period 2 versus constant birth rate
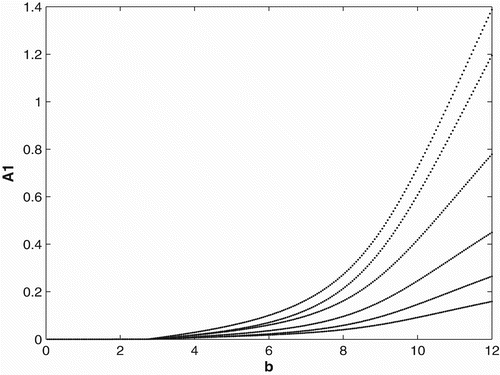
We are interested in understanding whether there are birth rates b for which seasonal breeding is advantageous over constant breeding. In particular, are there values of b in which a seasonal breeding strategy will result in an average adult population size that is larger than with a constant breeding strategy? To compare on an equal basis, we assume that over a complete cycle (composed of p time units), an adult produces the same number of juveniles whether breeding constantly or seasonally. For period 2, the constant breeders will produce b+b=2b offspring in one complete cycle. The seasonal breeders will produce offspring in one complete cycle. Therefore, we assume that [bcirc]=2 b. In the same light, we take
and
. In the following example, we show that for low birth rates the periodic strategy has an average adult population that is larger than that of the constant. While for large birth rates, the opposite happens.
For this example, we assume that the environment in patch 2 is better than that in patch 1. Therefore, we assume that the birth rate in patch 2 to be higher than that in patch 1, the dispersal rate from patch 1 to patch 2 is higher than that from patch 2 to patch 1, and the survivorship rate in patch 2 is higher than that in patch 1. In particular, we use ,
,
, and
where
, and
. We set b
1=b, b
2=b+1, m
12=0.04, and m
21=0.01. The initial conditions are
, A
1(0)=4, and A
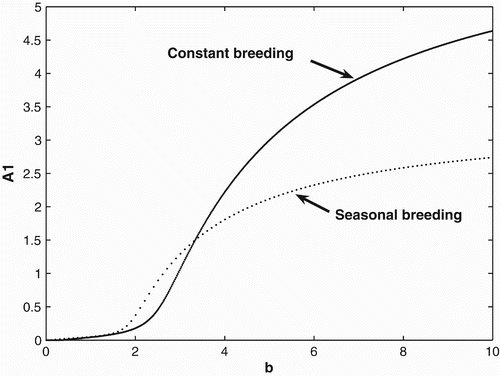
2(0)=5. A similar scenario seems to occur at the two locations of our motivating populations of green tree frogs. Among all the recaptures of green tree frogs in 2008, there were four times as many frogs migrating to the NWRC/EHCFC complex than to the LITE Center. In , we provide a bifurcation diagram for the constant and seasonal birth and migration rates with the birth rate used as a bifurcation parameter. This example shows that for birth rates less than ∼0.07, both populations with a seasonal breeding and constant breeding strategy will become extinct. We also can see that for birth rates
both the constant and seasonal breeders will survive, but seasonal breeding is advantageous over constant breeding. For birth rates b>3.36, we see that constant breeding is advantageous.
3.2 Constant and periodic breeding and dispersal rates
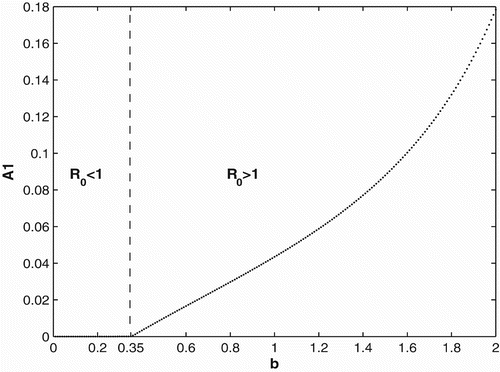
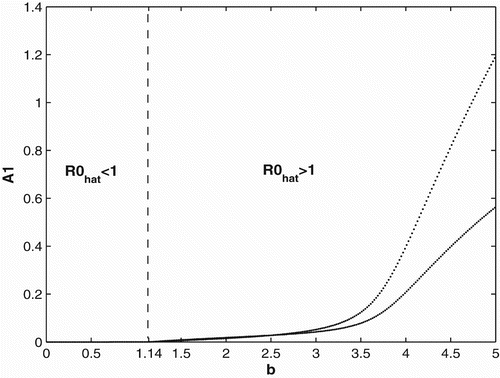
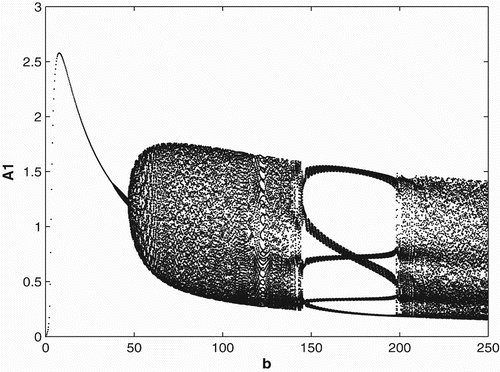
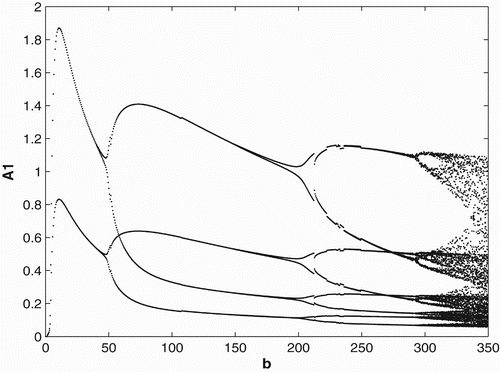
We first provide bifurcation diagrams illustrating our theoretical conclusions in Section 2. In and , we graph the population of adults in patch 1. The bifurcation diagram of the other adult population and the juveniles are similar and hence are not presented. We use the same survivorship functions and parameter values as in Section 3.1. is the bifurcation diagram for constant breeding and dispersal. For b<0.35, the population becomes extinct, while the population persists for b>0.35. is the bifurcation diagram for the period 2 birth and dispersal rates of the form used in Section 2. Extinction of the population occurs for b<1.14, while for b>1.14, the population persist and converges to a two-cycle.
In , we set a
1=0.5, a
2=0.4, a
3=a
4=0.6, . We consider period 3 birth rates of the form
,
and period 3 dispersal rates of the form
and
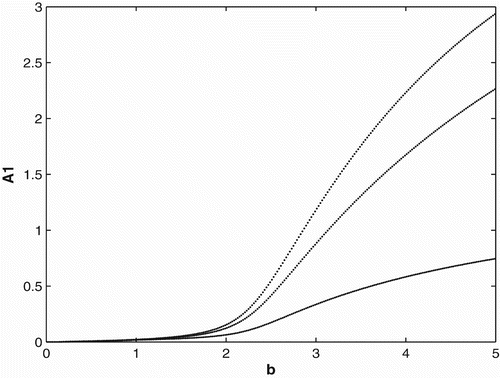
. In other words, if a full cycle is a year (composed of three time units, 4 months each), the populations would breed and disperse for only 8 months out of the year. We can see that when the birth rate gets large enough, the population persists and converges to three cycles.
Next we consider the case where birth rates are period 6 of the form ,
and dispersal rates are period 6 of the form
and
. This strategy suggests that dispersal begins before and ends after the breeding season. In this example, we let a
1=0.4, a
2=0.6, a
3=0.5, a
4=0.7,
. shows that when the birth rate gets large enough, the population persists and converges to six cycles.
3.3 Ricker-type non-linearities
In order to study the effects of survivorship functions that are strong non-linearities as opposed to weak non-linearities, we replace the Beverton–Holt non-linearities in our model used in Sections 3.1 and 3.2 with Ricker-type non-linearities. In , we consider Ricker-type survivorship functions with constant birth and dispersal rates, and in , we consider seasonal birth and dispersal rates of period 2. We use the following Ricker-type survivorship functions ,
,
,
. The parameter values and initial conditions are set to be the same as in Section 3.1. We see that the Ricker-type survivorship functions result in much richer dynamics than those with the Beverton–Holt functions used in Section 3.2, including chaotic dynamics. Observe, however, that in comparison with the constant case, seasonal birth and dispersal rates tend to stabilize the population dynamics. In particular, for some values of birth rates when the population has chaotic dynamics in the constant rate strategy, it has periodic dynamics under the seasonal rate strategy.
4. Conclusion
In this paper we develop and analyse a discrete juvenile–adult model Equation(1) with constant or seasonal reproduction and dispersion. When the population breeds and disperses with constant rates b
i
, i=1,2, and m
12, m
21, respectively, we show that when the net reproductive number
the extinction equilibrium E
0 is globally asymptotically stable; while if
, the model has a unique interior equilibrium which is globally attracting. When assuming the population breeds and disperses periodically with period 2, we get that if
, then the extinction equilibrium E
0 will be globally asymptotically stable. If
, then the model has a unique non-trivial periodic solution, which is globally attractive.
Bifurcation diagrams conclude that for low birth rates, the periodic strategy has an average adult population that is larger than that of the constant, while for large birth rates, the opposite happens. This indicate that seasonal breeding is advantageous for low birth rates and deleterious for high birth rates.
Finally, we consider the notion of using weak non-linearities as opposed to strong non-linearities for the survivorship functions in our models. In Section 3.2, we demonstrated bifurcation diagrams for Beverton–Holt non-linearities. The results indicate the existence of a periodic solution which emanates from the periodic environment. When replacing the Beverton–Holt non-linearities with Ricker-type non-linearities, the dynamics become much richer including chaotic dynamics. The data collected from 2004 to 2009 on our motivating population of green tree frogs indicates that the population oscillates and the length of the oscillation is 1 year. This suggests that weak non-linearities are likely to describe the type of competition occurring in this population and the fact that oscillation in this population arises from seasonality.
Acknowledgements
The work of A.S. Ackleh and P. Zhang was supported in part by the National Science Foundation under grant # DUE-0531915. The work of A.S. Ackleh was also supported in part by the National Science Foundation under grant # DMS-0718465. We would like to thank Dan Sutton and Tracey Robin for their participation in the summer research project related to the work in this paper. We would also like to thank the referees for their useful comments and suggestions.
References
- Ackleh , A. S. and Chiquet , R. A. 2009 . The global dynamics of a discrete juvenile-adult model with continuous and seasonal reproduction . J. Biol. Dyn. , 3 : 101 – 115 .
- Ackleh , A. S. and De Leenheer , P. 2008 . Discrete three-stage population model: Persistence and global stability results . J. Biol. Dyn. , 2 : 415 – 427 .
- Ackleh , A. S. and Jang , S. R.-J. 2007 . A discrete two-stage population model: Continuous versus seasonal reproduction . J. Diff. Equ. Appl. , 13 : 261 – 274 .
- Blaustein , A. R. , Wake , D. B. and Sousa , W. P. 1994 . Amphibian declines: Judging stability, persistence, and susceptibility of populations to local and global extinctions . Conserv. Biol. , 8 : 60 – 71 .
- Carr , L. W. and Fahrig , L. 2001 . Effect of road traffic on two amphibian species of differing vagility . Conserv. Biol. , 15 : 1071 – 1078 .
- Caswell , H. 2001 . Matrix Population Models , 2 , Sunderland : Sinauer .
- Cox , G. W. 1985 . The evolution of avian migration systems between temperate and tropical regions of the new world . Amer. Nat. , 126 : 451 – 474 .
- Cushing , J. M. 1998 . “ An Introduction to Structured Population Dynamics ” . Philadelphia : SIAM .
- Cushing , J. M. and Yicang , Z. 1994 . The net reporductive value and stability in matrix population models . Nat. Res. Model. , 8 : 297 – 333 .
- DiMilla , P. , Barbee , K. and Lauffenburger , D. A. 1991 . Mathematical model for the effects of adhesion and mechanics on cell migration speed . Biophys. J. , 60 : 15 – 37 .
- DiMilla , P. , Stone , J. A. , Quinn , J. A. , Albelda , S. M. and Lauffenburger , D. A. 1993 . Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength . J. Cell Biol. , 122 : 729 – 737 .
- Dingle , H. 1982 . Function of migration in the seasonal synchronization of insects . Entomol. Exp. Appl. , 31 : 36 – 48 .
- Dole , J. W. 1965 . Summer movements of adult leopard frogs, Rana Pipiens Schreber, in northern Michigan . Ecology , 46 : 236 – 255 .
- Elaydi , S. 2005 . An Introduction to Difference Equations , 3 , New York : Springer .
- Elzer , K. L. , Heitzman , D. A. , Chernin , M. I. and Novak , J. F. 2008 . Differential effects of serine proteases on the migration of normal and tumor cells: Implications for tumor microenvironment . Integr. Cancer Ther. , 7 : 282 – 294 .
- Gibbs , J. P. 1998 . Amphibian movements in response to forest edges, roads, and streambeds in Southern New England . J. Wildl. Manage. , 62 : 584 – 589 .
- Grayson , K. L. and Wilbur , H. M. 2009 . Sex- and context-dependent migration in a pond-breeding amphibian . Ecology , 90 : 306 – 312 .
- Halley , J. M. , Oldham , R. S. and Arntzen , J. W. 1996 . Predicting the persistence of amphibian populations with the help of a spatial model . J. Appl. Ecol. , 33 : 455 – 470 .
- Kirkland , S. , Li , C. and Schreiber , S. J. 2006 . On the evolution of dispersal in patchy landscapes . SIAM J. Appl. Math. , 66 : 1366 – 1382 .
- Levey , D. J. and Stiles , F. G. 1992 . Evolutionary precursors of long-distance migration: Resource availability and movement patterns in neotropical landbirds . Am. Nat. , 140 : 447 – 476 .
- Pham , L. , Boudreaux , S. , Karhbet , S. , Price , B. , Ackleh , A. S. , Carter , J. and Pal , N. 2007 . Population estimates of Hyla Cinerea (Schneider)(green tree frog) in an urban environment . Southeast. Nat. , 6 : 203 – 216 .
- Pilliod , D. S. , Peterson , C. R. and Ritson , P. I. 2002 . Seasonal migration of Columbia spotted frogs Rana luteiventris among complementary resources in a high mountain basin . Can. J. Zool. , 80 : 1849 – 1862 .
- Pope , S. E. , Fahrig , L. and Gray Merriam , H. 2000 . Landscape omplementation and metapopulation effects on leopard frog populations . Ecology , 81 : 2498 – 2508 .
- Roff , D. A. 1986 . The evolution of wing dimorphism in insects . Evolution , 40 : 1009 – 1020 .