 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We develop and analyse an ordinary differential equation model to investigate the transmission dynamics of releasing Wolbachia-infected mosquitoes to establish an endemic infection in a population of wild uninfected mosquitoes. Wolbachia is a genus of endosymbiotic bacteria that can infect mosquitoes and reduce their ability to transmit some viral mosquito-transmitted diseases, including dengue fever, chikungunya, and Zika. Although the bacterium is transmitted vertically from infected mothers to their offspring, it can be difficult to establish an endemic infection in a wild mosquito population. Our transmission model for the adult and aquatic-stage mosquitoes takes into account Wolbachia-induced fitness change and cytoplasmic incompatibility. We show that, for a wide range of realistic parameter values, the basic reproduction number,
, is less than one. Hence, the epidemic will die out if only a few Wolbachia-infected mosquitoes are introduced into the wild population. Even though the basic reproduction number is less than one, an endemic Wolbachia infection can be established if a sufficient number of infected mosquitoes are released. This threshold effect is created by a backward bifurcation with three coexisting equilibria: a stable zero-infection equilibrium, an intermediate-infection unstable endemic equilibrium, and a high-infection stable endemic equilibrium. We analyse the impact of reducing the wild mosquito population before introducing the infected mosquitoes and observed that the most effective approach to establish the infection in the wild is based on reducing mosquitoes in both the adult and aquatic stages.
1. Introduction
We use a disease transmission model to investigate the conditions for releasing Wolbachia-infected mosquitoes that will establish an endemic infection in a population of wild uninfected mosquitoes. The Wolbachia-infected mosquitoes are less able to transmit dengue, chikungunya, or Zika virus [Citation2,Citation10,Citation12,Citation14,Citation16,Citation32,Citation34,Citation39,Citation56]. The goal of the modeling effort is to better understand how this bacterium can be used to control vector-borne diseases. We observed that the basic reproductive number for the Wolbachia model is less than one for typical model parameters, hence small Wolbachia infestations will die out. Our model predicts that there is a critical threshold, and if a sufficient number of infected mosquitoes are released, then an endemic Wolbachia-infected population of mosquitoes can be established. We also investigated how this critical threshold can be reduced by first decreasing the population of wild mosquitoes.
Dengue fever, chikungunya, and Zika are some of the world's most significant and widespread arthropod-borne viral diseases [Citation13,Citation43]. The main vector, Aedes aegypti, is prevalent throughout the world and the secondary vector, Aedes albopictus keeps expanding its geographic distribution [Citation25]. There is no specific therapy available and even though the world first dengue vaccine was licensed for use last year, its efficacy is lower and varied between virus serotype, population age group, and country [Citation4,Citation55]. Traditional control strategies focused on reducing population of Aedes mosquito vectors have failed to significantly slow the current pandemics. There has been 30 fold increase in dengue fever cases over the past 50 years [Citation27]. Each year, approximately 400 million people are infected with dengue virus in more than 100 countries [Citation26], while the other one-third of the world's population is at risk. The ongoing chikungunya epidemics in the Americas, the Pacific Islands, and Indian Ocean Chikungunya causes an estimated three million infections each year [Citation44,Citation47]. Zika has been declared a global health security threat because of the severe birth defects and neurological problems caused by the disease [Citation42]. These diseases are driving a world-wide search for effective approaches to break the transmission cycles in Aedes aegypti and Aedes albopictus mosquitoes [Citation56].
Wolbachia induces resistance to dengue virus in Aedes aegypti [Citation1] and limits transmission of dengue virus in Aedes albopictus [Citation36]. Increasing attention has been paid to controlling the spread of these diseases by targeting mosquito longevity by introducing genetically modified mosquitoes or introducing endosymbiotic Wolbachia bacteria to shorten the mosquito lifespan [Citation2,Citation32,Citation56]. That is, Wolbachia-infected mosquitoes are released to create a sustained infection in the wild (Wolbachia-free) population. If the infection is sustained, then the wild infected mosquitoes will be less effective in transmitting these diseases. We create and analyse a mathematical model to help understand the underlying dynamics of Wolbachia-infected mosquitoes that are needed to create a sustained endemic Wolbachia infection. Once the Wolbachia disease transmission model is well understood, one of our future goals will be to couple this model with a mosquito-human model for the spread of dengue, chikungunya, and Zika.
1.1. Wolbachia bacteria
Wolbachia pipientis bacterium is a maternally inherited endosymbiont infecting more than of all insect species. The wMel strain of Wolbachia pipientis has the ability to alter host reproduction through parthenogenesis, which results in the development of unfertilized eggs, male killing, feminization, and cytoplasmic incompatibility (CI) [Citation3,Citation40] that prevents the eggs from forming viable offspring. The latter includes strategies for both the suppression and replacement of medically important mosquito populations. Cytoplasmic incompatibility is an incompatibility between the sperm and eggs induced by Wolbachia infection [Citation18] and has received considerable attention as a method to control vector-borne diseases [Citation51].
Uninfected females only mate successfully with uninfected males, while infected females can mate successfully with both uninfected and infected males [Citation8]. If a male fertilizes a female harbouring the same type of infection, the offspring still can survive [Citation32]. When Wolbachia-infected males mate with uninfected females, or females infected with a different Wolbachia strain, then the CI often results in killing the embryos. [Citation18,Citation48,Citation59]. Therefore, infected females have a reproduction advantage over uninfected females due to protection from CI [Citation58].
To successfully transmit the virus, a vector must imbibe virus particles during blood-feeding and survive to the point that the pathogen can be biologically transmitted to the next vertebrate host [Citation41]. This time period, called extrinsic incubation period , varies with ambient temperature, many other climatic factors, and characteristics of the vector-parasite system [Citation57]. Vectors that survive long enough to transmit the pathogen are called effective vectors [Citation41]. Typically, mosquito-borne viral diseases have an incubation period between one and two weeks to transmit through Aedes aegypti populations [Citation13]. A life-shortening strain of Wolbachia may halve the life span of Aedes aegypti [Citation34]. Wolbachia infection may reduce the rate of disease transmission due to the reduction on the lifespan of infected mosquitoes or the interference with mosquito susceptibility to the virus.
1.2. Existing mosquito-Wolbachia models
Ordinary differential equation (ODE) models have been developed to explore key factors that determine the success of applying Wolbachia to control viral diseases. A single-sex model for Wolbachia infection with both age-structured and unstructured models were presented to study the stability and equilibrium based on the assumption that Wolbachia infection leads to increased mortality or reduced birth rate [Citation11]. A model assuming a fixed ratio of females and males addressed how pathogen protection affects Wolbachia invasion [Citation49]. Age-structured and unstructured models combining males and females were found to be different in terms of existence and stability of equilibrium solutions [Citation11]. A stochastic model for female mosquitoes was developed to investigate the impact of introduction frequency on establishment of Wolbachia [Citation22].
Discrete-time models explored the impact of the type of immigration and the temporal dynamics of the host population on the spread of Wolbachia, assuming equal sex ratio between males and females [Citation15]. Discrete generation models for female mosquitoes were built to understand unstable equilibrium produced by reduced lifespan or lengthened development [Citation52]. Reaction–diffusion and integro-difference equation models have been used to analyse the impact of insect dispersal and infection spread on invasion of Wolbachia [Citation5,Citation19,Citation45,Citation49].
An ODE model was developed to evaluate the desirable properties of the Wolbachia strain to be introduced to female mosquitoes, assuming that Wolbachia-infected mosquitoes have reduced lifespan and reduced capability to transmit viral diseases, and an equal fraction of male and female mosquitoes [Citation20]. A continuous time non-spatial model and a reaction–diffusion model incorporating lifespan shortening and CI were developed to study factors that determine the spatial spread of Wolbachia through a population of female Aedes aegypti mosquitoes assuming constant population size and perfect maternal transmission of Wolbachia [Citation46]. A two-sex deterministic model with deterministic immature life stages and stochastic female adult life stage was developed to understand Wolbachia invasion into uninfected host population [Citation6].
A single-strain model, two-strain model, and spatial model were developed to study whether multiple stains of Wolbachia can coexist in a spatial context [Citation23]. A two-sex ODE model taking into account different death rates, but the same egg laying rates of Wolbachia-infected and Wolbachia uninfected mosquitoes [Citation24] showed the basic reproduction number is always less than one, and the complete infection equilibrium (CIE) is locally asymptotically stable (LAS) due to positive determinant of the Jacobian matrix for the system. Simulations showed that dengue epidemics will not occur when Wolbachia infection is sufficiently prevalent [Citation24]. A two-sex mosquito model assuming complete vertical transmission and equal death rates for male and female mosquitoes was developed and four steady states were found [Citation38].
Most of these models consider either a single-sex model for adult mosquitoes, or assume a fixed ratio between the number of male and female mosquitoes. Also, most of the models assume homogeneous death rates and egg laying rates for Wolbachia-free and Wolbachia-infected mosquitoes. Our model addresses both of these issues. Our emphasis is to understand how Wolbachia infection can be established in a wild population of mosquitoes.
1.3. Results
We proposed a compartmental two-sex model to investigate the underlying mechanisms that may contribute to invasion and sustainable establishment of Wolbachia in mosquito populations. We assigned female and male mosquitoes to different classes to understand corresponding roles that they are playing in the spread of Wolbachia in mosquito populations.
We showed that, for a wide range of realistic parameter values, the basic reproduction number, , for this model is less than one. Hence, the epidemic will die out if only a few
Wolbachia-infected mosquitoes are introduced into the wild population. Even though the basic reproduction number is less than one, an endemic Wolbachia infection can be established if a sufficient number of infected mosquitoes are released. This threshold effect can be explained as a backward bifurcation with three coexisting equilibria: a stable zero-infection equilibrium, an intermediate-infection unstable endemic equilibrium, and a high-infection stable endemic equilibrium.
If the number of infected individuals is below the unstable endemic equilibrium, then the infection decays to the zero-infection equilibrium. Conversely, if the number of infected mosquitoes is greater than unstable endemic equilibrium, then the solution tends to the stable high-infection equilibrium. We identified the relationships between dimensionless combinations of model parameters and the initial conditions for Wolbachia to be attracted to the high-infection state. As expected, the number of infected female mosquitoes needed to be released to establish the infection in a wild Wolbachia-free population decreases as increases to one. We analysed the impact of reducing the wild mosquito population before introducing the infected mosquitoes. We found that the most effective approach of reducing the number of infected mosquitoes needed to establish a wild Wolbachia-infected population requires reducing wild mosquito populations in both the adult and aquatic stages before the release. This could be accomplished by recursive spraying, or a combination of spraying and larvae control [Citation21].
Our main findings are:
Three equilibria, the disease-free equilibrium (DFE), the endemic equilibrium (EE), and the CIE coexist when
. The DFE is a steady state where all individuals are Wolbachia-free. The EE is a steady state where some individuals are Wolbachia-free, and the rest are infected with Wolbachia. The CIE is a steady state where all individuals are infected with Wolbachia.
The backward bifurcation analysis of our Wolbachia transmission model predicts that if
, then there is a critical threshold for the number of infected mosquitoes released in the wild before the infection can be established. If we release too few Wolbachia-infected mosquitoes, then the Wolbachia infection will die out.
Killing both aquatic state (eggs and larvae) and adult mosquitoes before releasing the infected mosquitoes greatly increases the chance that the infection will be established. Our model quantifies what fraction of wild mosquitoes must be killed before releasing Wolbachia-infected mosquitoes.
After introducing the mathematical model, we summarize the key results from the analysis and numerical simulations. We conclude with a discussion of the relevance, importance, and future directions for this work.
2. Description of model framework
We developed an ODE model incorporating adult females (F), adult males (M), and an aggregated aquatic (A) stage that includes the egg, larvae, and pupae stages. The population dynamics of mosquitoes without taking into account Wolbachia is in the appendix, Equation (A1). The vertical transmission of Wolbachia from infected females to their offspring is a key factor in establishing an endemic-infected population.
Mosquitoes are grouped into six compartments: susceptible aquatic stage, , infected aquatic stage,
, susceptible female mosquitoes,
, infected female mosquitoes,
, susceptible male mosquitoes,
, and infected male mosquitoes,
. The eclosion rates of susceptible female and male mosquitoes hatching from eggs are
and
, respectively. Similarly, the birth rates of infected female and male mosquitoes are
and
. Death rates of uninfected male mosquitoes and infected male mosquitoes are
and
. Death rates of uninfected female mosquitoes and infected female mosquitoes are
and
. Death rates of uninfected male mosquitoes and infected male mosquitoes are
and
. Development rates of uninfected aquatic stage and infected aquatic stage of mosquitoes are
and
. The model parameters are described in Table .
Table 1. State variables and parameters for the model (Equation1 (1a)
(1a)  (1a) ).
(1a) ).
The model (Figure ) describing population dynamics of aquatic stage, adult male, and adult female mosquitoes is given by
(1a)
(1a)
(1b)
(1b)
(1c)
(1c)
(1d)
(1d)
(1e)
(1e)
(1f)
(1f)
Figure 1. The birthing rates (Equation2(1b)
(1b) ) capture that when the uninfected males mate with uninfected females, they produce uninfected offspring. When infected males mate with uninfected females, then CI causes the embryos to die before hatching. Uninfected males mating with infected females produce a fraction, denoted by
, of infected offspring by vertical transmission. Cross of infected males with infected females produces a fraction of infected offspring.
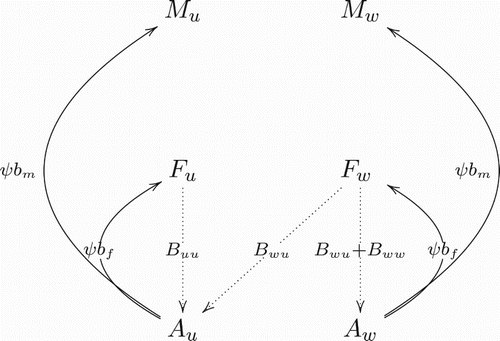
Wolbachia can be transmitted vertically from infected parents to their offspring where
Uninfected males mating with uninfected females produce uninfected offspring.
Infected males mating with uninfected females leads to death of embryos before hatching due to cytoplasmic incompatibility.
Uninfected males mating with infected females produce a fraction,
, of infected offspring by vertical transmission.
Infected males mating with infected females produces a fraction of infected offspring.
Because the vertical transmission and birth rates, , depend on the sex of the infected or uninfected parents, the model included the four egg laying situations
(2a)
(2a)
(2b)
(2b)
(2c)
(2c)
(2d)
(2d)
Here, and
are the fractions of uninfected and infected male mosquitoes.
is the egg laying rate of uninfected females mating with uninfected males.
is the egg laying rate of uninfected females mating with infected males.
is the egg laying rate of infected females mating with uninfected males.
is the total number of aquatic stage of mosquitoes, and
is carrying capacity of aquatic stage of mosquitoes.
3. Stability analysis of the equilibrium solutions
When , there are three equilibria: the DFE, the EE and the CIE. We analyse the stability of each equilibrium.
3.1. Stability of the DFE
We use the next generation matrix approach to compute the basic reproduction number [Citation53]. Only infected compartments are considered for ease of computation:
where is a vector for new infected, and
is a vector for transfer between compartments.
Jacobian matrices for transmission, F, and transition, V, [Citation53] are defined as
(3)
(3) where
represents the DFE, and
is the number or proportion of infected individuals in compartment j,
.
The unique DFE is
where
is the threshold for Wolbachia-free offspring,
is the total number of eggs laid by uninfected female mosquitoes,
is the probability that aquatic stage of mosquitoes survive to the point when they develop into adult mosquitoes.
is the number of female eggs that develop into adult mosquitoes. When
, then the mosquito population may grow; otherwise, the population will decrease.
The Jacobian matrix of evaluated at DFE is
where
.
The Jacobian matrix of evaluated at DFE is
The next generation matrix for infected compartments at DFE is
The basic reproduction number is the largest absolute eigenvalue of , denoted by
(4)
(4)
Wolbachia-infected and Wolbachia-free female mosquitoes produce and
eggs during their lifetime, respectively. Hence,
is the ratio of the number of eggs produced by
Wolbachia-infected females to the number of eggs produced by Wolbachia-free females during their lifetime.
is geometric mean of vertical transmission rate and the ratio of the total number of eggs produced by Wolbachia-infected females to the total number of eggs produced by Wolbachia-free females.
When the variables are ordered as , Jacobian matrix at equilibrium
for system of Equations (Equation1
(1a)
(1a) ) is
where
At DFE, D=0, then the eigenvalues of J are eigenvalues of A and B. Matrices A and B are
If , then all eigenvalues of A are negative. If
, then all eigenvalues of B are negative. Therefore, the system (Equation1
(1a)
(1a) ) at DFE is LAS whenever
and
.
3.2. Stability of the CIE
If and
and the vertical transmission is
, (
), then the ratio of the infected to the uninfected aquatic stage mosquitoes, k, is
and there is a unique EE:
(5a)
(5a)
(5b)
(5b)
(5c)
(5c)
(5d)
(5d)
(5e)
(5e)
(5f)
(5f)
where
.
If we further assume that , then
and the unique CIE is
Jacobian matrix of system of Equations (Equation1
(1a)
(1a) ) at CIE for
is
Eigenvalues of are composed of three eigenvalues of A and three eigenvalues of B. The eigenvalues of A are all negative. Characteristic polynomial of B is
(6)
(6) If
, all eigenvalues of B are negative. Therefore, the CIE is LAS whenever
, as shown in Figure (a).
Figure 2. Bifurcation diagrams for Wolbachia vertical transmission. and
are varying, and other parameter values are the same as those baseline values in Table . Denote the intersection of two endemic equilibria, that is, the intersection of the black dashed line and x-axis, as
. When
and
, no endemic equilibria exist. When
and
, as the vertical transmission rate increases so does
and the LAS equilibrium approaches a constant. If we increase the number of infected females, then the EE may become stable EE or CIE If we decrease the number of infected females at EE, then the EE may become DFE. WIF denotes Wolbachia-infected female mosquitoes.
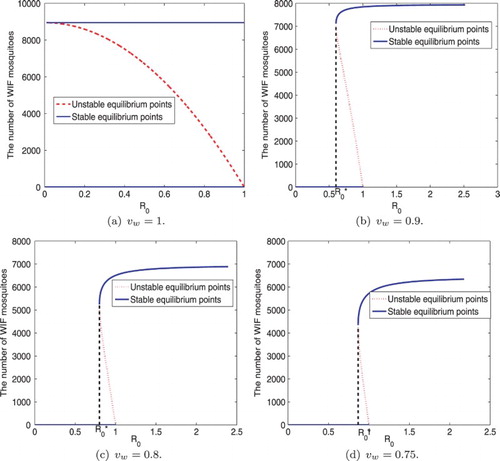
Table 2. Baseline values for parameters in Model (Equation1 (1a)
(1a)  (1a) ).
(1a) ).
3.3. Stability of the EE
When vertical transmission is incomplete, i.e. , then at EE,
(7)
(7) We assume
, and let
. When
, that is,
, then Equation (Equation6
(1f)
(1f) ) has two roots:
and
.
If and
, two endemic equilibria exist as shown in Figure (b), (c), and (d). When
, the EE is LAS. When
, the equilibrium is not LAS and backward bifurcation occurs. If
and
, then EE does not exist, only DFE exists. When
, then
. Let
, which is the intersection of unstable and stable EE. When
, a unique EE exists with
. It is shown to be LAS by numerical simulations.
4. Bifurcation analysis results
We observed that Wolbachia can persist when , where
is the turning point of the backward bifurcation. Three equilibria, namely, DFE, EE, and CIE coexist when
,
,
, and
as shown in Figure (a). The DFE is LAS whenever
and
, and CIE exists and is LAS as long as
. The unique EE is not LAS, and can become DFE by decreasing the number of infected individuals or become CIE by increasing the number of infected individuals. Figure (b)–( d) showed that two endemic equilibria exist when
and
, and only one of them is LAS as shown by numerical simulations. When
is larger,
is closer to one. The conditions for the existence of the equilibria and their stability are summarized in Table .
Table 3. Threshold condition for existence of DFE, EE, and CIE and their stability.
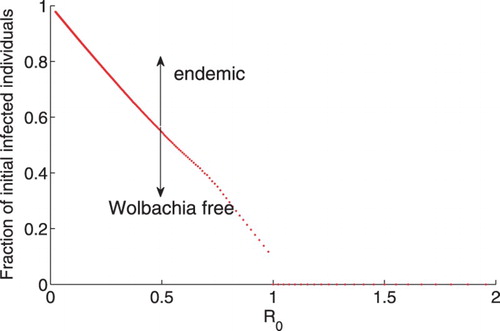
Initial condition thresholds for an epidemic to occur vary with the vertical transmission rate as shown in Table . When , the epidemic will spread if at least
of the population are initially infected. When
, the epidemic will spread if at least
of the population are initially infected. When
, the epidemic will spread if at least
of the population are initially infected. When the vertical transmission rate is high, the threshold for the number of initially infected individuals to start Wolbachia epidemic is low.
Table 4. Initial condition thresholds for epidemic to occur with different vertical transmission rates.
The initial condition threshold for an epidemic to occur varies with the ratio of death rates of Wolbachia-infected male mosquitoes to death rates of Wolbachia-free male mosquitoes, , while fixing other parameters. The larger
is, the larger the initial number of infected individuals required to start a Wolbachia epidemic. With higher vertical transmission rate, a smaller percentage of Wolbachia carriers can invade, as shown in Table . Similarly, if we increase
or
, or decrease
or
, then the threshold for initial infection is smaller. Increasing
will increase the epidemic threshold for initial infection. The thresholds for fraction of initial infections decreasing with the basic reproduction number are shown in Figure .
Figure 3. Thresholds for fraction of infected individuals vary with reproduction number. ,
,
. When
, the smaller
is, the larger number of infected female mosquitoes are needed to be released for Wolbachia to be endemic. The Wolbachia infection is only sustained if the fraction of WIF mosquitoes is above the red dotted line.
The reproduction number is very sensitive to the vertical transmission rate, egg laying rates of Wolbachia-infected mosquitoes, egg laying rates of Wolbachia-free mosquitoes, and death rates of Wolbachia-infected female mosquitoes and Wolbachia-free female mosquitoes as shown in Equation (Equation4(1d)
(1d) ). The reproduction number varies directly with either the vertical transmission rate, the egg laying rates of
Wolbachia-infected mosquitoes, or the death rates of Wolbachia-free female mosquitoes. The reproduction number varies inversely with egg laying rates of
Wolbachia-free mosquitoes, or the death rates of Wolbachia-infected female mosquitoes.
We compared five strategies before the release of Wolbachia-infected female mosquitoes:
DFE: releasing Wolbachia-infected female mosquitoes at the DFE,
KHA: first killing half of the aquatic stage of mosquitoes,
KHM: first killing half of the wild adult mosquitoes,
KHM2: first killing half of the wild adult mosquitoes, and then killing half of the adult mosquitoes again after two weeks,
KHMA: first killing half of the wild mosquitoes and half aquatic stage of mosquitoes.
The ratios of the minimum number of Wolbachia-infected female mosquitoes that can lead to persistence of Wolbachia to the number of female mosquitoes at DFE are listed in Table in decreasing order. Notice that killing the adult mosquitoes only once is not an effective strategy because the aquatic stage mosquitoes hatch and quickly replace the wild uninfected population. Killing both the adult and aquatic (larvae) stage mosquitoes before releasing the infected mosquitoes is the most effective strategy.
Table 5. Different population suppression strategies applied before release of Wolbachia infected female mosquitoes can reduce the minimum number of Wolbachia-infected mosquitoes that can lead to persistence of Wolbachia.
The results section should provide details of all of the experiments that are required to support the conclusions of the paper. There is no specific word limit for this section, but details of experiments that are peripheral to the main thrust of the article and that detract from the focus of the article should not be included. The section may be divided into subsections, each with a concise subheading. Large datasets, including raw data, should be submitted as supporting files; these are published online alongside the accepted article. The results section should be written in the past tense.
5. Discussion
We developed a model considering two sex of aquatic stage and adult mosquitoes, diversity in the death rates of Wolbachia-infected mosquitoes and Wolbachia-free mosquitoes, and egg laying rates of Wolbachia-infected female mosquitoes and Wolbachia-free female mosquitoes. The general model is not constrained to particular weather condition, specific Wolbachia strains, or specific mosquito species, and it can be easily adapted to Aedes aegypti or Aedes albopictus at any location with parameters calibrated using realistic environmental factors, such as temperature and rainfall .
We found conditions for the existence of multiple equilibria and backward bifurcation. If vertical transmission is complete, then a unique EE exists and is not LAS when and
. A backward bifurcation occurs when EE changes into DFE if we decrease the initial number of infected individuals, or it reaches another LAS equilibrium if we increase the number of initially infected individuals. When vertical transmission is incomplete but more than
, and
, two endemic equilibria coexist, but only one EE is LAS such that it becomes DFE or EE by perturbation. Since Wolbachia-infected mosquitoes are less capable of transmitting the virus, complete Wolbachia-infection is the ideal case for disease control.
When , Wolbachia can spread with a small number of initially infected mosquitoes, although very slowly. Population replacement may occur. When
, Wolbachia can spread if initial number of infected individuals exceeds a threshold, which depends on the vertical transmission rate, ratio of egg laying rates of Wolbachia-infected females to egg laying rates of Wolbachia-free female mosquitoes, and ratio of death rates of infected female mosquitoes to death rates of uninfected female mosquitoes. A smaller number of
Wolbachia-infected female mosquitoes is needed to be released for persistence of Wolbachia if a population suppression strategy is implemented before the release.
The reproduction number is the product of the vertical transmission rate, ratio of the egg laying rates of Wolbachia-infected mosquitoes to egg laying rates of Wolbachia-free mosquitoes, and the ratio of death rates of Wolbachia-free mosquitoes to death rates of Wolbachia infected mosquitoes. If the total number of eggs produced by
Wolbachia-infected mosquitoes through vertical transmission is more than the total number of eggs produced by Wolbachia-free mosquitoes, then , such that CIE or EE is LAS.
The number of Wolbachia-infected mosquitoes required for sustained Wolbachia infection depends on vertical transmission rate, , the ratio of the number of eggs laid by
Wolbachia-infected mosquitoes to the number of eggs laid by Wolbachia-free mosquitoes,
, the ratio of the death rates of Wolbachia-free females to death rates of
Wolbachia-infected females,
, and the ratio of the death rates of Wolbachia-free males to death rates of Wolbachia-infected males
. These parameters depend on partculiarWolbachia strain. If the life span of a mosquito is shorter, then the mosquitoes will lay fewer eggs. Once we know the specific Wolbachia strain that a specific mosquito species, such as Aedes aegypti or Aedes albopictus is carrying, we can estimate the number of infected individuals needed to be released for sustainable Wolbachia establishment using this model.
We find that reducing both the aquatic stage and adult uninfected mosquitoes before releasing Wolbachia-infected female mosquitoes is the most effective strategy to reduce the number of Wolbachia-infected female mosquitoes needed for Wolbachia persistence (Table ). The second most effective strategy was to repeatedly kill the wild uninfected mosquitoes (to reduce both the adult and the aquatic stage mosquitoes) before releasing the infected mosquitoes.
The model and analysis can help in understanding how Wolbachia can invade and persist in mosquito populations. In future research, we will couple our model to a transmission model and analyse the impact that a Wolbachia-infected mosquito population has on the spread of dengue fever, chikungunya, and Zika. Previous studies have shown spatial heterogeneity in the mosquito population can be an important factor in establishing a sustained Wolbachia infected population [Citation5,Citation19,Citation49]. We will also add heterogeneity to our Wolbachia transmission models to better understand the impact of the diffusion of mosquitoes.
The discussion should spell out the major conclusions of the work along with some explanation or speculation on the significance of these conclusions. How do the conclusions affect the existing assumptions and models in the field? How can future research build on these observations? What are the key experiments that must be done? The discussion should be concise and tightly argued. The results and discussion may be combined into one section, if desired.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the National Institutes of Health. The authors thank Michael Robert for his many helpful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- G. Bian, Y. Xu, P. Lu, Y. Xie, and Z. Xi, The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti, PloS Neglected Trop. Dis. 6 (2010).
- M.S.C. Blagrove, C. Arias-Goeta, A. -B. Failloux, and S.P. Sinkins, Wolbachia strain wmel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus, Proc. National Acad. Sci. USA 109 (2012), pp. 255–260. doi: 10.1073/pnas.1112021108
- K. Bourtzis and S.L. O'Neill, Wolbachia infections and arthropod reproduction, Bioscience 480 (1998), pp. 287–293. doi: 10.2307/1313355
- M.R. Capeding, N.H. Tran, S.R. Hadinegoro, H.I. Ismail, T. Chotpitayasunondh, M.N. Chua, C.Q. Luong, K. Rusmil, D.N. Wirawan, R. Nallusamy, P. Pitisuttithum, U. Thisyakorn, I.-K. Yoon, D. van der Vliet, E. Langevin, T. Laot, Y. Hutagalung, C. Frago, M. Boaz, T.A. Wartel, N.G. Tornieporth, M. Saville, and A. Bouckenooghe, Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in asia: a phase 3, randomised, observer-masked, placebo-controlled trial, Lancet 384 (2014), pp. 1358–1365. doi: 10.1016/S0140-6736(14)61060-6
- M.H. Chan, P.S. Kim, and R. Marangell, Stability of travelling waves in a Wolbachia invasion, preprint (2016). Available at arXiv:1603.05744.
- P.R. Crain, J.W. Mains, E. Suh, Y. Huang, P.H. Crowley, and S.L. Dobson, Wolbachia infections that reduce immature insect survival: predicted impacts on population replacement, BMC Evol. Biol. 11 (2011), pp. 290–2148–11–290. doi: 10.1186/1471-2148-11-290
- H. Delatte, G. Gimonneau, A. Triboire, and D. Fontenille, Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian ocean, J. Med. Entomol. 46 (2009), pp. 33–41. doi: 10.1603/033.046.0105
- S.L. Dobson, E.J. Marsland, and W. Rattanadechakul, Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive, Genetics 160 (2002), pp. 1087–1094.
- Y. Dumont and J.M. Tchuenche, Mathematical studies on the sterile insect technique for the chikungunya disease and Aedes albopictus, J. Math. Biol. 65 (2012), pp. 809–854. doi: 10.1007/s00285-011-0477-6
- H.L.C. Dutra, M.N. Rocha, F.B.S. Dias, S.B. Mansur, E.P. Caragata, and L.A. Moreira, Wolbachia blocks currently circulating zika virus isolates in brazilian aedes aegypti mosquitoes, Cell Host Microbe (2016).
- J.Z. Farkas and P. Hinow, Structured and unstructured continuous models for Wolbachia infections, Bull. Math. Biol. 72 (2010), pp. 2067–2088. doi: 10.1007/s11538-010-9528-1
- N.M. Ferguson, D.T. Hue Kien, H. Clapham, R. Aguas, V.T. Trung, T.N. Bich Chau, J. Popovici, P.A. Ryan, S.L. O'Neill, E.A. McGraw, V.T. Long, L.T. Dui, H.L. Nguyen, N.V. Vinh Chau, B. Wills, and C.P. Simmons, Modeling the impact on virus transmission of wolbachia-mediated blocking of dengue virus infection of aedes aegypti, Sci. Translational Med. 7 (2015), pp. 279ra37–279ra37. doi: 10.1126/scitranslmed.3010370
- R.V. Gibbons and D.W. Vaughn, Dengue: An escalating problem, BMJ (Clin. Res. ed.) 324 (2002), pp. 1563–1566. doi: 10.1136/bmj.324.7353.1563
- C. Gunaratne, M.I. Akbas, I. Garibay, and O. Ozmen, Evaluation of zika vector control strategies using agent-based modeling, preprint (2016). Available at arXiv:1604.06121.
- P.A. Hancock, S.P. Sinkins, and H.C. Godfray, Population dynamic models of the spread of Wolbachia, Amer Nat. 177 (2011), pp. 323–333. doi: 10.1086/658121
- P.A. Hancock, S.P. Sinkins, H.C.J. Godfray, and S.L. O'Neill, Strategies for introducing wolbachia to reduce transmission of mosquito-borne diseases, PLoS Neglected Trop. Dis. 5 (2011), p. e1024. doi: 10.1371/journal.pntd.0001024
- J. Hofbauer and J.W.H. So, Uniform persistence and repellers for maps, Proc AMS 107 (1980), pp. 1137–1142. doi: 10.1090/S0002-9939-1989-0984816-4
- A.A. Hoffmann and M. Turelli, Cytoplasmic incompatibility in insects, in Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, S.L. O'Neill, A.A. Hoffmann, and J.H. Werren, eds., Oxford University Press, Oxford, 1997, pp. 42–80.
- M. Huang, M. Tang, and J. Yu, Wolbachia infection dynamics by reaction–diffusion equations, Sci/ China Math. 58 (2015), pp. 77–96. doi: 10.1007/s11425-014-4934-8
- H. Hughes and N.F. Britton, Modelling the use of Wolbachia to control dengue fever transmission, Bull. Math. Biol. 75 (2013), pp. 796–818. doi: 10.1007/s11538-013-9835-4
- S.P. Jacups, T.S. Ball, C.J. Paton, P.H. Johnson, and S.A. Ritchie, Operational use of household bleach to ‘crash and release’ aedes aegypti prior to wolbachia-infected mosquito release, J. Med. Entomol. 50 (2013), pp. 344–351. doi: 10.1603/ME12043
- V.A. Jansen, M. Turelli, and H.C. Godfray, Stochastic spread of Wolbachia, Proc. Biol. Sci./R. Soc. 275 (2008), pp. 2769–2776. doi: 10.1098/rspb.2008.0914
- M.J. Keeling, F.M. Jiggins, and J.M. Read, The invasion and coexistence of competing Wolbachia strains, Heredity 91 (2003), pp. 382–388. doi: 10.1038/sj.hdy.6800343
- J. Koiller, M.A. da Silva, M.O. Souza, C. Codeco, A. Iggidr, and G. Sallet, Aedes, Wolbachia and Dengue, Technical Report, Institute for Research in Computer Science and Automation, 2014.
- M.U. Kraemer, M.E. Sinka, K.A. Duda, A.Q. Mylne, F.M. Shearer, C.M. Barker, C.G. Moore, R.G. Carvalho, G.E. Coelho, W. Van Bortel, G. Hendrickx, F. Schaffner, I.R. Elyazar, H.-J. Teng, O.J. Brady, J.P. Messina, D.M. Pigott, T.W. Scott, D.L. Smith, G.W. Wint, N. Golding, and S.I. Hay, The global distribution of the arbovirus vectors aedes aegypti and ae. albopictus, Elife 4 (2015), p. e08347. doi: 10.7554/eLife.08347
- J.L. Kyle and E. Harris, Global spread and persistence of dengue, Annu. Rev. Microbiol. 62 (2008), pp. 71–92. doi: 10.1146/annurev.micro.62.081307.163005
- Z. Lam, D. Burke, M.R. Capeding, and C.K. Chong, Preparing for introduction of a dengue vaccine: Recommendations from the 1st dengue v2v asia-pacific meeting, Vaccine 29 (2011), pp. 9417–9422. doi: 10.1016/j.vaccine.2011.08.047
- M. Li and L. Wang, Global stability in some SEIR epidemic models, IMA 126 (2002), pp. 295–311.
- L.P. Lounibos and R.L. Escher, Sex ratios of mosquitoes from long-term censuses of florida tree holes, J. Amer. Mosquito Control Assoc. 24 (2008), pp. 11–15. doi: 10.2987/5656.1
- J. Mallet-Paret and G. Sell, The poincaré–bendixson theorem for monotone cyclic feedback systems with delay, J Differ Equ 125 (1996), pp. 441–489. doi: 10.1006/jdeq.1996.0037
- W.C. Marquardt and B.C. Kondratieff, Biology of Disease Vectors, Elsevier Academic Press, Burlington, MA, 2005.
- C.J. McMeniman, R.V. Lane, B.N. Cass, A.W.C. Fong, M. Sidhu, Y.-F. Wang, and S.L. O'Neill, Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti, Science 323 (2009), pp. 141–144. doi: 10.1126/science.1165326
- L.C. Monteiro, J.R. de Souza, and C.M. de Albuquerque, Eclosion rate, development and survivorship of Aedes albopictus (Skuse)(Diptera: Culicidae) under different water temperatures, Neotropical Entomol. 36 (2007), pp. 966–971. doi: 10.1590/S1519-566X2007000600021
- L.A. Moreira, I. Iturbe-Ormaetxe, J.A. Jeffery, G. Lu, A.T. Pyke, L.M. Hedges, B.C. Rocha, S. Hall-Mendelin, A. Day, M. Riegler, L.E. Hugo, K.N. Johnson, B.H. Kay, E.A. McGraw, A.F. van den Hurk, P.A. Ryan, and S.L. O'Neill, A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, Plasmodium, Cell 139 (2009), pp. 1268–1278.
- D. Moulay, M.A. Aziz-Alaoui, and M. Cadivel, The chikungunya disease: Modeling, vector and transmission global dynamics, Math. Biosci. 229 (2011), pp. 50–63. doi: 10.1016/j.mbs.2010.10.008
- L. Mousson, K. Zouache, C. Arias-Goeta, V. Raquin, P. Mavingui, and A.B. Failloux, The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus, PLoS Neglected Trop. Dis. 6 (2012), p. e1989. doi: 10.1371/journal.pntd.0001989
- J.M. Muldowney, Compound matrices and ordinary differential equations, Rocky Mountain J. Math. 20 (1990), pp. 857–872. doi: 10.1216/rmjm/1181073047
- M.Z. Ndii, R.I. Hickson, and G.N. Mercer, Modelling the introduction of Wolbachia into Aedes aegypti mosquitoes to reduce dengue transmission, ANZIAM J 53 (2012), pp. 213–227.
- M.Z. Ndii, R.I. Hickson, D. Allingham, and G.N. Mercer, Modelling the transmission dynamics of dengue in the presence of wolbachia, Math. Biosci. 262 (2015), pp. 157–166. doi: 10.1016/j.mbs.2014.12.011
- S.L. O'Neill, A. Hoffman, and J.H. Werren, Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, Oxford University Press, 1997.
- J.L. Rasgon, L.M. Styer, and T.W. Scott, Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods, J. Med. Entomol. 40 (2003), pp. 125–132. doi: 10.1603/0022-2585-40.2.125
- S.A. Rasmussen, D.J. Jamieson, M.A. Honein, and L.R. Petersen, Zika virus and birth defects-reviewing the evidence for causality, N Engl. J. Med. 2016 (2016), pp. 1981–1987. doi: 10.1056/NEJMsr1604338
- A. Roth, A. Mercier, C. Lepers, D. Hoy, S. Duituturaga, E. Benyon, L. Guillaumot, and Y. Souares, Concurrent outbreaks of dengue, chikungunya and zika virus infections-an unprecedented epidemic wave of mosquito-borne viruses in the pacific 2012–2014, Euro Surveillance 19 (2014), p. 20929. doi: 10.2807/1560-7917.ES2014.19.41.20929
- I.-C. Sam, S.-K. Loong, J.C. Michael, C.-L. Chua, W.Y.W. Sulaiman, I. Vythilingam, S.-Y. Chan, C.-W. Chiam, Y.-S. Yeong, and S. AbuBakar, et al., Genotypic and phenotypic characterization of chikungunya virus of different genotypes from malaysia, PloS One 7 (2012), p. e50476. doi: 10.1371/journal.pone.0050476
- P. Schofield, Spatially explicit models of turelli-hoffmann Wolbachia invasive wave fronts, J. Theoret. Biol. 215 (2002), pp. 121–131. doi: 10.1006/jtbi.2001.2493
- J.G. Schraiber, A.N. Kaczmarczyk, R. Kwok, M. Park, R. Silverstein, F.U. Rutaganira, T. Aggarwal, M.A. Schwemmer, C.L. Hom, R.K. Grosberg, and S.J. Schreiber, Constraints on the use of lifespan-shortening Wolbachia to control dengue fever, J. Theoret. Biol. 297 (2012), pp. 26–32. doi: 10.1016/j.jtbi.2011.12.006
- N. Seppa, Chikungunya: Is on the move: The virus has found a new hemisphere and might get a new latitude, Sci. News 187 (2015), pp. 16–20. doi: 10.1002/scin.2015.187012017
- S.P. Sinkins, Wolbachia and cytoplasmic incompatibility in mosquitoes, Insect Biochem Molecular Biol. 34 (2004), pp. 723–729. doi: 10.1016/j.ibmb.2004.03.025
- C. Souto-Maior, J.S. Lopes, E. Gjini, C.J. Struchiner, L. Teixeira, and M.G.M. Gomes, Heterogeneity in symbiotic effects facilitates wolbachia establishment in insect populations, Theoret. Ecol. 8 (2015), pp. 53–65. doi: 10.1007/s12080-014-0235-7
- L.M. Styer, S.L. Minnick, A.K. Sun, and T.W. Scott, Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood, Vector Borne Zoonotic Dis. (Larchmont, N.Y.) 7 (2007), pp. 86–98. doi: 10.1089/vbz.2007.0216
- E. Suh and S.L. Dobson, Reduced competitiveness of Wolbachia infected Aedes aegypti larvae in intra- and inter-specific immature interactions, J. Invertebrate Pathol. 114 (2013), pp. 173–177. doi: 10.1016/j.jip.2013.08.001
- M. Turelli, Cytoplasmic incompatibility in populations with overlapping generations, Evol: Int. J. Organic Evol. 64 (2010), pp. 232–241. doi: 10.1111/j.1558-5646.2009.00822.x
- P. van den Driessche and J. Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission, Math. Biosci. 180 (2002), pp. 29–48. doi: 10.1016/S0025-5564(02)00108-6
- M. Vidyasagar, Decomposing techniques for large-scale systems, IEEE Trans. Autom. Control AC-25 (1980), pp. 892–898.
- L. Villar, G.H. Dayan, J.L. Arredondo-García, D.M. Rivera, R. Cunha, C. Deseda, H. Reynales, M.S. Costa, J.O. Morales-Ramírez, G. Carrasquilla, L.C. Rey, R. Dietze, K. Luz, E. Rivas, M.C. Miranda Montoya, M. Cortés Supelano, B. Zambrano, E. Langevin, M. Boaz, N. Tornieporth, M. Saville, and F. Noriega, Efficacy of a tetravalent dengue vaccine in children in latin america, New Engl. J. Med. 372 (2015), pp. 113–123. doi: 10.1056/NEJMoa1411037
- T. Walker, P.H. Johnson, L.A. Moreira, I. Iturbe-Ormaetxe, F.D. Frentiu, C.J. McMeniman, Y.S. Leong, Y. Dong, J. Axford, P. Kriesner, A.L. Lloyd, S.A. Ritchie, S.L. O'Neill, and A.A. Hoffmann, The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations, Nature 476 (2011), pp. 450–453. doi: 10.1038/nature10355
- D.M. Watts, D.S. Burke, B.A. Harrison, R.E. Whitmire, and A. Nisalak, Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus, Amer J. Trop. Med. Hygiene 36 (1987), pp. 143–152.
- A.R. Weeks, M. Turelli, W.R. Harcombe, K.T. Reynolds, and A.A. Hoffmann, From parasite to mutualist: rapid evolution of Wolbachia in natural populations of drosophila, PLoS Biol. 5 (2007), p. e114. doi: 10.1371/journal.pbio.0050114
- X. Zhang, S. Tang, and R.A. Cheke, Birth-pulse models of wolbachia-induced cytoplasmic incompatibility in mosquitoes for dengue virus control, Nonlinear Anal: Real World Appl. 22 (2015), pp. 236–258. doi: 10.1016/j.nonrwa.2014.09.004
Appendix
The system of equations for mosquito population dynamics is
(A1a)
(A1a)
(A1b)
(A1b)
(A1c)
(A1c)
The parameters for this model are described in Table .
Theorem A.1
The zero equilibrium for mosquito population dynamics is LAS when while the EE is LAS when
where
.
Proof.
Jacobian matrix of system (A1) is
The Jacobian matrix for no-infection equilibrium is
The characteristic polynomial of
is
(A2)
(A2)
If , then all eigenvalues are negative, the zero equilibrium is LAS.
The Jacobian matrix at EE is
Characteristic polynomial of is
(A3)
(A3)
If , then all eigenvalues of
are negative, and the EE is LAS.
Theorem A.2
The zero equilibrium: is globally asymptotically stable
GAS
when
. The EE:
is GAS when
.
Proof.
We follow the approach in [Citation9]. First, we consider the subsystem:
(A4a)
(A4a)
(A4b)
(A4b)
where
is
on an open set
.
Let ,
,
, and
. According to the definition of tridiagonal feedback [Citation9], system (A4) is a monotone tridiagonal feedback system with Poincaré–Bendixson property [Citation30].
Recall Theorem 2 in [Citation28]. If the systems of ODEs satisfies:
The system exists on a compact absorbing set
.
A unique equilibrium point E exists and is LAS.
The system has Poincaré–Bendixson property.
Each periodic orbit of the system is asymptotically stable.
Then E is globally asymptotically stable in D.
To prove that each periodic orbit of system (A4) is asymptotically stable, we follow [Citation37] and Theorem 3 in [Citation9]. We need to prove that the linear system
is asymptotically stable, where
is the second additive compound matrix of the Jacobian matrix
associated with system (A4). For system (A4),
. We build the following linear system with one equation and the right-hand side is the compound matrix of the Jacobian matrix
.
Let Lyapunov function
. The right derivative of V along the solution paths (X) and
is:
, which implies that
, and
as
. Therefore, the linear system (A4) is asymptotically stable, and the solution
is asymptotically orbitally stable with asymptotic phase.
By the same argument in [Citation9], the system (A4) is uniformly persistent in . The zero equilibrium
is isolated and the largest compact invariant outside D is
, which is absorbing and the system (A4) is uniformly persistent [Citation17]. The conditions for Theorem 2 in [Citation28] are all satisfied. Therefore,
is GAS whenever
, and
exists and is GAS when
.
Following [Citation54] and Theorem 4 in [Citation9], is GAS whenever
and
is GAS whenever
.
Theorem A.3
(A5)
(A5) is an invariant region under the flow induced by Equation (A1).
Proof.
The proof directly follows the proofs for Lemmas 4.2 and 4.3 in [Citation35].
