Abstract
The basic reproduction number ℛ0 for a compartmental disease model is often calculated by the next generation matrix (NGM) approach. When the interactions within and between disease compartments are interpreted differently, the NGM approach may lead to different ℛ0 expressions. This is demonstrated by considering a susceptible–infectious–recovered–susceptible model with free-living pathogen (FLP) growing in the environment. Although the environment could play different roles in the disease transmission process, leading to different ℛ0 expressions, there is a unique type reproduction number when control strategies are applied to the host population. All ℛ0 expressions agree on the threshold value 1 and preserve their order of magnitude. However, using data for salmonellosis and cholera, it is shown that the estimated ℛ0 values are substantially different. This study highlights the utility and limitations of reproduction numbers to accurately quantify the effects of control strategies for infections with FLPs growing in the environment.
1. Introduction
The basic reproduction number, ℛ0, is considered as one of the most practical tools that mathematical thinking has brought to epidemic theory Citation26. ℛ0 is defined as the average number of secondary infections produced by a single infectious host introduced into a totally susceptible population Citation1. In most cases, if , then the outbreak generates an epidemic; whereas, if
, then the infection will disappear from the population. Since ℛ0 synthesizes important elements of the infection transmission process, it identifies the most important factors in the infection transmission cycle. A method often used to derive ℛ0 expression is the next generation matrix (NGM) approach Citation18
Citation19.Footnote
As noted in previous works Citation19, depending on the biological interpretations of the disease compartments, different ℛ0 expressions can be derived for a compartmental model. Our study highlights the issue of calculating a valid ℛ0 expression for diseases transmitting through the contaminated environment. Previous studies Citation7 Citation13 Citation41 Citation45 differ fundamentally in the way they treat the environment compartment; therefore, the ℛ0 expressions derived are substantially different. For instance, in Citation13 Citation41 Citation45, the derived ℛ0 is represented as a sum of two separate terms corresponding to the host-to-host and environment-to-host transmission pathways. This may suggest the independence of these pathways in the disease transmission cycle. Whereas the ℛ0 derived in Citation7 has a square root term suggesting a more complicated interaction between the host-to-host and environment-to-host transmission pathways. The present work demonstrates the properties and behaviours of possible ℛ0 expressions resulting from different ways of interpreting the role of the environment in the transmission cycle of infection. To avoid the issue of multiple ℛ0 expressions, the NGM approach is used to derive a unique threshold quantity known as a type reproduction number Citation27 Citation43.
Although host-to-host disease transmission has been traditionally considered as the main cause of infection spread, the role of environment-to-host disease transmission is becoming more evident. A contaminated environment such as food, water, soil, objects and contact surfaces may transmit infection to susceptible hosts Citation6
Citation12
Citation44. Pathogens in a free-living state adapt to the environment by morphological and physiological changes that promote their survival Citation5 and even growth Citation36 in the environment. In addition, the presence of a free-living pathogen (FLP) in the environment can be replenished by infectious hosts that excrete the pathogen for a considerable amount of time. In contrast, the natural decay of a pathogen and decontamination practices reduce environmental persistence. By taking into account the above-mentioned factors and waning host immunity typical for certain infections Citation1, we extend a susceptible–infectious–recovered–susceptible (SIRS) model to an model that includes FLP capable of growth and survival in the environment.
The main objective of this study is to deepen the understanding of how multiple transmission pathways and pathogen growth in the environment affect measures of control efforts required to eradicate or reduce the infection in the host population. Depending on how the pathogen interactions within the environment and between the host and environment are interpreted, different ℛ0 expressions corresponding to the model are derived. In particular, the pathogen shedding from infectious hosts into the environment and FLP growth in the environment are considered as transition among infectious states of an infectious host, generation of secondary infectious agents or a combination of both. The former is a progression of an already infectious host through the environment (i.e. pathogen shedding and growth represent extensions of the host's infectiousness to the environment). The latter corresponds to the appearance of secondary FLP in the environment generated by an infectious host or through the growth of pathogen in the environment. While the derived ℛ0 expressions are different, they all intersect at the threshold value 1 and preserve their order of magnitude below and above 1. Although the global stability results obtained in this study are valid for any ℛ0 expression, the differences between ℛ0 values can be extremely large depending on the parameter values. This is numerically illustrated using data of salmonellosis and cholera infections. When the pathogen is unable to maintain itself in the environment, the host population becomes disease-free when a type reproduction number is less than 1, thus this number can be used to accurately guide disease control strategies.
2. The model
The model consists of the standard SIRS model Citation9, where S, I, R denotes the number of susceptible, infectious, and recovered hosts, respectively, and a compartment P that indicates the FLP load in the environment. Susceptible individuals become infectious either by adequate contacts with infectious individuals or the contaminated environment. Infectious individuals contaminate the environment by shedding pathogen that is capable of growth and survival in the environment. Hence, the set of ordinary differential equations (ODEs) representing the SIRSP model is given by
Figure 1. A schematic representation of the SIRSP model. Solid and dashed lines indicate the dynamics of host and FLP, respectively.
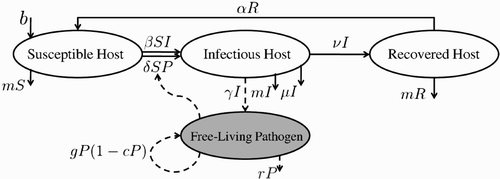
Table 1. Variables and parameters with units for the SIRSP model.
3. Host–pathogen dynamics
3.1. Feasible region and the equilibria
For the model Equation(1)–Equation(4)
, it can be verified that all solutions with non-negative initial conditions remain non-negative. Letting N=S+I+R and adding Equations (1)–(3) gives
, which implies that
As a consequence, EquationEquation (4)
yields
, and thus there exists some M>0 such that
. The constant M can be chosen as the unique positive zero of the quadratic polynomial
. Hence, the feasible region
The disease-free equilibrium (DFE) is given by (S
0, 0, 0, 0) with S
0=b/m. As shown in Section 3.3 and Appendix 1, under a stated condition, there exists a unique endemic equilibrium (EE) with
in the interior of Γ, which is denoted by
.
3.2. Basic reproduction numbers
EquationEquations (2) and Equation(4)
form a subsystem describing the generation and transition of infectious hosts and FLP. The Jacobian matrix associated with the linearized subsystem at the DFE is given by
Figure 2. A conceptual representation of the secondary FLP and infectious hosts generated in the cycle of disease transmission. Here, H 0 and H 1 are the initial and secondary infectious host, respectively, while P 0 and P 1 are the initial and secondary FLP.
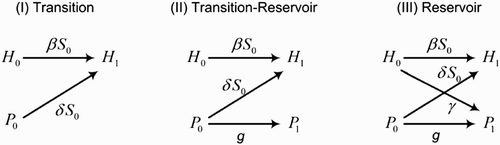
(I) Transition. Assume that the FLP population cannot maintain itself through growth in the environment (i.e. the FLP growth rate g is always less than the FLP decay rate r). Then, the environment is considered as an extended state of host infectiousness, where the pathogen shedding into and growth within the environment are considered as transitions within the initial infectious state of the host population. Therefore, the shedding and growth rate of the pathogen (i.e. γ and g) are placed in the V matrix rather than the F matrix, giving
Although specific forms of and
are only related to the model Equation(1)
–Equation(4)
, several studies consider the same scenario and derive an
with the same structure as in Equation(7)
. For example, without pathogen growth in the environment (i.e. g=0), this approach has been used in studies of cholera infection Citation14
Citation25
Citation45, multi-strain disease transmission Citation10 and salmonellosis Citation13.
(II) Transition–Reservoir. Similar to the previous scenario, the environment is assumed to act as an extended state of host infectiousness for pathogen shed by an infected host. However, the environment is assumed to also act as a reservoir of infection. Here, the growth of FLP can be regarded as vertical transmission of infectious pathogen in the environment. Using the extended definition of the F matrix Citation30, the entry (i, j) of matrix F represents the rate at which secondary individuals appear in class i per individual of type j. Therefore, the FLP growth rate g represents the rate of secondary FLP generated in the environment. Under these assumptions, the F, V and K matrices are changed to
(III) Reservoir. The environment is assumed to act as a reservoir, where secondary FLP are added into the environment both through FLP growth and pathogen shedding by infectious hosts. Hence, both the shedding and growth rates γ and g are placed in the F matrix. Then,
Without pathogen growth in the environment and host-to-host disease transmission (i.e. g=β=0), the NGM approach presented here has already been used in previous studies. This includes studies of schistosomiasis Citation21, toxoplasmosis Citation37 and low-pathogenic avian influenza Citation7 transmission dynamics.
Assuming r>g, it is fairly straightforward to show that all three basic reproduction numbers agree at the threshold value, i.e. . In particular, for a fixed set of parameter values, they are all greater, equal or less than 1. When a basic reproduction number is greater than 1, provided r>g, it can be shown that they are always in the order
. The order is reversed if the basic reproduction number is less than 1. While
is limited to the case r>g,
and
do not have such a limitation and
for g>r. The differences between the three reproduction numbers are negligible when
is small and
. Nonetheless, as numerically illustrated in Sections 3.4 and 4.1, the differences among the reproduction numbers can be large when
is large.
3.3. Disease invasion and persistence
Using Theorem 2 of Citation19, the DFE is locally asymptotically stable if , and unstable if
, where ℛ0 refers to any reproduction number in the form of Equation(6)
, Equation(8)
or Equation(9)
. Moreover, as indicated in the following theorems,
is a necessary and sufficient condition for the existence and uniqueness of the EE, which is then locally asymptotically stable. The proofs of Theorems 3.1 and 3.2 are provided in Appendix 1.
Theorem 3.1
Model
Equation(1)–Equation(4)
admits a unique EE if and only if
.
Theorem 3.2
The EE is locally asymptotically stable if
.
Model Equation(1)–Equation(4)
is said to be uniformly persistent
Citation11 in
if there exists constant c>0 such that
Theorem 3.3
The following results hold for model
Equation(1)–Equation(4)
.
(1) If
| |||||
(2) If
| |||||
As established in the next result, if the infection is assumed to confer permanent immunity, then irrespective of the initial number of infectious hosts, the infection becomes endemic when .
Theorem 3.4
Assume that α=0. If
then the unique EE is globally asymptotically stable in
.
The proofs of Theorems 3.3 and 3.4 are given in Appendices 2 and 3, respectively. Note that the global stability of the EE remains an open problem when α>0 (i.e. when individuals recovered from infection lose their immunity after an average period 1/α). However, the numerical simulations suggest that the result of Theorem 3.4 remains valid for .
3.4. Examples
We consider two examples of infection: Equation(1) salmonellosis in a dairy herd, and Equation(2)
cholera in a large human population. The specific parameter values and references related to the salmonellosis and cholera examples are given in and , respectively. Note that these parameters give r>g for both examples. The parameter values related to cholera are mainly taken from Citation46, which studies the nineteenth century cholera outbreak in London, UK. Note that some of the parameter values have been converted from weeks or years to days. Dynamics of salmonellosis and cholera are explored by numerically solving model Equation(1)
–Equation(4)
with different initial conditions. Using these parameter values,
and the DFE is not achieved for either the salmonellosis or cholera.
Table 2. Model parameters values related to salmonellosis in a dairy herd.
Table 3. Model parameters and values related to cholera in humans.
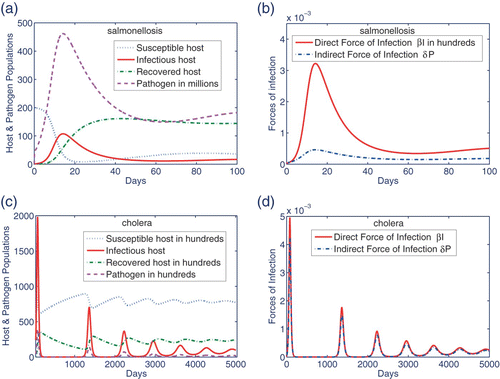
refers to salmonellosis, where solutions of model Equation(1)–Equation(4)
tend to the EE
,
. Note that the epidemic peaks of infection in the host population and those of FLP in the environment are synchronized. As shown in , for these chosen parameter values, the direct force of salmonellosis is much higher than the indirect force. The reproduction numbers for salmonellosis are given by
,
,
,
,
and
, which indicates
, agreeing with the direct force being more important. shows that the dynamics of cholera tends to the EE
in an oscillatory fashion. The epidemic waves become more frequent but with shorter amplitudes as they tend to the EE. Therefore, long-term oscillatory behaviour simulated in Citation46 may occur without imposing the seasonality in the environment-to-host transmission pathway. In addition, prevalence at the EE could be higher than the previous results []Citation46. More importantly, the number of infectious individuals between the first three epidemic waves falls below 1, which highlights the role of the environment as a reservoir of infection and the importance of stochasticity. represents the forces of infection with almost equal contributions of infectious host and environment in the generation of secondary infections. Note that for the cholera parameters
,
,
,
,
and
Figure 3. Using the parameter values indicated in and , dynamics of salmonellosis and cholera are numerically simulated. (a) Salmonella infection tends to an EE after the first epidemic wave. (b) Considering the infectious host and environment as generators of salmonellosis, the former corresponds to a force significantly higher than the latter. This is in agreement with . (c) After several epidemic waves, cholera becomes endemic and remains persistent in the host population and the environment. (d) Based on the parameter values, the direct and indirect infection forces play almost equal roles in generation of cholera infection.
As quantified in a previous study Citation3, reducing the contacts with the infectious host or the contaminated environment can be used as control measures to eradicate or reduce the infection. Specifically for the cholera parameters, when β falls below (or δ is less than 10−8), ℛ0 becomes less than 1 and the population becomes disease-free.
4. Control of infection
4.1. Impacts of misinterpretation
The basic reproduction number can be used to measure the control efforts required to reduce or eliminate an infection Citation1
Citation18. In the event of a disease outbreak, health officials may consider various control measures including Equation(1) more aggressive decontamination of the environment (i.e. increasing the value of r by additional cleaning and disinfecting), Equation(2)
antibiotic treatments (i.e. assuming that the pathogen is not resistant to antibiotics, the recovery rate ν is increased and the shedding rate γ is decreased), Equation(3)
reducing contacts with infectious host (i.e. the host-to-host transmission rate β is reduced) and Equation(4)
reducing contacts with contaminated environment (i.e. the environment-to-host transmission rate δ is reduced) by approaches such as isolation, training and advisory services. Assuming a unique ℛ0 expression, the efficacy of each control measure can be quantified Citation3. Specifically, using the partial derivatives of ℛ0 with respect to the above-mentioned parameters, the rate of reduction in ℛ0 can be determined for each of these control measures. The issue arises when the ℛ0 expression is non-unique and therefore partial derivatives of different reproduction numbers may endorse different control measures.
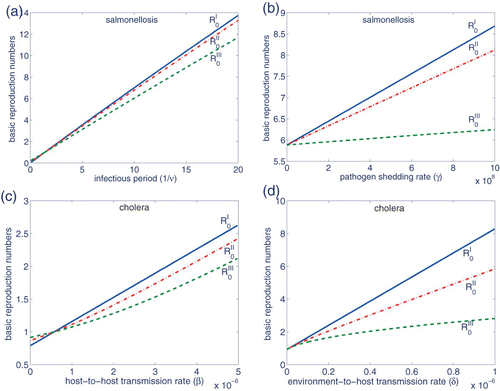
Considering the salmonellosis and cholera examples of Section 3.4, shows the changes in the basic reproduction numbers ,
and
due to changes in 1/ν and γ, for salmonellosis, and changes in β and δ for cholera. All other parameter values are as given in and . Note that all reproduction numbers intersect only at 1. As stated in Section 3.2, for values less than 1,
, whereas the inequalities are reversed for values greater than 1. Moreover, as shown in and , the differences between the reproduction numbers can be quite substantial. An overestimated ℛ0 may give rise to public panic, unnecessary control efforts and economic losses, whereas underestimating ℛ0 may result in choosing control policies that are not sufficiently intensive. For instance, considering culling as a disease control policy in livestock or poultry, the former may lead to a huge economic loss, whereas the latter may result in culling a proportion of the population that is not sufficient to eradicate the infection.
Figure 4. The top and bottom plots represent, respectively, changes in reproduction numbers for the salmonellosis and cholera parameters. Changes in the parameter values of (a) 1/ν, (b) γ, (c) β and (d) δ may result in substantially different ℛ0 values.
Under certain conditions, the ℛ0 expressions are reduced to simpler forms. Nevertheless, they remain substantially different. To show this, we consider three different limiting cases as the following parameter values tend to zero: (a) FLP growth rate g, (b) host-to-host transmission rate β and (c) both g and β.
(a) Neglecting FLP growth. The growth rate of several pathogens such as Salmonella and Vibrio cholerae tends to zero under low temperature conditions (see, for example, Citation23). In this case, both reproduction numbers and
coincide, that is
; whereas,
(b) Neglecting host-to-host transmission. Assuming low mixing in the host population, the value of β can be negligible. Also for several infections, such as Clostridium difficile, vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, the environment-to-host pathway is the predominant route of infection transmission (see, for example, Citation29). In this case, all three reproduction numbers are substantially different. Specifically,
and
.
(c) Neglecting FLP growth and host-to-host transmission. Similar to case (a), both reproduction numbers and
coincide, that is,
, whereas
.
Hence, misinterpreting the role of the environment gives rise to an incorrect ℛ0 expression and possible choice of an inefficient control policy. As shown in the next section, despite initial assumptions about the role of the environment, a unique control measure is obtained when the pathogen is unable to maintain itself in the environment (i.e. r>g).
4.2. Type reproduction number
If the NGM K of order n is known, then using the approach provided in Citation27 Citation43 a type reproduction number can be calculated. In particular, the disease and the environment compartments may represent different population types. The type reproduction number T s associated with the population type s has the following expression Citation27 Citation43
Suppose that an ODE model has n disease compartments, and that the interactions within and between m disease compartments (m<n) are interpreted differently. The Jacobian matrix is decomposed as in Section 3.2, leading to different NGMs, and
. Without loss of generality, assume that V
j
=V
i
+U
m
and F
j
=F
i
+U
m
, where U
m
is a matrix with m non-zero rows (say, rows
, which correspond with the m disease compartments above) and n−m zero rows. Then, the following result indicates that, regardless of how the interactions are interpreted, the type reproduction number related to any disease compartment other than those m compartments is unique. The proof is provided in Appendix 4.
Theorem 4.1
Let
and
be the type reproduction numbers associated with population type s defined by
Equation(10)
and, respectively, derived from the NGMs K
i
and K
j
. If
and both
and
are well defined, then
.
Concerning the SIRSP model, denote the infectious host and FLP populations as type 1 and type 2 population, respectively. Infection can be eradicated by applying a control measure (e.g. vaccination) to type 1 population. If r>g, then is well defined for
and III, corresponding to the cases (I), (II) and (III) of Section 3.2. Thus, using Theorem 4.1
Now assume that a control measure (e.g. pathogen removal by decontamination) is applied only to type 2 population, then
Case j=I is degenerate, since the FLP in the environment is not assumed as an independent population type. Instead, it is considered as an adjunct part of the infectious host population. Therefore, provided that
.
For j=II,
For j=III,
5. Discussion
The present work highlights the utility of the type reproduction number when our knowledge regarding certain disease compartments is limited. For instance, if we are confident that net growth of FLP is negative, the unique expression related to the host of interest (e.g. humans, livestock or wildlife) leads to a correct estimation of the control efforts required to eradicate the infection.
The SIRP model proposed in this study is an extension of the SIR model and considers both host-to-host and environment-to-host disease transmission pathways. The pathogen shed by infectious hosts is assumed to be capable of growth and survival in the environment. Assuming that the environment acts as a transition, transition-reservoir or reservoir of infection, three different ℛ0 expressions are derived. Several studies Citation7
Citation13
Citation41
Citation45 consider one of these scenarios and derive ℛ0 that has the same structure as or
. Although all ℛ0 expressions of the SIRP model intersect at the threshold value 1 and preserve their order of magnitude, the differences between the ℛ0 values can be large. Therefore, misinterpreting the role of pathogen growth and shedding may result in overestimating or underestimating the control efforts required in eradicating the infection. We showed that the issue is partly resolved when the type reproduction number is used instead of ℛ0. In particular, regardless of how the pathogen shedding and growth are interpreted in the NGM, the type reproduction number related to the host population is the same. However, this is meaningful only when the environmental decontamination keeps the net FLP growth rate negative (i.e. r>g). Otherwise, the environment becomes a reservoir of the pathogen and targeting the host population will not be sufficient to eradicate the infection.
Noting that all ℛ0 expressions coincide at the threshold value 1, the local and global stability results presented in this work remain valid for any ℛ0 expression in the form of Equation(6), Equation(8)
or Equation(9)
. Here, we showed that the DFE is globally stable when
and the unique locally (and globally) stable endemic equilibria emerges when ℛ0 exceeds 1. Furthermore, the system Equation(1)
–Equation(4)
is uniformly persistent when
. The uniqueness of the EE is highly dependent on the pathogen growth and decay behaviours in the environment. In particular, considering a generalized logistic growth Citation42 of FLP in the environment and limiting the pathogen decay rate to a certain range, there will be multiple EE, leading to rich dynamics of the SIRSP model. For the indirect transmission, a more realistic threshold of pathogen is incorporated in the disease transmission term in Citation32, but leads to a non-smooth system. This incidence can be approximated by a saturating incidence (rather than mass action) as considered in Citation14
Citation25. Our methods for the analysis of the DFE can be extended to include such incidence.
In conclusion, the basic reproduction number ℛ0 provides a reliable threshold for control and prevention of infection. When the interactions between and within the disease compartments are unclear, the NGM approach may lead to a unique expression for the type reproduction number associated with a population type and resolve the issue of multiple ℛ0 expressions.
Acknowledgements
This work was supported by the National Science Foundation grant NSF-EF-0913367 to RI funded under the American Recovery and Reinvestment Act of 2009. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The research of Zhisheng Shuai and P. van den Driessche is partially supported by an NSERC Postdoctoral Fellowship, an NSERC Discovery Grant and Mprime.
Notes
This is a paper based on an invited talk given at the 3rd International Conference on Math Modeling & Analysis, San Antonio, USA, October 2011.
References
- Anderson , R. M. and May , R. M. 1991 . “ Infectious Diseases of Humans: Dynamics and Control ” . Oxford : Oxford University Press .
- Ayliffe , G. A.J. , Babb , J. R. and Taylor , L. J. 1999 . Hospital-Acquired Infection: Principles and Prevention , 3 , Oxford : Butterworth-Heinemann .
- Bani-Yaghoub , M. , Gautam , R. , Döpfer , D. , Kaspar , C. W. and Ivanek , R. 2012 . Effectiveness of environmental decontamination in control of infectious diseases . Epidemiol. Infect. , 140 : 542 – 553 .
- Beretta , E. and Takeuchi , Y. 1995 . Global stability of an SIR epidemic model with time delays . J. Math. Biol. , 33 : 250 – 260 .
- Bolton , D. J. , Byrne , C. M. , Sheridan , J. J. , McDowell , D. A. and Blair , I. S. 1999 . The survival characteristics of a non-toxigenic strain of Escherichia coli O157:H7 . J. Appl. Microbiol. , 86 : 407 – 411 .
- Boone , S. A. and Gerba , C. P. 2007 . Significance of fomites in the spread of respiratory and enteric viral disease . Appl. Environ. Microbiol. , 73 : 1687 – 1696 .
- Bourouiba , L. , Teslya , A. and Wu , J. 2011 . Highly pathogenic avian influenza outbreak mitigated by seasonal low pathogenic strains: Insights from dynamic modeling . J. Theor. Biol. , 271 : 181 – 201 .
- Boyce , J. M. , Potter-Bynoe , G. , Chenevert , C. and King , T. 1997 . Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications . Infect. Control Hosp. Epidemiol. , 18 : 622 – 627 .
- Brauer , F. , van den Driessche , P. and Wu , J. 2008 . “ Mathematical Epidemiology ” . In Lecture Notes in Mathematics 1945 , Edited by: Brauer , F. , van den Driessche , P. and Wu , J. Berlin : Springer .
- Breban , R. , Drake , J. M. and Rohani , P. 2010 . A general multi-strain model with environmental transmission: Invasion conditions for the disease-free and endemic states . J. Theor. Biol. , 264 : 729 – 736 .
- Butler , G. J. , Freedman , H. I. and Waltman , P. 1986 . Uniformly persistent systems . Proc. Amer. Math. Soc. , 96 : 425 – 430 .
- Caraco , T. and Wang , I.-N. 2008 . Free-living pathogens: Life-history constraints and strain competition . J. Theor. Biol. , 250 : 569 – 579 .
- Chapagain , P. P. , van Kessel , J. S. , Karns , J. S. , Wolfgang , D. R. , Hovingh , E. , Nelen , K. A. , Schukken , Y. H. and Gröhn , Y. T. 2007 . A mathematical model of the dynamics of Salmonella Cerro infection in a US dairy herd . Epidemiol. Infect. , 136 : 263 – 272 .
- Codeço , C. T. 2001 . Endemic and epidemic dynamics of cholera: The role of the aquatic reservoir . BMC Infect. Dis. , 1 : 1
- Cozad , A. and Jones , R. D. 2003 . Disinfection and the prevention of infectious disease . Amer. J. Infect. Control , 31 : 243 – 254 .
- Danforth , D. , Nicolle , L. E. , Hume , K. , Alfieri , N. and Sims , H. 1987 . Nosocomial infections on nursing units with floors cleaned with a disinfectant compared with detergent . J. Hosp. Infect. , 10 : 229 – 235 .
- Daschner , F. and Schuster , A. 2004 . Disinfection and the prevention of infectious disease: No adverse effects? . Amer. J. Infect. Control , 32 : 224 – 225 .
- Diekmann , O. and Heesterbeek , J. A.P. 2000 . “ Mathematical Epidemiology of Infectious Diseases ” . Chichester : John Wiley & Sons .
- van den Driessche , P. and Watmough , J. 2002 . Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission . Math. Biosci. , 180 : 29 – 48 .
- Freedman , H. I. , Ruan , S. and Tang , M. 1994 . Uniform persistence and flows near a closed positively invariant set . J. Dyn. Differ. Equat. , 6 : 583 – 600 .
- Gao , S. , Liu , Y. , Luo , Y. and Xie , D. 2011 . Control problems of a mathematical model for schistosomiasis transmission dynamics . Nonlinear Dyn. , 63 : 503 – 512 .
- Gardner , I. A. , Hird , D. W. , Utterback , W. W. , Danaye-Elmi , C. , Heron , B. R. , Christiansen , K. H. and Sischo , W. M. 1990 . Mortality, morbidity, case-fatality, and culling rates for California dairy cattle as evaluated by the National Animal Health Monitoring System, 1986–1987 . Prev. Vet. Med. , 8 : 157 – 170 .
- Gautam , R. , Bani-Yaghoub , M. , Neill , W. H. , Döpfer , D. , Kaspar , C. W. and Ivanek , R. 2011 . Modeling impact of seasonal variation in ambient temperature on the transmission dynamics of a pathogen with a free-living stage: Simulation of Escherichia coli O157:H7 prevalence in a dairy herd . Prev. Vet. Med. , 102 : 10 – 21 .
- Guo , H. , Li , M. Y. and Shuai , Z. 2006 . Global stability of the endemic equilibrium of multi-group SIR epidemic models . Can. Appl. Math. Q. , 14 : 259 – 284 .
- Hartley , D. M. , Morris , J. G. Jr. and Smith , D. L. 2006 . Hyperinfectivity: A critical element in the ability of V. Cholerae to cause epidemics? . PLOS Med , 3 : 63 – 69 .
- Heesterbeek , J. A.P. and Dietz , K. 1996 . The concept of R0 in epidemic theory . Stat. Neerl. , 50 : 89 – 110 .
- Heesterbeek , J. A.P. and Roberts , M. G. 2007 . The type-reproduction number T in models for infectious disease control . Math. Biosci. , 206 : 3 – 10 .
- Himathongkham , S. , Bahari , S. , Riemann , H. and Cliver , D. 1999 . Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow slurry . FEMS Microbiol. Lett. , 178 : 251 – 257 .
- Hota , B. 2004 . Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? . Clin. Infect. Dis. , 39 : 1182 – 1189 .
- Hurford , A. , Cownden , D. and Day , T. 2010 . Next-generation tools for evolutionary invasion analyses . J. R. Soc. Interface , 7 : 561 – 571 .
- Jensen , M. A. , Faruque , S. M. , Mekalanos , J. J. and Levin , B. R. 2006 . Modeling the role of bacteriophage in the control of cholera outbreaks . PNAS , 103 : 4652 – 4657 .
- Joh , R. I. , Wang , H. , Weiss , H. and Weitz , J. S. 2009 . Dynamics of indirectly transmitted infectious diseases with immunological threshold . Bull. Math. Biol. , 71 : 845 – 862 .
- Kabir , S. 2005 . Cholera vaccines: The current status and problems . Rev. Med. Microbiol. , 16 : 101 – 116 .
- Lanzas , C. , Brien , S. , Ivanek , R. , Lo , Y. , Chapagain , P. P. , Ray , K. A. , Ayscue , P. , Warnick , L. D. and Gröhn , Y. T. 2008 . The effect of heterogeneous infectious period and contagiousness on the dynamics of Salmonella transmission in dairy cattle . Epidemiol. Infect. , 136 : 1496 – 1510 .
- LaSalle , J. P. 1976 . “ The Stability of Dynamical Systems ” . In Regional Conference Series in Applied Mathematics , Philadelphia : SIAM .
- LeJeune , J. T. , Besser , T. E. and Handcock , D. D. 2001 . Cattle water troughs as reservoirs of Escherichia coli O157 . Appl. Environ. Microbiol. , 67 : 3053 – 3057 .
- Lélu , M. , Langlais , M. , Poulle , M.-L. and Gilot-Fromont , E. 2010 . Transmission dynamics of Toxoplasma gondii along an urban-rural gradient . Theor. Popul. Biol. , 78 : 139 – 147 .
- Li , M. Y. , Graef , J. R. , Wang , L. and Karsai , J. 1999 . Global dynamics of a SEIR model with varying total population size . Math. Biosci. , 160 : 191 – 213 .
- Maaløe , O. and Richmond , M. H. 1962 . The rate of growth of Salmonella typhimurium with proline or glutamate as sole C source . J. Gen. Microbiol , 27 : 269 – 284 .
- Maki , D. G. , Alvarado , C. J. , Hassemer , C. A. and Zilz , M. A. 1982 . Relation of the inanimate hospital environment to endemic nosocomial infection . New Engl. J. Med. , 307 : 1562 – 1566 .
- Mukandavire , Z. , Liao , S. , Wang , J. , Gaff , H. , Smith , D. L. and Morris , J. G. Jr. 2011 . Estimating the reproductive number for the 2008–2009 cholera outbreaks in Zimbabwe . PNAS , 108 : 8767 – 8772 .
- Peleg , M. , Corradini , M. G. and Normand , M. D. 2007 . The logistic (Verhulst) model for sigmoid microbila growth curves revisited . Food Research Interna. , 40 : 808 – 818 .
- Roberts , M. G. and Heesterbeek , J. A.P. 2003 . A new method for estimating the effort required to control an infectious disease . Proc. R. Soc. Lond. B , 270 : 1359 – 1364 .
- Scott , E. and Bloomfield , S. F. 1990 . The survival and transfer of microbial contamination via cloths, hands and utensils . J. Appl. Bacteriol. , 68 : 271 – 278 .
- Tien , J. H. and Earn , D. J.D. 2010 . Multiple transmission pathways and disease dynamics in a waterborne pathogen model . Bull. Math. Biol. , 72 : 1506 – 1533 .
- Tien , J. H. , Poinar , H. N. , Fisman , D. N. and Earn , D. J.D. 2011 . Herald waves of Cholera in nineteenth century London . J. R. Soc. Interface , 8 : 756 – 760 .
- Woodward , W. E. 1971 . Cholera reinfection in man . J. Infect. Dis. , 123 : 61 – 66 .
- Xiao , Y. , Bowers , R. G. , Clancy , D. and French , N. P. 2005 . Understanding the dynamics of Salmonella infections in dairy herds: A modelling approach . J. Theor. Biol. , 233 : 159 – 175 .
Appendix 1. Existence and stability of the EE
Proof of Theorem 3.1
The equilibrium equations of model Equation(1)–Equation(4)
are given by
Proof of Theorem 3.2
Let be the unique EE of model Equation(1)
–Equation(4)
provided
. The linearization at the EE, setting
, gives
Appendix 2. Global stability of the DFE
Proof of Theorem 3.3
Let x=(I, P). Since
If , L′=0 implies that u
T
x=0 by Equation(A6)
, and thus I=0 or P=0. It follows from Equation(2)
and Equation(4)
that the largest invariant set where L′=0 satisfies δ SP=0 or γ I=0, respectively; thus, I=P=0 since
. EquationEquations (1)
and Equation(3)
lead to S=S
0 and R=0 in the above invariant set, that is, the singleton
. By LaSalle's invariance principle Citation35, the DFE is the globally asymptotically stable in Γ if
.
If , L′=0 implies that S=S
0, by Equation(A3)
, Equation(A5)
and the fact that u
T
V
−1>0. Then, EquationEquation (1)
gives
. Adding EquationEquations (2)
and Equation(3)
gives
, for which the invariant set satisfies I+R=0, and thus I=R=0. EquationEquation (2)
gives δ SP=0, and thus P=0. Therefore, the largest invariant set where L′=0 is the singleton
, and by LaSalle's invariance principle, the DFE is globally asymptotically stable in Γ if
.
If , then by continuity, L′>0 in a neighbourhood of the DFE in
. Solutions in
sufficiently close to the DFE move away from the DFE, implying that the DFE is unstable. Using a uniform persistence result from Citation20 and an argument as in the proof of Proposition 3.3 of Citation38, it can be shown that, when R
0>1, instability of the DFE implies uniform persistence of model Equation(1)
–Equation(4)
. ▪
Appendix 3. Global stability of the EE
Proof of Theorem 3.4
If , then by Theorem 3.1, an EE
exists, where
satisfy the equilibrium equations Equation(A1)
. Following Citation45, consider a Lyapunov function
Appendix 4. Type reproduction number
Proof of Theorem 4.1
Using Equation(10) it follows that