?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Optimal control methods are applied to a deterministic mathematical model to characterize the factors contributing to the replacement of hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA) with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), and quantify the effectiveness of three interventions aimed at limiting the spread of CA-MRSA in healthcare settings. Characterizations of the optimal control strategies are established, and numerical simulations are provided to illustrate the results.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacteria that is resistant to many antibiotics. Hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA) has long been a serious infection problem in hospitals and other healthcare settings. In 2011, there were nearly 460,000 hospitalizations involving HA-MRSA diagnoses according to hospital billing data collected by the US Agency for Healthcare Research and Quality [Citation7]. In the United States, approximately 60% of staphylococcal infections in the intensive care unit are now caused by HA-MRSA, and percentages continue to rise [Citation26]. The data suggest that these hospitalizations resulted in nearly 23,000 deaths.
Relatively recently, a new strain of MRSA has emerged and spread outside of hospitals, causing serious infections in even young and healthy individuals, including school children, military personnel, prison inmates, and even NFL players [Citation10, Citation17, Citation20, Citation28, Citation31]. In the community setting, most MRSA infections are skin infections, whereas in medical facilities, MRSA causes life-threatening bloodstream, pneumonia, and surgical site infections. The community-acquired strain of MRSA (CA-MRSA) has rapidly spread throughout the world [Citation12, Citation13, Citation33, Citation36]. CA-MRSA has not only invaded hospital settings, but in some cases, has replaced the hospital-acquired strains [Citation6, Citation21, Citation24, Citation25, Citation29]. This replacement of strains has serious potential consequences for hospital care, since CA-MRSA is implicated in severe infections, especially in patients in seriously compromised health situations [Citation18].
In community settings CA-MRSA infections in children continue to rise, increasing 10% a year among youths under 17 years. A large share of infections in children have been linked to community strains that can pass from child to child. CA-MRSA also exhibits broader antibiotic resistance susceptibility than does HA-MRSA [Citation26]. With the use of mathematical models, it has been shown that the presence of a CA-MRSA community reservoir has a major impact on the control of MRSA in hospitals [Citation3, Citation15, Citation27, Citation28]. In [Citation4, Citation5] deterministic differential equations models were developed to model the transmission dynamics of CA-MRSA in a prototype 400 bed hospital. The analysis in [Citation4, Citation5] strongly implied that CA-MRSA may eventually become dominant in hospital settings.
Intervention measures, such as improved hygiene, screening and decolonization of CA-MRSA carriers, and isolation of CA-MRSA infected patients, offer possibilities for epidemic control. Evaluation of the efficacy of infection control strategies is difficult, particularly during outbreaks. Some groups have advocated ‘search and destroy’ policies that recommend routine screening for MRSA to identify, isolate, and treat carriers, with the ultimate goal of eradicating the pathogen from healthcare facilities [Citation19]. Numerous governmental agencies have mandated MRSA screening programs. Some authorities in infection control organizations, however, have questioned the appropriateness of mandated screening [Citation22]. For example, from October 2007 through June 2010, there were 1,934,598 admissions, transfers or discharges from inpatient units in VA hospitals. During this period, the percentage of patients screened for MRSA at admission rose from 82% to 96%, meaning that VA hospitals performed 1.7 million screening tests in less than three years. The mean prevalence of MRSA colonization at the time of admission during that period was only 13.6%. Patients that test positive can be kept isolated from other patients during the length of their stay at VA hospitals [Citation30]. Harbarth et al. [Citation9] argue, however, that new findings do not support the recommendation for universal screening for MRSA on hospital admission to reduce the rate of hospital-acquired infections in surgical patients.
Decolonization, on the other hand, has been an effective control strategy. But the question still remains for how long should the decolonization regimen be continued. There is currently no consensus on the optimal duration of systemic antibiotic treatment to eradicate MRSA carriage [Citation1]. More importantly, due to the competition between HA-MRSA and CA-MRSA strains in hospitals, the question of what are the most effective strategies for dealing with patients colonized or infected with the new CA-MRSA strain remains unanswered.
Our objective here is to focus on optimal cost-effective strategies for combinations of decolonization of colonized CA-MRSA patients, and screening of colonized and infected CA-MRSA patients. We formulate a system of deterministic differential equations for the transmission dynamics of CA-MRSA in hospital settings. Our optimal control problem is to maximize the uncolonized and uninfected patients, minimize the infected CA-MRSA and HA-MRSA patients, while minimizing the cost to apply decolonization of colonized CA-MRSA patients, and screening of patients colonized and infected with CA-MRSA. In Section 2, we present a five-compartment model with three control strategies. In Section 3 we derive the adjoint equations and the characterization of optimal control strategies for a 400-bed hospital setting. Parameter estimates were obtained from the Beth Israel Deaconess Medical Centers computerized database system, which provides patient and infection control data [Citation4]. Numerical simulations are provided in Section 4.
2. The model
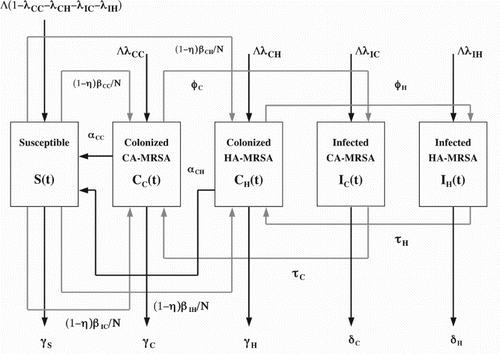
The model in [Citation4, Citation5] was formulated for patients and Health Care Workers (HCWs) divided into the following five compartments (see Figure ):
,
,
,
,
.
The parameters of the model are as follows: Patients are admitted at a rate of Λ per day, with the fractions of CA-MRSA colonized, CA-MRSA infected, HA-MRSA colonized and HA-MRSA infected patient admissions respectively. Susceptible patients have an average length of stay
, and colonized CA-MRSA and colonized HA-MRSA have average
and
, respectively. The colonization rates of susceptible patients to the colonized CA-MRSA compartment are
and
, and to the colonized HA-MRSA compartment are
and
. Here η is the compliance with hand washing hygiene (with
corresponding to
compliance and
corresponding to
compliance),
the colonization transmission rates of patients from HCWs contaminated by colonized CA-MRSA, infected CA-MRSA, colonized HA-MRSA and infected HA-MRSA patients, respectively, and N is the total number of patients in the hospital. The rates of infection of colonized CA-MRSA and colonized HA-MRSA patients are
and
, respectively. The cure rates of infected CA-MRSA and HA-MRSA patients are
and
, respectively. The hospital exit rates of susceptible, colonized CA-MRSA, colonized HA-MRSA, infected CA-MRSA, and infected HA-MRSA patients are
,
,
,
and
, respectively. The rates of decolonization of colonized CA-MRSA and colonized HA-MRSA patients are
and
, respectively. The model in [Citation4, Citation5] strongly suggests that CA-MRSA will become the dominant MRSA strains in hospitals and healthcare facilities. Improving compliance with hand hygiene and screening for and decolonization of CA-MRSA carriers are effective strategies. In this paper, our goal is to find the optimal cost-effective strategies using multiple intervention methods consisting of decolonization of colonized CA-MRSA patients, and screening of the patients colonized and infected with CA-MRSA, respectively.
Let be functions of time, then
are our control variables, and the state equations are:
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
with initial conditions
and
.
The control set is
(6)
(6) where the constants
are the maximum control efforts for decolonization of colonized CA-MRSA patients, screening of colonized and infected CA-MRSA patients, respectively.
Our goal is to maximize the objective functional:
(7)
(7) with the cost coefficients
, i.e. we want to maximize the uncolonized and uninfected patients, minimize the infected CA-MRSA and HA-MRSA patients, while minimizing the cost to apply decolonization of colonized CA-MRSA patients, and screening the patients colonized and infected with CA-MRSA. Note that the cost for decolonization depends on the number of colonized patients, so we add the
term.
3. The adjoint system and the characterization of optimal controls
This state system with Lebesque measurable coefficients has a unique non-negative bounded solution on the finite time interval [Citation16]. Note for this system, the control set and the objective functional have the appropriate compactness and convexity assumptions to guarantee the existence of an optimal control pair and the corresponding states [Citation8, Citation14]. Having the existence of an optimal control, we can now apply Pontryagin's Maximum Principle to obtain a characterization of the optimal control [Citation23].
Theorem 3.1.
Given optimal controls and the corresponding state solutions
,
in (Equation1
(1)
(1) )–(Equation5
(5)
(5) ), there exist adjoint variables
satisfying the system
(8)
(8)
(9)
(9)
(10)
(10)
(11)
(11)
(12)
(12)
with the transversality conditions
(13)
(13) Furthermore, the optimal controls are given by
(14)
(14)
(15)
(15)
(16)
(16)
Proof.
We set up the Hamiltonian using Pontryagin's Maximum principle [Citation23]:
(17)
(17)
We obtain the adjoint variables as follows:
(18)
(18)
(19)
(19)
(20)
(20)
(21)
(21)
(22)
(22) with the transversality conditions:
(23)
(23)
We obtain the characterization of the optimal controls by setting
From
, we have
(24)
(24) which implies
(25)
(25) Using
, we have
(26)
(26) which implies
(27)
(27) From
, we have
(28)
(28) which implies
(29)
(29)
Taking the upper and lower bounds for into account, we have the characterization of the optimal controls
(30)
(30)
(31)
(31)
(32)
(32)
The state equations (Equation1(1)
(1) )–(Equation5
(5)
(5) ) and the adjoint equations (Equation8
(8)
(8) )–(Equation12
(12)
(12) ), together with the characterization of the optimal control (Equation14
(14)
(14) )–(Equation16
(16)
(16) ) and the boundary conditions, are called the optimality system. The strict concavity of the objective functional
, as well as the Lipschitz continuity of the right-hand side of the state equations and the adjoint equations in the state and adjoint variables, yields the uniqueness of solutions of the optimality system for small time [Citation14].
4. Numerical results
Parameter estimates were obtained from the Beth Israel Deaconess Medical Center's computerized database system, which provides patient and infection control data [Citation4] (see Table ).
Table 1. Parameter values [Citation4].
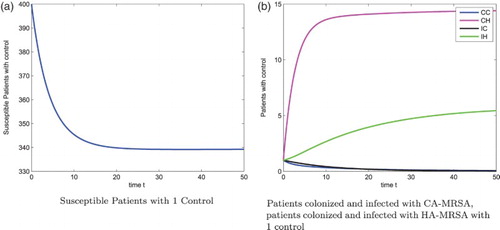
Figure shows the susceptible patients, patients colonized with the CA-MRSA, patients infected with the CA-MRSA, patients colonized with the HA-MRSA, and patients infected with the HA-MRSA, respectively, without any control strategies for . Optimal 3-control strategies dramatically reduce the severity of the patients colonized with the CA-MRSA, patients infected with the CA-MRSA, patients colonized with the HA-MRSA, and patients infected with the HA-MRSA, respectively, see Figure . The corresponding 3-control strategies are in shown Figure (a), with
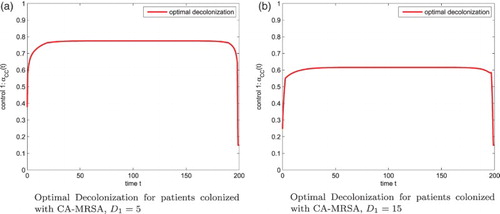
. Our numerical results also show that the optimal screening for the colonized and infected patients are 1.5%, 1.5%, respectively, see Figure .
Figure 2. Patients: without control, N=400. (a) Susceptible Patients w/o control (b) Patients colonized and infected with CA-MRSA, patients colonized and infected with HA-MRSA w/o control.
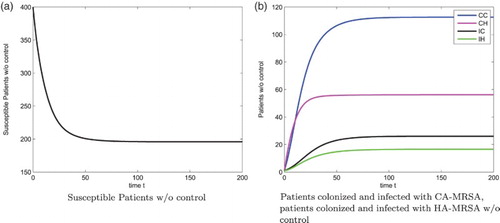
Figure 3. Patients: with 3 controls,
. (a) Susceptible Patients with 3 Controls (b) Patients colonized and infected with CA-MRSA, patients colonized and infected with HA-MRSA with 3 controls.
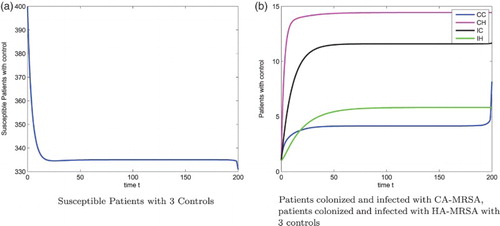
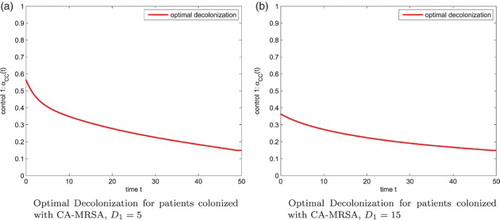
Figure 4. Optimal Decolonization for patients colonized with CA-MRSA, . (a) Optimal Decolonization for patients colonized with CA-MRSA,
(b) Optimal Decolonization for patients colonized with CA-MRSA,
.
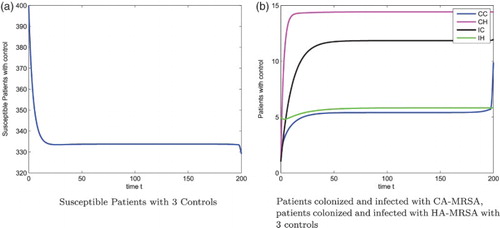
Figure 5. Optimal Screening for patients colonized and infected with CA-MRSA, . (a) Optimal Screening for patients colonized with CA-MRSA (b) Optimal Screening for patients infected with CA-MRSA.
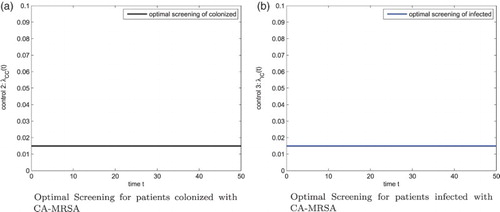
When the decolonization for patients colonized with the CA-MRSA is more expensive, that is, we increase , the corresponding optimal control effort is reduced, but the optimal control strategies for the screening of patients colonized and infected with CA-MRSA are still 1.5%, 1.5%, respectively. Figure (b) gives the optimal decolonization strategy of the colonized CA-MRSA patients. The figure of susceptible patients, patients colonized and infected with CA-MRSA, and patients colonized and infected with HA-MRSA, are shown in Figure .
Figure 6. Patients: with 3 controls,
. (a) Susceptible Patients with 3 Controls (b) Patients colonized and infected with CA-MRSA, patients colonized and infected with HA-MRSA with 3 controls.
The optimal control problem can be reformulated to find the optimal strategy of each control method when used alone. Based on our numerical findings above, the optimal screening for the colonized and infected patients are 1.5%, 1.5%, respectively, so we set these two controls to be zero in the system (Equation1(1)
(1) )–(Equation5
(5)
(5) ) and in the objective functional (Equation7
(7)
(7) ). Then the optimal decolonization for patients colonized with CA-MRSA is determined numerically. See Figure for the susceptible patients, patients colonized and infected with CA-MRSA, patients colonized and infected with HA-MRSA, respectively. We observe more reduction in the patients colonized and infected with CA-MRSA compared with 3-control case, see Figures (b) and
(b). Figure (a) gives the optimal decolonization strategy. We observe a different pattern for optimal decolonization of 1 or 3-control when comparing Figures (a) with (a). Figure (b) gives the optimal decolonization for
.
5. Conclusion and discussion
We have provided an optimal control analysis that evaluates the dynamic factors involved in specific interventions of CA-MRSA and HA-MRSA infections in hospital settings. Parameter estimates were obtained from the Beth Israel Deaconess Medical Center's computerized database system, which provides patient and infection control data [Citation4]. Our study indicates that emphasis on CA-MRSA decolonization, rather than CA-MRSA screening and isolation, can be a patient-outcome effective and cost-effective strategy for substantial reduction in these infections in hospital settings.
Although our modeling result is consistent with a patient cohort study in [Citation9], which reported that a universal, rapid MRSA admission screening strategy did not reduce nosocomial MRSA infection in a surgical department with endemic MRSA prevalence but relatively low rates of MRSA infection; our finding is controversial [Citation22]: numerous government agencies have mandated MRSA screening programs, and yet several authorities in infection control organizations have questioned the appropriateness of mandated screening. In [Citation32], it is argued that universal MRSA screening strategies are far more cost-intensive than the targeted screening approaches. In addition, it was demonstrated that all targeted screening strategies produce lower costs than not performing a screening at all. Other modelling approaches have emphasized that decolonization should be accompanied by emphasized patient isolation, patient screening, healthcare worker hygiene measures, and stewardship of antimicrobial regimens [Citation2, Citation11, Citation34, Citation35]. The cost-effectiveness of identifying colonized patients, conducting decolonization treatments on a suitably limited fraction of them, and preventing the emergence of resistant strains from decolonization pharmaceuticals involves complex interrelated dynamic elements that require further modelling studies.
Acknowledgments
We are grateful to Dr Erika M.C. D'Agata at Beth Israel Deaconess Medical Center, Boston, Massachusetts for her useful discussions on our project and choices of the decolonization of colonized CA-MRSA patients, the screening of patients colonized and infected with CA-MRSA as control strategies.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- S.F. Bradley, Eradication or decolonization of methicillin-resistant Staphylococcus aureus carriage: What are we doing and why are we doing it?, Clin. Infect. Dis. 44(2) (2007), pp. 186–189.
- F. Chamchod and S. Ruan, Modeling methicillin-resistant Staphylococcus aureus in hospitals: Transmission dynamics, antibiotic usage and its history, Theoret. Biol. Med. Mod. 9(25) (2012).
- B.S. Cooper, G.F. Medley, S.P. Stone, C.C. Kibbler, B.D. Cookson, J.A. Roberts, G. Duckworth, R. Lai, and S. Ebrahim, Methicillin-resistant Staphylococcus aureus in hospitals and the community: Stealth dynamics and control catastrophes, Proc. Natl. Acad. Sci. USA 101 (2004), pp. 10223–10228.
- E.M.C. D'Agata, G.F. Webb, M.A. Horn, R.C. Moellering, and S. Ruan, Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals, Clin. Infect. Dis. 48 (2009), pp. 274–284.
- E.M.C. D'Agata, G.F. Webb, M.A. Horn, R.C. Moellering, and S. Ruan, Competition of hospital-acquired and community-acquired methicillin-resistant Staphylococcus aureus strains in hospitals, J. Biol. Dyn. 4(1) (2010), pp. 115–129.
- S.L. Davis, M.J. Rybak, M. Amjad, G.W. Kaatz, and P.S. McKinnon, Characteristics of patients with healthcare-associated infection due of SCCmec type IV methcillin-resistant Staphylococcus aureus, Infect. Control Hosp. Epidemiol. 27 (2006), pp. 1025–1031.
- P. Eisler, Dangerous MRSA Bacteria Expand into Communities, Usatoday.com, Gannett, 16 Dec. 2013. Web. 01 Apr. 2014 27.
- W.H. Fleming and R.W. Rishel, Deterministic and Stochastic Optimal Control, Springer-Verlag, New York, 1975.
- S. Harbarth, C. Fankhauser, J. Schrenzel, J. Christenson, P. Gervaz, C. Bandiera-Clerc, G. Renzi, N. Vernaz, H. Sax, and D. Pittet, Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial Infection in surgical patients, JAMA 299 (2008), pp. 1149–1157.
- B.C. Herold, L.C. Immergluck, and L.C. Maranan, et al., Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk, JAMA 279 (1998), pp. 593–598.
- A. Hurford, A.M. Morris, D.N. Fisman, and J. Wu, Linking antimicrobial prescribing to antimicrobial resistance in the ICU: Before and after an antimicrobial stewardship program, Epidemics 4 (2012), pp. 203–210.
- E. Klein, D.L. Smith, and R. Laxminarayan, Hospitalizations and deaths caused by methicillin resistant Staphylococcus aureus, United States, 1999–2005, Emerg. Infect. Dis. 12 (2007), pp. 1840–1846.
- M.F.Q. Kluytmans-VandenBergh and J.A.J.W. Kluytmans, Community-acquired methicillin-resistant Staphylococcus aureus: Current perspectives, Clin. Microbiol. Infect. 12 (2006(S1)), pp. 9–15.
- S. Lenhart and J.T. Workman, Optimal Control Applied to Biological Models, CRC Press, New York, 2007.
- B.R. Levin, Minimizing potential resistance: A population dynamics view, Clin. Infect. Dis. 33 (2001), pp. S161–S169.
- D.L. Lukes, Differential Equations: Classical to Controlled, Academic Press, New York, 1982.
- Centers for Disease Control, Methicillin-resistant Staphylococcus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR 52 (2003), pp. 793–795.
- L.G. Miller, F. Perdreau-Remington , G. Rieg, S Mehdi, J. Perlroth, A.S. Bayer, A.W. Tang, T.O. Phung, and B. Spellberg, Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles, New Engl. J. Med. 352 (2005), pp. 1445–1453.
- C.A. Muto, J.A. Jernigan , B.E. Ostrowsky, H.M. Richet, W.R. Jarvis, J.M. Boyce, and B.M. Farr, SHEA guidelines for preventing nosocomial transmission of multidrug resistant strains of Staphylococcus aureus and Enterococcus, Infect. Control Hosp. Epidemiol. 24 (2003), pp. 362–86.
- J.A. Otter and G.L. French, Nosocomial transmission of community-associated meticillin-resistant Staphylococcus aureus: An emerging threat, Lancet Infect. Dis. 6 (2006), pp. 753–755.
- M. Patel, K.B. Waites, C.J. Hoesley, A.M. Stamm, K.C. Canupp, and S.A. Moser, Emergence of USA300 MRSA in a tertiary medical centre: Implications for epidemiological studies, J. Hosp. Infect. 68 (2008), pp. 208–213.
- L. Peterson and D. Diekema, To screen or not to screen for methicillin-resistant Staphylococcus aureus, J. Clin. Microbiol. 48 (2010), pp. 683–689.
- L. Pontryagin, V. Boltyanskii, R. Gamkrelidze, and E. Mishchenko, The Mathematical Theory of Optimal Processes, Wiley-Interscience, New York, 1962.
- K. Popovich, R. Weinstein, and B. Hota, Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains?, Clin. Infect. Dis. 46 (2008), pp. 795–798.
- K. Prabaker and R.A. Weinstein, Trends in antimicrobial resistance in intensive care units in the United States, Curr. Opin. Crit. Care 17 (2011), pp. 472–479.
- L.B. Rice, Antimicrobial resistance in gram-positive bacteria, Am. J. Infect. Control (2006), Jun;34(5 Suppl 1):S11-9; discussion S64-73.
- J.V. Robotham, C.A. Scarff, D.R. Jenkins, and G.F. Medley, Meticillin-resistant Staphylococcus aureus (MRSA) in hospitals and the community: model predictions based on the UK situation, J. Hosp. Infect. 65 (2007(S2)), pp. 93–99.
- L. Saiman, M.O. Keefe, P.L. Graham, F. Wu, B.S. Salim, B. Kreiswirth, A. LaSala, P.M. Schlievert, and P.D. Latta, Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women, Clin. Infect. Dis. 37 (2003), pp. 1313–1319.
- U. Seybold, E.V. Kourbatova, J.G. Johnson, S.J. Halvosa, Y.F. Wang, M.D. King, S.M. Ray, and H.M. Blumberg, Emergence of Community-Associated Methicillin-Resistant Staphylococcus aureus USA300 Genotype as a Major Cause of Health Care–Associated Blood Stream Infections, Clin. Infect. Dis. 42 (2006), pp. 647–656.
- Study: Resistant Infections Plummet at VA Hospitals Because of MRSA Initiative 2011 Issues, Department of Veterans Affairs (VA), Infectious Disease, May 2011.
- A. Tristan, M. Bes , H. Meugnier, G. Lina, B. Bozdogan, P. Courvalin, M.-E Reverdy, M.C. Enright, F. Vandenesch, and J. Etienne, Global distribution of pantonvalentine leukocidin-positive methicillin-resistant Staphylococcus aureus, 2006, Emerg. Infect. Dis. 13 (2007), pp. 594–600.
- A. Tübbicke, C. Hübner, N.-O. Hüner, C. Wegner, A. Kramer, and S. Fleßa, Cost comparison of MRSA screening and management - a decision tree analysis, BMC Health Serv. Res. 12(438) (2012).
- P. van den Driessche and J. Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Math. Biosci. 180 (2002), pp. 29–48.
- J. Wang, L. Wang, P. Magal, Y. Wang, J. Zhuo, X. Lu, and S. Ruan, Modelling the transmission dynamics of meticillin-resistant Staphylococcus aureus in Beijing Tongren hospital, J. Hosp. Infect. 79 (2011), pp. 302–308.
- X. Wang, S. Panchanathan, and G. Chowell, A data-driven mathematical model of CA-MRSA transmission among age groups: Evaluating the effect of control interventions, PLoS Comp. Biol. 9(11) (2013).
- N. Zetola, J.S. Francis, E.L. Nuermberger, and W.R. Bishai, Community-acquired meticillin-resistant Staphylococcus aureus: An emerging threat, Lancet Infect. Dis. 5 (2005), pp. 275–286.