Abstract
Influenza A virus evolves through two types of evolutionary mechanisms – drift and shift. These two evolutionary mechanisms allow the pathogen to infect us repeatedly, as well as occasionally create pandemics with large morbidity and mortality. Here we introduce a novel model that incorporates both evolutionary mechanisms. This necessitates the modelling of three types of strains – seasonal human strains, bird-to-human transmittable H5N1 strains and evolved pandemic H5N1 strain. We define reproduction and invasion reproduction numbers and use them to establish the presence of dominant and coexistence equilibria. We find that the amino acid substitution structure of human influenza can destabilize the human influenza equilibrium and sustained oscillations are possible. We find that for low levels of infection in domestic birds, these oscillations persist, inducing oscillations in the number of humans infected with the avian flu strain. The oscillations have a period of 365 days, similar to the one that can be observed in the cumulative number of human H5N1 cases reported by the World Health Organization (WHO). Furthermore, we establish some partial global results on the competition of the strains.
1. Introduction
The world is recovering from the most recent flu pandemic, the so-called ‘swine flu’ H1N1 virus emerged in Mexico and swept across the planet. The relative mildness of the ‘swine flu’ pandemic, which generated disease-induced mortality comparable to the one of seasonal flu Citation37, should not prevent us from keeping the dangers of pandemic influenza in focus, as the three big pandemics in 1918, 1957 and 1968 combined took the lives of an estimated 40–50 million people. What makes this supposedly ‘mild’ pathogen, which we experience every year, capable of so much devastation? The answer lies in the unique evolutionary capabilities of the flu virus. The pathogen that causes human influenza evolves through two types of evolutionary mechanisms – drift and shift. Drift describes small and gradual changes in the surface proteins (antigens) of the virus through random mutational processes. Although each strain is believed to impart permanent immunity to the host Citation5, drift allows the pathogen to evade our immune response and infect repeatedly. Since the newly infectious strain is structurally similar to the previous one, our immune system is still quite effective against the new strain and thus capable of protecting us against serious health risk.
Shift evolution occurs when an influenza virus of subtype that normally does not infect humans, becomes adapted to the human population. Influenza viruses of different subtypes infects many other species – predominantly birds both domestic and wild, also pigs, horses, marine mammals, cats and others Citation6. Differences in the surface proteins prevent these viruses from jumping across the species barrier and causing infection in humans Citation19. However, highly pathogenic H5N1 viruses have succeeded in crossing the species barrier and have started infecting humans, creating conditions for shift evolution to occur. First human infections with the H5N1 subtype were reported in Hong Kong in 1997 Citation39. As of March 2011, WHO has reported 526 cases with 311 deaths – mortality of 59% Citation40. Most infections this far are predominantly from bird-to-human transmission. The highly pathogenic H5N1 can become pandemic among humans if it acquires a highly efficient human-to-human transmission mechanism, while retaining high pathogenicity. This can happen if an H5N1 strain can exchange genetic components via reassortment with seasonal human-to-human transmittable influenza strain within a common host. The shift mechanism gives rise to a new evolved strain. A novel strain, obtained through shift evolution, is structurally different from its parent strains and represents adaptation of new influenza subtype to humans with potentially dangerous consequences.
In this article, we introduce a new model that incorporates both the drift and the shift mechanisms of influenza A evolution. Those two influenza A evolution processes were modelled separately. The drift evolution in seasonal influenza was first modelled as a partial differential equation in Citation29. The shift expected in avian influenza was recently modelled in a sequence of articles by Iwami et al. Citation13 Citation14 Citation15. We draw on these results to build the model that incorporates both drift and shift as evolution mechanisms of influenza. The main question that we address is how the presence of antigenic drift in human influenza impacts the dynamics and the competition in the system human influenza–avian influenza.
The threat of a pandemic caused by H5N1 has motivated a stream of research on avian influenza. Mathematical modelling is at the core of this development, providing valuable insights on epidemic scenarios and control strategies in case an influenza pandemic occurs. Many of these models, particularly those with elaborate realistic features, are necessarily simulational Citation7 Citation8 Citation22 Citation27. The spread of pandemic influenza has also been explored with deterministic and stochastic metapopulation-type multi-city models Citation3 Citation4 Citation9. All these models on pandemic preparedness and containment are focused on the human population. Other models study the distribution of the avian influenza virus in wild birds and domestic poultry. Much of the transmission of avian influenza, particularly transmission among wild birds, is environmental. Environmental transmission potentially plays an important role in generating the oscillatory pattern, observed in data Citation2. Most animal-based models focus on domestic birds. Spatial farm-based model treating poultry-farms as units Citation20, SIR model for within flock transmission of H5N1 Citation36, and a model with LPAI and HPAI Citation23 were considered. Early models basically ignored the complexities of the multi-species avian influenza transmission. However, recently models with at least two species have appeared Citation1 Citation10 Citation13 Citation14 Citation15. Time dependent optimal control strategies for the prevention of avian influenza pandemic were discussed in Citation17. Spatial spread of avian influenza was the focus of Citation16 Citation18 Citation21.
This article is organized as follows. In Section 2 we introduce a novel influenza A model with both drift and shift. As the model involves three strains, in Section 3 we investigate separately single strain equilibria and introduce each strain reproduction number. Section 4 is devoted to coexistence equilibria. Two scenarios are investigated: continuous mutation or instantaneous mutation into a pandemic strain. Section 5 presents the analysis of the local stability of equilibria. Section 6 derives conditions for global stability. We summarize our results in Section 7.
2. A deterministic evolutionary model of avian flu
In this section we introduce an evolutionary model of influenza A which includes for the first time, both the drift and shift evolutionary mechanisms of influenza A.
Shift in the evolution of influenza A occurs when strains which normally circulate in other species become adapted to humans. We model the transition domestic birds humans of influenza A H5N1 subtype. Highly pathogenic avian influenza (HPAI) strains from the subtype H5N1 are currently endemic in some domestic bird populations Citation23. They have evolved sufficiently to infect humans through bird
human transmission. This defines the current pre-pandemic scenario which does not involve human-to-human transmission of H5N1. To model this scenario, we model the infection in the domestic bird population. Denote by B(t) the number of healthy domestic birds at time t, and by Y(t) the number of infected domestic birds. We model the epidemic among the domestic birds by an SI model that assumes no recovery, based on observed high mortality and culling. The model is given by
To introduce the human epidemic model, let S(t) be the number of susceptible human individuals and I(t) the number of those infected with human influenza. We model the drift evolution of influenza by a(τ) the number of the amino acid substitutions from the strain circulating at time t, where τ is time-since-recovery. We assume the rate of change of a(τ) to be constant a′(τ)=k Citation29 Citation32. With this notation, the quantity r(a, t) represents the density of individuals recovered from human influenza strain that differs by a(τ) amino acid substitutions from the strain at time t, that is
Table 1. List of dependent variables.
The human epidemic model is built on the foundation of Pease's influenza drift model Citation29. Pease constructed his model without demographics to show that due to the drift process influenza A can persist, even if there is no recruitment into the population. In contrast to Pease's model, we include a susceptible class and demography. This is necessary since the H5N1 ‘spill over’ infection has already been continuing for more than 10 years, so we are looking at long time-scales for the demographics to be ignored. Susceptible humans are recruited at birth rate Λ, and die at a natural death rate μ. Susceptible individuals become infected with seasonal human flu at a rate β, with avian flu from birds with rate β Y , and with pandemic flu, when such occurs at rate β Z . Recovered individuals from seasonal human flu become reinfected at a rate γ(a) and return to the human flu-infected class. Those infected with human influenza can also become infected with avian influenza at a rate β J . Infected with seasonal flu individuals recover at a rate α. Recovered individuals are assumed to become infected with avian flu or pandemic flu at the same rates as susceptible: β Y and β Z , respectively. Individuals infected with avian influenza leave the class at a rate ν which includes recovery and death. Since nearly 60% die, and those who recover cannot be reinfected with avian influenza, those individuals leave the system. The exit rates from the jointly infected class and the class of those infected with pandemic flu is ν J and ν Z , respectively. Pandemic influenza emerges at a mutation/reassortment rate ρ. We will assume that it may occur on continuous bases ρ≠0 or instantaneously, in which case ρ=0 and the process is modelled by Dirac delta function. Parameters are listed in . The human part of the influenza epidemic model with drift and shift is given by
Table 2. List of parameters.
In this model, we consider an invasion scenario. This scenario focuses on the early stages of the invasion of the avian strain as human-to-human transmittable strain. This scenario is characterized by lack of drift evolution of the pandemic strain — that is, upon recovery from the human-to-human transmittable strain, recovered individuals are protected from future infection with it. We model that by lumping the recovery and death into the per capita rate ν Z .
In the next section, we investigate the single strain equilibria and introduce the reproduction numbers of the seasonal flu, avian flu and pandemic flu.
3. Single strain equilibria
Equilibria are time-independent solutions of the system. Since the avian dynamics is independent of the human dynamics, we first consider the equilibria in the avian system. Those satisfy the system
This system always has the avian population disease-free equilibrium, obtained by setting Y=0 and given by
The equilibria of the human dynamics satisfy the following system:
The system always has the disease-free equilibrium for which I=0, I
b=0, J=0, and Z=0. These conditions, considering the equations above, also imply that r(a)=0. We note that the human population disease-free equilibrium exists only if the avian flu strain has not established itself in the bird population. Thus, we can have scenario in which in the long run both the avian and the human populations are disease-free, but we cannot have a scenario that the disease has established itself in the avian population but the human population is disease-free. Consequently, we have the following disease-free equilibrium of the full system Equation(1) and Equation(2)
:
To obtain the equilibria we integrate the differential expression in EquationEquation (5) to get
To see the existence of a dominance endemic equilibrium of the human influenza, assume that . Then
Proposition 3.1
Let
. Then, there is at least one dominance endemic equilibrium of the human influenza
We note that all our simulations in the case resulted in a unique endemic equilibrium of the human flu strain. We illustrate this scenario in . When
EquationEquation (8)
may or may not have solutions. It will have solutions if the bifurcation at the critical value of the reproduction number
(obtained when I=0) is backward bifurcation. If we assume that EquationEquation (8)
defines β implicitly as function of I, and we differentiate in EquationEquation (8)
with respect to I, then the bifurcation at the critical value of the reproduction number
will be backward if and only if β′(0)<0. Replacing
with
, differentiating implicitly with respect to I and setting I=0 we get the following expression for β′(0):
We summarize this result in the following proposition.
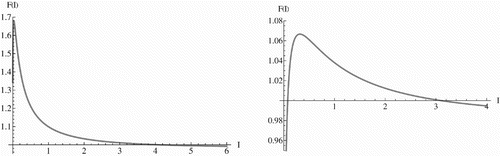
Figure 1. The left figure shows a graph of the function F(I) in the case ℛ1>1. The graph clearly intersects the horizontal line y=1 at around I*≈ 3.5. The right figure shows a graph of the function F(I) in the case ℛ1<1. The graph clearly intersects the horizontal line y=1 at two points I*1 ≈ 0.1 and I 2*≈ 3.0. The parameters for the two figures are taken as Λ=13.3 births per year, μ=0.0133 (years)−1, α=52 (years)−1, k=3.6 (years)−1, γ(a)=γ a with γ=0.005 (amino acid substitution)−1 (years)−1 (person)−1. The left figure has β=0.07 cases per person per year, while the right figure has β=0.02 cases per person per year. The reproduction numbers for the two figures are ℛ1=1.34 for the left figure, and ℛ1=0.38 for the right figure.
Proposition 3.2
Assume
. Let
. Then, there may be no dominance endemic equilibrium of the human influenza. If backward bifurcation occurs, that is if condition
Equation(12)
is satisfied, then there are at least two endemic equilibria
Inaba Citation12 established for Pease's model Citation29, which does not involve demography, that it has a unique endemic equilibrium. That endemic equilibrium also cannot be explicitly computed. Even with demography and a susceptible class incorporated, Thieme and Yang Citation35 find that there is a unique endemic equilibrium in an influenza epidemic model with variable reinfection rate. Thus, our results of occurrence of backward bifurcation in the human influenza model somewhat contrast the results in these two studies. Part of the reason is the constitutive form of γ(a). Pease Citation29 fitted the percentage infected with a strain from a given year against the year when the immunizing strain was isolated for several studies, and found that this relationship is linear. Thus, he assumed that . However, the data he used were for a period of time spanning eight years. It is probably unlikely that this relationship will persist since the immunized individuals will be eventually completely naive with respect to the challenging strain. Thus, it is reasonable to assume that γ(a) is an increasing but bounded function:
Proposition 3.3
Assume that γ(a) is increasing and bounded so that
. If
then, there is a unique dominance endemic equilibrium of the human influenza
The number of humans infected with the avian flu will be nonzero at equilibrium only if the avian flu is at nontrivial equilibrium in the bird population. In the absence of human flu, that is I=0, the nontrivial equilibrium of the avian flu strain in the human population will have I b≠0 with J=0 and Z=0. We summarize the result on the avian flu strain in humans infection equilibrium in the following proposition.
Proposition 3.4
Assume
. Then, there exists a unique dominance equilibrium of the avian flu virus in the human population:
A couple of remarks are in order. First, the equilibrium of the bird-to-human transmittable avian flu strain is unique and can be explicitly computed. Second, it exists based solely on the assumption that guarantees persistence of the disease in the bird population; there is no additional reproduction number or threshold condition that gives its persistence in the human population. This is a result of the fact that if the avian flu strain has become established in the bird population, which happens if , then it is automatically established in the human population (a ‘spill over infection’). We conclude that, if the avian flu strain is endemic in the bird population, it will be endemic in the human population too.
We introduce the reproduction number of the human-to-human transmittable pandemic flu strain
There exists only one equilibrium of the pandemic avian strain which can be explicitly computed. We summarize that in the following proposition.
Proposition 3.5
If
then there is a unique dominance endemic equilibrium of the human-to-human transmittable avian influenza
4. Coexistence equilibria
The results in the previous section show that there could be three different strains that can exist in the human population independently: the human influenza, the bird-to-human avian influenza, and the pandemic avian influenza. In terms of coexistence, in the case of continuous mutation into the pandemic strain (ρ≠0), there are three possible scenarios: in absence of the human influenza, bird-to-human and pandemic avian influenza may coexist, in the absence of bird-to-human avian influenza, human and pandemic influenza may coexist, and all three strains may coexist. This case is investigated in the first subsection.
The current pre-pandemic scenario where the human influenza and bird-to-human strain coexist but there is no pandemic strain only occurs in model Equation(3) if the appropriate reassortment into a pandemic strain has not occurred, that is, if ρ=0. If the reassortment that creates the pandemic strain is spontaneous, then the conditions for invasion of the pandemic strain with ρ=0 are different. The case ρ=0 is investigated in the second subsection.
4.1 Coexistence in the case of continuous mutation (ρ≠0)
This section investigates the coexistence equilibria in the case when all parameters, including ρ are non-zero. Conditions for existence of coexistence equilibria depend on the reproduction numbers and invasion reproduction numbers. The invasion reproduction number of strain i at the equilibrium of strain j gives the number of secondary infections one strain-i infected individual will produce in a population where strain j is at equilibrium during his lifespan as infected. Mathematically, the invasion reproduction number of strain i at the equilibrium of strain j is computed similarly to the basic reproduction number, that is as a criterion for stability of the equilibrium of strain j. Reproduction numbers and invasion reproduction numbers are listed in .
Table 3. List of reproduction and invasion reproduction numbers and their interpretation.
The coexistence equilibria satisfy the system Equation(4). One can solve for I
b* and J*:
Case 1
Coexistence of bird-to-human transmittable and pandemic strains. In this case I*=0, that is, there is no human strain. We define the invasion reproduction number of the pandemic strain when the bird-to-human strain is at equilibrium:
Proposition 4.1
If
and the invasion reproduction number of the pandemic avian influenza strain
then there exists a unique coexistence equilibrium of bird-to-human and pandemic influenza strains
The proof of Proposition 4.1 is straightforward and is omitted.
Case 2
Coexistence of the human and pandemic strains. In this case, Y*=0. This also implies that I
b*=0 and J*=0. From the last equation in EquationEquation (4) we have
The following gives coexistence of human and pandemic influenza:
Proposition 4.2
Assume γ(a) is increasing and
. Assume
and
. Further, assume that the human strain can invade the equilibrium of the pandemic strain
and that the pandemic strain can invade the equilibrium of the human strain,
. Then there exists at least one coexistence equilibrium of the human and pandemic influenza strains
Case 3
Coexistence of all three strains. With ρ≠0 if the human and the bird-to-human strains coexist, there always exists the pandemic strain too. This gives coexistence of all three strains. The analysis of the full coexistence equilibrium is similar to the analysis of the coexistence equilibrium of the human and bird-to-human strains. As in the case of coexistence of the human and pandemic strains, we integrate the differential equation in system Equation(4) and add the equations for the susceptible individuals, individuals infected with the human strain and recovered individuals which gives:
Proposition 4.3
Assume
and
. Further assume that the pandemic strain can invade the bird-to-human strain, that is
. Finally assume that the human strain can invade the equilibrium of the bird-to-human and the pandemic strains, that is
. Then there exists an equilibrium of all three strains:
Proof
Denote by
As in the case of human influenza equilibria, we cannot rule out the possibility of multiple coexistence equilibria. In the case ρ≠0, there is no condition for invasion of the pandemic strain. Because of the continuous mutation, the pandemic strain exists, and is non-zero, as long as both the bird-to-human strain and the human strain exist.
4.2 Coexistence in spontaneous mutation ρ=0 – pre-pandemic scenario and invasion of the pandemic strain
In this subsection, we investigate the current pre-pandemic scenario, and the invasion of the pandemic strain.
Case 1
Current pre-pandemic scenario: coexistence of bird-to-human and human strains. Occurs in model
Equation(4)
only if ρ=0. Currently, two types of strains circulate in the human population – regular seasonal strains, and we include in this category the Swine flu strains, and a bird-to-human transmittable avian influenza strain. The bird-to-human cases occur through a ‘spill over infection’ from domestic birds. Because model Equation(3)
includes a continuous mutation from the coinfected state into the pandemic strain, when the human and the bird-to-human strains are present, that necessarily leads to the presence of the pandemic strain, if ρ≠0. Thus, the current pre-pandemic scenario is not part of the model Equation(3)
if all parameters are nonzero. However, the only reason that the pandemic avian influenza strain has not occurred is the absence of an appropriate mutation into such a strain. To have the current pre-pandemic situation, we should have ρ=0. In this subsection, we investigate the equilibria with ρ=0, and the distinctly different scenario when the pandemic strain has to invade the pre-pandemic strains to become established.
When ρ=0, possibility for an equilibrium exists with Z=0. Integrating the differential equation in system Equation(4) and adding the three equations for the human flu we arrive at:
Proposition 4.4
Assume ρ=0,
and
. Assume further that the invasion number of the human influenza at the bird-to-human influenza
. Then there is a pre-pandemic equilibrium
Case 2
Invasion of the pandemic strain. Occurs in model
Equation(4)
only if ρ=0. In this case, we are looking for a solution with Z≠0. To express Z as a function of I, we add the susceptible, infected and recovered from human flu in system Equation(4)
which gives:
As in Proposition 4.2 a function can be defined. If we assume that
, we have that
We can now formulate the theorem that gives coexistence of all three strains, after invasion of the pandemic strain of the pre-pandemic scenario.
Proposition 4.5
Assume ρ=0,
and
. Assume further that the invasion number of the human influenza at the bird-to-human influenza
and that the invasion number of the pandemic influenza at the bird-to-human influenza
. Furthermore, assume that the human strain can invade the equilibrium of the pandemic and bird-to-human strains:
and that the pandemic strain can invade the equilibrium of the bird-to-human and human strains:
. Then there exists an equilibrium in which all strains coexist:
Conditions for existence of equilibria are summarized in .
Table 4. List of equilibria and conditions for existence.
5. Local stability of equilibria
In this section we derive conditions for local stability of equilibria. Those conditions typically depend on the reproduction numbers and the invasion reproduction numbers. To introduce the linearized system we define the perturbations: ,
,
,
,
,
,
,
, where the quantities with superscript ‘*’ denote any of the equilibria. We also use
and
. Looking for exponential solutions with growth rate λ, we arrive at the following linear eigenvalue problem. The system for the bird population takes the form
Proposition 5.1
The equilibrium
is locally asymptotically stable if
and unstable otherwise. The endemic equilibrium
is locally asymptotically stable whenever it exists, that is, if
.
The linearized system for the human population takes the form:
Proposition 5.2
The bird–human system disease-free equilibrium ℰ0 is locally asymptotically stable if the following inequalities all hold:
In what follows we investigate the local stability of the single-strain equilibria.
5.1 Human influenza equilibrium
The human influenza equilibrium has the most complex stability pattern, as, because of the presence of the amino acid substitution structure a, the equilibrium can be destabilized and sustain oscillations are possible Citation34. Recently, the presence of oscillations in Pease's model has been established rigorously, and through simulations Citation24. We consider the stability of the human influenza equilibrium in the context of the presence of the remaining strains, and refer the reader to Citation24 for a more detailed discussion of the human influenza only case. In the human equilibrium case we have Y*=0, I
b*=0, J*=0 and Z*=0, but I*≠0 and r*(a)≠0. To guarantee the local stability of the equilibrium we assume throughout this subsection that
. We consider system Equation(32)
with
and Y*=0. The eigenvalues of the system are
,
,
, and all λ’s that satisfy the human influenza system:
We note that if λ is taken different from all other eigenvalues, that implies y=0 and z=0. Hence, we have . Solving the first equation for x, the last equation for w and substituting in the second equation, we obtain the following characteristic equation
If the pandemic strain can invade a given equilibrium of the human strain, that is if for that I*, then that equilibrium is unstable.
Proposition 5.3
Assume the pandemic strain can invade a given equilibrium of the human strain, that is if
and if
does not belong to the spectrum Σ, then that equilibrium of the human strain is unstable. In addition, if the bird-to-human strain can persist, that is if
and
does not belong to Σ, then any equilibrium of the human influenza only is unstable.
In what follows we will assume that the pandemic strain cannot invade the given equilibrium of the human strain, that is for that I*, in addition to the assumption that
. Then the stability of the human strain equilibrium is controlled by the solutions of the characteristic Equationequation (35)
. In investigating the characteristic Equationequation (35)
a key role is played by the equation in I for the equilibrium. EquationEquation (8)
given by
Recall that for there are multiple simple equilibria, defined in Proposition 3.2 under the assumption that
. For the lower equilibrium
inequality Equation(38)
does not hold, while for the next equilibrium
inequality Equation(38)
holds. Thus, the lower equilibrium
is unstable.
We turn to investigating the stability of equilibria for which EquationEquation (38) does hold. We investigate the case when
is a constant and does not depend on a and refer to Citation24 for the general case. The characteristic equation of the corresponding ordinary differential equation model when γ is constant, can be obtained from EquationEquation (35)
, which simplifies to:
Proposition 5.4
Assume
and
. Then the endemic equilibrium for which Equation
Equation(38)
holds is locally asymptotically stable.
Proof
Clearly, a
2>0. Condition Equation(38) implies that a
0>0. This implies that
The proposition below follows from the results in Citation24.
Proposition 5.5
Assume
and γ(a) depends on a. Then the endemic equilibrium for which Equation
Equation(38)
holds can become unstable in the sense that Equation
Equation(35)
can have complex solution λ with positive real part. In this case sustained oscillations are possible.
5.2 Pandemic influenza strain only
When the pandemic influenza strain is the only strain present, then I*=0, Y*=0, r*(a)=0, and I
b=0, but Z*≠0. We assume again that , which implies that one of the eigenvalues
. We will use the linearized human system Equation(32)
with I*=0, Y*=0, r*(a)=0, and I
b=0, but Z*≠0. From the sixth equation we obtain
. The equation for i gives an eigenvalue
Theorem 5.6
Assume
and that the human strain cannot invade the equilibrium of the pandemic strain, that is
. Then the pandemic influenza equilibrium is locally asymptotically stable. If
then the pandemic influenza equilibrium is unstable.
5.3 Bird-to-human strain only
In this section, we investigate the conditions for local stability of the bird-to-human equilibrium. In this case I*=0, r*(a)=0, J*=0, Z*=0, but Y*≠0 and . We assume that
, a condition that guarantees that Y*≠0. We use system Equation(32)
with I*=0, r*(a)=0, J*=0, Z*=0, but Y*≠0 and
.
From the equation for i, we have the first eigenvalue:
Theorem 5.7
Assume
. If the human and the pandemic strains cannot invade the equilibrium of the bird-to-human strain, that is if
5.4 Avian and pandemic strain coexistence equilibrium
In the absence of the human strain, we can expect that the avian and the pandemic strains coexistence equilibrium is stable. Indeed, in this case we have I*=0, r*(a)=0, J*=0, but Y*≠0, I
b≠0, and Z*≠0. We use system Equation(32) with I*=0, r*(a)=0, J*=0, but Y*≠0, I
b≠0, and Z*≠0.
From the equation for i we have the eigenvalue , which is negative if and only if the human influenza strain cannot invade the equilibrium of the bird-to-human and pandemic strains:
. If we look at eigenvalues that differ from λ1, then i=0, which, in turn implies that w(a)=0. From the equation for j we have that either
or j=0. From the equations for x and z we obtain the following quadratic characteristic equation
Theorem 5.8
Assume
and
. Assume further that the human influenza cannot invade the equilibrium of the bird-to-human and pandemic coexistence strains, that is, assume
. Then the equilibrium of the bird-to-human and pandemic strains is locally asymptotically stable. If
it is unstable.
5.5 Avian and human strains (ρ=0)
Investigations on the Pease's model for human strain evolution through drift have shown that the presence of amino acid evolution can destabilize the equilibrium, and sustained oscillations are possible Citation24. It is expected that in the presence of the bird-to-human strain, at least when the prevalence of infection in birds is relatively low, the oscillations would continue to exist. A more interesting outcome of the stability analysis here is that bird-to-human strain in a sufficiently high level of prevalence has the potential to stabilize the human strain equilibrium, particularly when coinfection is very low, as it is in reality. We consider the linearized system Equation(32) for the coexistence equilibrium of the bird-to-human and human strains with J*=0 and Z*=0 (ρ=0). For the bird-to-human strain to exist, we need that
, so that y=0. In the equation for z, we use
Theorem 5.9
Assume
. If
Proof
We denote the left-hand side of the characteristic Equationequation (45) by F(λ) and the right-hand side by G(λ). For λ with
, inequality Equation(49)
implies that
If inequality Equation(48) fails for the equilibrium ℰ6, then that equilibrium is unstable. We have the following result.
Proposition 5.10
If
and
or inequality
Equation(48)
fails, then equilibrium ℰ6
is unstable.
Proof
If , then there is a positive eigenvalue, and the equilibrium ℰ6 is unstable. Assume now inequality Equation(48)
fails. Then F(0)<G(0). On the other hand for λ real,
as
, while
. Thus, the equation
has a real positive solution. ▪
Example
In what follows, we will exhibit a specific example in which sustain oscillations occur. We make the following assumptions:
Assumption 5.1
Assume
| • | The reinfection rate is a step function | ||||
| • | Infection of susceptible and recovered individuals occurs at the same rate: | ||||
| • | Coinfection in humans does not occur, that is β J =0. | ||||
In the case there is a unique equilibrium that can be explicitly computed. The density of the recovered individuals in that equilibrium is given by
This is a transcendental equation. We can use the analytical methods in Citation38 to show that an equation of that form can exhibit Hopf bifurcation. Those methods can be used for Equationequation (58) to establish this result. Here, we take an approach that will allow us to determine parameter values that give periodic solutions Citation24
Citation28. Let
. Separating the real and imaginary part in the equation above we obtain the following system
Figure 2. The left figure shows parametric plot in the (γ I*,αγ I*) plane. The right figure shows α as a function of w. The plots are made with w∈[π/32, π/16].
![Figure 2. The left figure shows parametric plot in the (γ I*,αγ I*) plane. The right figure shows α as a function of w. The plots are made with w∈[π/32, π/16].](/cms/asset/e3d68078-04f8-4292-a732-4670e250af49/tjbd_a_573866_o_f0002g.gif)
Table 5. List of parameter values for simulation. See Citation25 for discussion.
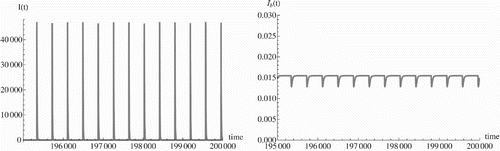
We construct a finite difference method along the characteristic lines. The characteristic lines of system Equation(2) are the lines a=kt. Approximation of the partial derivative is done similarly as in the case k=1; however, the step in a is k times the step in t. The parameter k can be obtained from Citation32 and is estimated there as k=3.6 years−1. It turns out that for realistic values of the parameters, as discussed in Citation25, the oscillations in the human influenza still remain. Although we have showed that for large enough prevalence of avian influenza in birds and humans, stabilization may occur, it seems that for the parameters and values we obtain from the WHO data in Citation25 stabilization does not occur. We present the results of simulations in . We note that the oscillations have period of 365 days. The period of the oscillations appears to be equal to A
k
.
6. Global results on coexistence and competitive exclusion
The results in the previous section are local, that is, if an equilibrium is locally asymptotically stable, solutions only converge to it, if the initial conditions are sufficiently close to the equilibrium. In this section, we derive global results on persistence and extinction of the strains. As before, we strive to derive those results based on the reproduction and invasion reproduction numbers.
We begin with the global stability of the disease-free equilibrium.
Theorem 6.1
Assume
and
and
. Then the disease-free equilibrium is globally asymptotically stable.
We note that the condition for the global stability of the disease-free equilibrium cannot be omitted. In other words, global stability cannot be established based only on the assumptions
,
,
. As we saw earlier, if the condition
is not satisfied, backward bifurcation and subthreshold equilibria may exist. In this case, the disease-free equilibrium is not globally asymptotically stable.
Proof
To see the global stability of the disease-free equilibrium, we first show that Y(t)→0 as t→∞. From system Equation(1) we know that
. Hence, given ε>0,
for t>T. From the second expression in EquationEquation (1)
, and after shifting the systems, we have:
Before we consider the global results for the whole system, we investigate the global stability of the bird system Equation(1). This is established in the following Proposition.
Proposition 6.2
Assume
. The system
Equation(1)
has no periodic solutions, homoclinic loops, or oriented phase polygons inside the first quadrant. Consequently, the equilibrium E
b
is globally stable.
Proof
We recall that E b is locally stable. Dulac criterion implies the global stability. We introduce the notation:
The global stability of the equilibria of the domestic bird population, and the fact that the bird system is linked to the human system but it is not dependent on it, suggests that depending on the reproduction number we are essentially investigating two human systems Equation(2)
– one where Y=0, and another, where Y=Y*. The following Proposition states the global stability of the bird-to-human equilibrium under the very restrictive conditions that the reproduction numbers of the human and pandemic influenza are smaller than one. This proposition can be established with techniques similar to the ones used for the global stability of the disease-free equilibrium.
Proposition 6.3
Assume
and
and
. Then the bird-to-human equilibrium is globally asymptotically stable.
Next we formulate and establish a much more serious global stability result, one that we establish with conditions similar to the conditions for the local stability of the bird-to-human equilibrium. Those state that the human and pandemic strains would not persist, if their invasion reproduction numbers are smaller than one. We note that in the Theorem below, we do not assume that the reproduction numbers of human and pandemic influenza are smaller than one.
Theorem 6.4
Assume
. Assume that human influenza and pandemic influenza cannot invade the equilibrium of the bird-to-human influenza, that is, assume
and
. Assume further that
and
. Then the bird-to-human equilibrium is globally asymptotically stable.
Proof
To see the claim, we integrate the partial differential equation directly, and add the equations for S, I, and R. If , we obtain the following differential inequality for N
H:
Proposition 6.5
Assume
. Assume that human influenza can invade the equilibrium of the bird-to-human influenza, that is assume
while pandemic influenza cannot invade the equilibrium of the bird-to-human strain, that is and
. Assume further that
and ρ=0. Then the bird-to-human and human influenza persist, while the pandemic strain cannot persist.
Proof
We sketch the proof here. For a more detailed proof of persistence, we refer to Citation26. Similar reasoning as in Theorem 6.4 leads to Z(t)→0 as t→∞. We will say that human influenza persists, if it is uniformly strongly persistent, that is, if there is η>0, such that
(1) It is uniformly weakly persistent, that is, there is η>0 independent of the initial conditions such that | |||||
(2) The solutions of the system Equation(2) | |||||
7. Discussion
Influenza A has the unique capability to evolve through both drift and shift and allow the virus to infect us repeatedly, as well as reassort with variants that normally infect other species to generate a novel strain capable of creating pandemics with high mortality. In this paper we introduce for the first time a model that incorporates both the drift and the shift mechanisms of evolution of influenza A. The model combines strains from the seasonal human flu that evolves through drift with strains of the H5N1 subtype which normally affect avian species but recently have started infecting humans as well. This is the pre-pandemic scenario which is currently in place. The H5N1 subtype can create a pandemic among humans if strains from that subtype become fully human-to-human transmittable. We incorporate this hypothetical pandemic scenario also in the model. Thus, the model involves three possible strains: human flu strain, avian strain that circulates in domestic birds and through ‘spill over’ infection in humans, and a hypothetical pandemic strain. We compute the reproduction numbers of all three strains. We find the following novel results related to the dynamics of this three-strain system.
(1) Regarding human influenza, we find that there could be more than one dominance equilibrium corresponding to human influenza, some of which may be arising through backward bifurcation. Pease's drift influenza model has been studied in several articles Citation11 Citation12 Citation24 but multiple equilibria associated with influenza drift model are found here for the first time. | |||||
(2) Coexistence of all three strains in the case ρ=0 will occur if a pandemic strain emerges through a spontaneous mutation, and invades the equilibrium of the human and bird-to-human strains. Thus, for a pandemic to occur, it is necessary that invasion number of the pandemic strain at the human and bird-to-human coexistence equilibrium to be larger than one. The value of this invasion number is inversely proportional to the level of infection in birds, and the seasonal flu levels in humans. This implies that control measures directed to eliminating or reducing those infections essentially increase the invasion capabilities of the pandemic strain and may facilitate a pandemic. This result is similar to the one obtained by Iwami Citation14, that the elimination of the avian influenza in birds can lead to more infections of humans by the pandemic strain and is a manifestation of the competition among the strains. | |||||
(3) The disease-free equilibrium is locally stable if all three reproduction numbers are below one. The disease-free equilibrium is globally stable if all three reproduction numbers are below one and | |||||
(4) In a recent article, Magal and Ruan Citation24 find that the human influenza equilibrium can become destabilized by the drift evolution and oscillations are possible in the Pease's model. The same holds for the human strain equilibrium in our model. Furthermore, we show that the current pre-pandemic coexistence equilibrium, in which the human strain coexists with the bird-to-human strain, can also become destabilized and oscillations are possible. Our results also determine that sufficiently high levels of infection in birds (see, inequality Equation(49) | |||||
We also obtain several novel global stability and persistence results in multi-strain systems. We note that these global stability results are established despite the presence of oscillations. In particular, we show the following.
(1) If the reproduction number of the bird-to-human strain is above one, but the other two reproduction numbers are below one, then the bird-to-human equilibrium is globally stable. We also establish a stronger result: if the invasion reproduction numbers of the human strain and the pandemic strain at the equilibrium of the bird-to-human strain are below one (with some additional conditions), then the bird-to-human strain equilibrium is globally stable. | |||||
(2) Finally we establish persistence of the human strain and bird-to-human strain and extinction of the pandemic strain if the invasion reproduction number of the pandemic strain at the bird-to-human equilibrium is below one, while the invasion reproduction number of the human strain is above one. As we show this persistence may be in an oscillatory way. | |||||
Acknowledgements
The author acknowledges support from the NSF under grant DMS-0817789. The manuscript has benefitted from discussions with Manojit Roy. The author thanks Horst Thieme for pointing her to reference Citation24. Furthermore, the author thanks two knowledgeable referees, who contributed enormously to the improvement of the manuscript.
References
- Agusto , F. B. and Gumel , A. B. 2010 . Theoretical assessment of avian influenza vaccine . Dis. Cont. Dyn. Sys. , B 13 ( 1 ) : 1 – 25 .
- Breban , R. , Drake , J. M. , Stallknecht , D. E. and Rohani , P. 2009 . The role of environmental transmission in recurrent avian influenza epidemics . PLoS Comput. Biol. , 5 ( 4 ) : e1000346
- Colizza , V. , Barrat , A. , Barthelemy , M. , Valleron , A.-J. and Vespignani , A. 2007 . Modeling worldwide spread of pandemic influenza: baseline case and containment interventions . PLoS Med. , 4 ( 1 ) : 0095 – 0110 .
- Cooper , B. S. , Pitman , R. J. , Edmunds , J. and Gay , N. J. 2006 . Delaying the international spread of pandemic influenza . PLoS Med. , 3 ( 6 ) : 0845 – 0854 .
- Earn , D. J.D. , Doushoff , J. and Levin , S. A. 2002 . Ecology and evolution of influenza . Trends Ecol. Evol. , 17 ( 7 ) : 334 – 340 .
- Enserink , M. 2005 . Pandemic influenza: Global update . Science , 309 : 370 – 371 .
- Ferguson , N. M. , Cummings , D. A.T. , Cauchemez , S. , Fraser , C. , Riley , S. , Meeyai , M. , Iamsirithaworn , S. and Burke , D. S. 2005 . Strategies for containing an emerging influenza pandemic in Southeast Asia . Nature , 437 : 209 – 214 .
- Germann , T. C. , Kadau , K. , Longini , I. M. Jr and Macken , C. A. 2006 . Mitigation strategies for pandemic influenza in the United States . Proc. Natl Acad. Sci. USA , 103 ( 15 ) : 5935 – 5940 .
- Grais , R. F. , Ellis , J. H. and Glass , G. E. 2003 . Assessing the impact of airline travel on the geographic spread of pandemic influenza . Eur. J. Epidemiol. , 18 : 1065 – 1072 .
- Gumel , A. B. 2009 . Global dynamics of a two-strain avian influenza model . Int. J. Comput. Math. , 86 : 85 – 108 .
- Inaba , H. 1998 . “ Mathematical analysis for an evolutionary epidemic model ” . In Mathematical Models in Medical and Health Sciences , Edited by: Horn , M. A. , Simonett , G. and Webb , G. F. 213 – 236 . Nashville , TN : Vanderbilt University Press .
- Inaba , H. 2002 . “ Endemic threshold and stability in an evolutionary epidemic model ” . In Mathematical Approaches for Emerging and Reemerging Infectious Diseases: Models, Methods and Theory , Edited by: Casillo-Chavez , C. , Blower , S. , van den Driessche , P. , Kirchner , D. and Yakubu , A. A. Vol. 126 , 337 – 359 . New York : IMA, Springer .
- Iwami , S. , Takeuchi , Y. and Liu , X. 2007 . Avian-human influenza epidemic model . Math. Biosci. , 207 : 1 – 25 .
- Iwami , S. , Takeuchi , Y. , Korobeiniov , A. and Liu , X. 2008 . Prevention of avian influenza epidemic: What policy should we choose? . J. Theor. Biol. , 252 : 732 – 741 .
- Iwami , S. , Takeuchi , Y. and Liu , X. 2009 . Avian flu pandemic: Can we prevent it? . J. Theor. Biol. , 257 : 181 – 190 .
- Iwami , S. , Takeuchi , Y. , Liu , X. and Namaoka , S. 2009 . A geographical spread of vaccine-resistence in avian influenza epidemics . J. Theor. Biol. , 259 : 219 – 228 .
- Jung , E. , Iwami , S. , Takeuchi , Y. and Jo , T.-C. 2009 . Optimal control strategy for prevention of avian influenza pandemic . J. Theor. Biol. , 260 ( 2 ) : 220 – 229 .
- Kim , K. I. , Lin , Z. and Zhang , L. 2010 . Avian-human influenza epidemic model with diffusion . Nonlinear Anal. Real. World. Appl. , 11 : 313 – 322 .
- Kuiken , T. , Holmes , E. C. , McCauley , J. , Rimmelzwaan , G. F. , Williams , C. S. and Grenfell , B. T. 2006 . Host species barriers to influenza virus infections . Science , 312 : 394 – 397 .
- Le Menach , A. , Vargu , E. , Grais , R. F. , Smith , D. L. and Flahault , A. 2006 . Key strategies for reducing spread of avian influenza among commercial poultry holdings: lessons for transmission to humans . Proc. R. Soc. , B 273 : 2467 – 2475 .
- Liu , R. , Duvvuri , V. R.S.K. and Wu , J. 2008 . Sread pattern formation of H5N1-avian influenza and its implications for control strategies . Math. Model. Nat. Phenom , 3 ( 7 ) : 161 – 179 .
- Longini , I. M. , Nizam , A. , Xu , S. , Ungchusak , K. , Hanshaoworakul , W. , Cummings , D. A.T. and Halloran , M. E. 2005 . Containing pandemic influenza at the source . Science , 309 : 1083 – 1087 .
- Lucchetti , J. , Roy , M. and Martcheva , M. 2009 . “ An avian influenza model and its fit to human avian influenza cases ” . In Advances in Disease Epidemiology , Edited by: Tchuenche , J. M. and Mukandavire , Z. 1 – 30 . New York : Nova Science Publishers .
- Magal , P. and Ruan , S. 2010 . Sustained oscillation in an evolutionary epidemiological model of influenza drift . Proc. R. Soc. , A 466 : 965 – 992 .
- Martcheva , M. “ Avian influenza: modeling and implications for control ” . (in preparation)
- Martcheva , M. and Thieme , H. R. 2003 . Progression age enhanced backward bifurcation in an epidemic model with super-infection . J. Math. Biol. , 46 : 385 – 424 .
- Mills , C. E. , Robins , J. M. , Bergstrom , C. T. and Lipsitch , M. 2006 . Pandemic influenza: risk of multiple introductions and the need to prepare for them . PLoS Medicine , 3 ( 6 ) : 1 – 5 .
- Milner , F. A. and Pugliese , A. 1999 . Periodic solutions: a robust numerical method for am S-I-R model of epidemics . J. Math. Biol. , 39 : 471 – 492 .
- Pease , C. M. 1987 . An evolutionary epidemiological mechanism with applications to type A influenza . Theor. Popul. Biol. , 31 : 422 – 452 .
- Scholtissek , C. , Rohde , W. , Vonhoyningen , V. and Rott , R. 1978 . Origin of human influenza-virus subtypes H2N2 and H3N2 . Virology , 87 : 13 – 20 .
- Shaefer , J. R. , Kawaoka , Y. , Bean , W. J. , Suss , J. , Senne , D. and Webster , R. G. 1993 . Origin of the pandemic 1957 H2 influenza-A viruses and the persistence of its possible progenitors in the avian reservoir . Virology , 194 : 781 – 788 .
- Smith , D. J. , Lapedes , A. S. , de Jong , J. C. , Bestebroer , T. M. , Rimmelzwaan , G. , Osterhaus , A. D.M.E. and Fauchier , R. A.M. 2004 . Mapping the antigenic and genetic evolution of influenza virus . Science , 305 : 371 – 376 .
- Thieme , H. R. 2000 . Uniform persistence and permanence for non-autonomous semiflows in population biology . Math. Biosci. , 166 : 173 – 201 .
- Thieme , H. R. and Castillo-Chavez , C. 1993 . How may infection-age-dependent infectivity affect the dynamics of HIV/AIDS? . SIAM J. Appl. Math. , 53 ( 5 ) : 1447 – 1479 .
- Thieme , H. R. and Yang , J. 2002 . An epidemic model with variable reinfection rate and applications to influenza . Math. Biosci. , 180 : 207 – 235 .
- Tiensin , T. , Nielen , M. Vernooij , H. 2007 . Transmission of the highly pathogenic avian influenza virus H5N1 within flocks during the 2004 epidemic in Thailand . JID , 196 : 1679 – 1684 .
- Viboud , C. , Miller , M. , Olson , D. and Osterholm , M. Preliminary Estimates of Mortality and Years of Life Lost Associated with the 2009 A/H1N1 Pandemic in the US and Comparison with Past Influenza Seasons . PLoS Currents , 20 March (2010). Available at http://knol.google.com/k/preliminary-estimates-of-mortality-and-years-of-life-lost-associated-with-the#
- Wei , H. M. , Li , X. Z. and Martcheva , M. 2008 . An epidemic model of a vector-borne disease with direct transmission and time delay . J. Math. Anal. Appl. , 342 ( 2 ) : 895 – 908 .
- WHO, H5N1 avian influenza: timeline of major events http://www.who.int/csr/disease/avian_influenza/ai_timeline/en/index.html
- World Health Organization, Cumulative Number of Confirmed Human Cases of Avian Influenza A (H5N1) Reported to WHO. Available at http://www.who.int/csr/disease/avian_influenza/country/ cases_table_2006_05_12/en/index.html
- Yosida , K. 1995 . “ Functional Analysis ” . In , 6 , New York : Springer .