?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this paper, the dynamical behaviours for a five-dimensional virus infection model with three delays which describes the interactions of antibody, cytotoxic T-lymphocyte (CTL) immune responses and Beddington–DeAngelis incidence are investigated. The reproduction numbers for virus infection, antibody immune response, CTL immune response, CTL immune competition and antibody immune competition, respectively, are calculated. By using the Lyapunov functionals and linearization method, the threshold conditions on the local and global stability of the equilibria for infection-free, immune-free, antibody response, CTL response and interior, respectively, are established. The existence of Hopf bifurcation with immune delay as a bifurcation parameter is investigated by using the bifurcation theory. Numerical simulations are presented to justify the analytical results.
1. Introduction
In recent years, the virus infection models provide comprehensive views for our understanding of diseases, such as HIV, influenza, HBV, Ebola, HTLV and HCV (see [Citation1–21,Citation23,Citation24]). Theoretical analysis for these mathematical models are important to obtain complete insights for the viral dynamics in vivo. In particular, the stability and the bifurcation will provide specific information for our understanding about disease control.
The adaptive immune system reacts against virus and infected cells during virus infections. The antibody and cytotoxic T-lymphocyte (CTL) responses play the significant role in preventing infections. Hence, effective strategies to prevent virus infection need both antibody and CTL responses (see [Citation1,Citation14,Citation17,Citation19]). In [Citation17], Wodarz proposed a basic model to describe the interactions of antibody and CTL immune responses with bilinear incidence which includes uninfected target cells
, productively infected cells
, free virus
, CTL response cells
and antibody response cells
. Then, Yousfi et al. [Citation20] gave the global analysis for this model. Yan and Wang [Citation19] incorporated an intracellular delay into the infected cell equation in the model and studied the effect of the delay on the global dynamics. However, Wang and Xu [Citation15] suggested that the incidence rate is probably not linear over the large number of virus and susceptible cells. Balasubramaniam et al. [Citation1] developed a HIV model with Beddington–DeAngelis incidence and investigated the global stability and the existence of Hopf bifurcation.
However, only single immune response delay is considered in [Citation1]. We know that there are usually three delays in a virus infection disease with the interactions of antibody and CTL immune responses: the intracellular delay, virus replication delay and immune response delay in the transmission process of virus infection. An important and interesting problem is how dynamical properties in virus infection disease will befallen when three delays exist simultaneously. Particularly, how stability properties will occur at possible equilibrium stations.
Therefore, in this paper we consider a five-dimensional virus infection model with three time delays which describes the interactions of antibody, CTL immune responses and Beddington–DeAngelis incidence rate
(1)
(1) where Λ, k, c and g are the birth rate of the uninfected cells, the virus, the CTL responses and the antibody responses, respectively; β is the infection rate; d,r,u,h and α represent the death rate of uninfected target cells, productively infected cells, virus, CTL responses and antibody responses, respectively; p represents the killing rate of infected cells by CTL response cells; q is the B cells neutralize rate;
denotes the intracellular delay, and
denotes the surviving rate of infected cells during delay period
(see [Citation4,Citation7–10,Citation12,Citation19,Citation24]);
is virus replication delay, and
denotes the surviving rate of virus during delay period
(see [Citation5,Citation18]) and
denotes immune response delay which is suggested in [Citation1,Citation8,Citation11,Citation23].
In this paper, our purpose is to investigate the dynamical properties of model (Equation1(1)
(1) ), expressly the stability of equilibria and the existence of Hopf bifurcation. The organization of our paper is as follows. In Section 2, the basic properties of model (Equation1
(1)
(1) ) for the non-negativity and boundedness of solutions, the threshold values and the existence of five equilibria are discussed. In Section 3, the threshold conditions on the global stability and instability for the infection-free equilibrium, infection equilibrium without immune response and infection equilibrium with only antibody response are stated. When
, the threshold conditions on the global stability and instability for the infection equilibrium with only CTL response and infection equilibrium with both CTL and antibody responses are proved. In Section 4, when
the sufficient conditions on the existence of Hopf bifurcation for the infection equilibrium with only CTL response and infection equilibrium with both CTL and antibody responses are established. In Section 5, the numerical simulations are presented to further illustrate the dynamical behaviour of the model. Finally, we will give a conclusion.
2. Boundedness and equilibrium
Let and
denotes the space of continuous functions mapping interval
into
with norm
for any
.
The initial conditions for model (Equation1(1)
(1) ) are given as follows
(2)
(2) where
By the fundamental theory of functional differential equation [Citation6], It is easy to see that model (Equation1
(1)
(1) ) admits a unique solution
satisfying initial conditions (Equation2
(2)
(2) ). We have the following basic result of model (Equation1
(1)
(1) ).
Theorem 2.1
Let be the solution of model (Equation1
(1)
(1) ) satisfying initial conditions (Equation2
(2)
(2) ), then
and
are positive and ultimately bounded.
Proof.
It is now easy to show that all solutions of model (Equation1(1)
(1) ) with initial conditions (Equation2
(2)
(2) ) are defined on
and remain positive for all
. Denote
Calculating the derivative of
along solutions of model (Equation1
(1)
(1) ), we have
where
. This implies that
is ultimately bounded for large t. So,
and
are also ultimately bounded.
Next, we discuss the existence of equilibria of model (Equation1(1)
(1) ). Firstly, we directly obtain that model (Equation1
(1)
(1) ) always has a unique infection-free equilibrium
with
The basic reproductive number of viral infection for model (Equation1(1)
(1) ) is
Here, k is the rate of the newly virus produced by infected cells,
is the surviving period of virus,
is the surviving rate of newly virus in
,
denotes the newly infected cells which are infected by the first virus,
is the the surviving rate of newly infected cells in
, and
is the surviving period of infected cells. Therefore, we easily see that
denotes the average number of the free virus released by the infected cells which are infected by the first virus.
Obviously, implies that
and
When , model (Equation1
(1)
(1) ) has a unique immune-free equilibrium
where
The antibody immune reproductive number for model (Equation1(1)
(1) ) is
Note that when
model (Equation1
(1)
(1) ) has a unique immune-free equilibrium
. This shows that virus infection is successful and the number of free virus at equilibrium
is
. Furthermore, we have that
is the average life-span of antibody cells, g is birth rate of the antibody response. Hence,
denotes the average number of the antibody immune cells activated by virus when virus infection is successful and CTL responses have not been established.
The CTL immune reproductive number for model (Equation1(1)
(1) ) is
Here,
denotes the average number of the CTL immune cells activated by infected cells when virus infection is successful and antibody immune responses have not been established. Note that the number of infected cells at equilibrium
is
,
is the average life-span of CTL cells and c is the rate at which the CTL responses are produced.
We see that is equivalent to
, and
is equivalent to
When , model (Equation1
(1)
(1) ) has a unique infection equilibrium with only antibody response
, where
and
is the unique positive zero point of the following function
In fact, from , by
we obtain
. From the expression of
it follows that the existence of equilibrium
is equivalent to
. Noticing that
is a quadratic function and
, we know that the existence and uniqueness of equilibrium
is equivalent to
Since
from
, we have
Therefore, when
we get
When , model (Equation1
(1)
(1) ) has a unique infection equilibrium with only CTL response
, where
and
is the unique positive zero point of the following function
In fact, since , by
we obtain
. From the expression of
it follows that the existence of CTL-present infection equilibrium
is equivalent to
. Noticing that
is a quadratic function and
, we know that the existence and uniqueness of CTL-present equilibrium
is equivalent to
Since
from , we have
Therefore, when
we get
The CTL immune competitive reproductive number for model (Equation1(1)
(1) ) is
In fact, when
, model (Equation1
(1)
(1) ) has a unique infection equilibrium with only antibody response
. This predicates that CTL immune responses have been established, and the number of infected cells at equilibrium
is
. Hence,
denotes the average number of the CTL immune cells activated by infected cells under the condition that antibody immune responses have been established.
The antibody immune competitive reproductive number for model (Equation1(1)
(1) ) is
In fact, when
, model (Equation1
(1)
(1) ) has a unique infection equilibrium with only CTL response
. This predicates that antibody immune responses have been established, and the number of the virus at equilibrium
is
. Hence,
denotes the average number of the antibody immune cells activated by viruses under the condition that CTL immune responses have been established.
When and
, model (Equation1
(1)
(1) ) has a unique infection equilibrium with CTL and antibody responses
, where
In fact, from the above discussion on the existence of equilibrium we directly have
and
From
we have
. From the expression of
, we further have
. Hence,
, which implies
. Furthermore, from
we also have
. This shows that equilibrium
uniquely exists.
3. Stability analysis
Theorem 3.1
If
then the infection-free equilibrium
is globally asymptotically stable.
If
then the equilibrium
is unstable.
Proof.
Consider conclusion (a). Define a Lyapunov functional
Calculating the derivative of
along any positive solution of model (Equation1
(1)
(1) ) and noting that
, we can obtain
If
, then
for any
. We have
if and only if
, v=0, z=0 and w=0. From the LaSalle's invariance principle [Citation6], we have that
is globally asymptotically stable when
.
Next, we consider conclusion . The characteristic equation of the linearized system of model (Equation1
(1)
(1) ) at the equilibrium
is
where
If
, we have
and
. Hence, there is at least a positive
such that
. Therefore, when
is unstable. This completes the proof.
Remark 3.2
Theorem 3.1 shows that if only infection-free equilibrium exists, then it is globally asymptotically stable, and delays
,
and
do not impact the stability of
Biologically, we see that in this case the virus is cleared up.
Theorem 3.3
Let .
If
and
then the immune-free equilibrium
is globally asymptotically stable.
If
or
then the equilibrium
is unstable.
Proof.
Let , we have
for all
and
if and only if
. Consider conclusion
. Define a Lyapunov functional
Calculating the derivative of
along the solution of model (Equation1
(1)
(1) ) gives
Notice that
Hence,
and
if and only if
,
and
From the LaSalle's invariance principle [Citation6], we have that
is globally asymptotically stable when
,
and
.
Next, we consider conclusion (b). The characteristic equation of the linearized system of model (Equation1(1)
(1) ) at the equilibrium
is
where
When
, we have
. Hence, there is a positive root
. When
, we have
and
. Hence, there is at least a positive root
such that
. Therefore, when
or
,
is unstable. This completes the proof.
Remark 3.4
Theorem 3.3 shows that delays ,
and
do not impact the stability of
Biologically, we see that when
,
and
then the establishments of both CTLs and antibody immune responses are unsuccessful.
Theorem 3.5
Let and
.
If
then the infection equilibrium
with only antibody response is globally asymptotically stable;
If
then the equilibrium
is unstable.
Proof.
Consider conclusion (a). Define a Lyapunov functional
Calculating the derivative of
along the solution of model (Equation1
(1)
(1) ), it follows that
Notice that
Hence,
and
if and only if
and
From the LaSalle's invariance principle [Citation6], we have that
is globally asymptotically stable when
,
and
.
Next, we consider conclusion . The characteristic equation of the linearized system of model (Equation1
(1)
(1) ) at the equilibrium
is
where
where
If
, then we have
and
. Hence, there is at least a positive root
such that
. Therefore, when
is unstable. This completes the proof.
Remark 3.6
From Theorem 3.5 we see that delays ,
and
do not impact the stability of
Biologically, Theorem 3.5 implies that when
and
, the antibody immune response can be established, but the infected cells are too weak so that it can not stimulate CTL immune response.
Theorem 3.7
Let and
.
If
and
, then the infection equilibrium
with only CTL response is globally asymptotically stable;
If
then the equilibrium
is unstable.
Proof.
Consider conclusion (a). Define a Lyapunov functional
Calculating the derivative of
along the solution of model (Equation1
(1)
(1) ), we have
Notice that
Hence,
and
if and only if
and
From the LaSalle's invariance principle [Citation6], we have that the equilibrium
is globally asymptotically stable when
,
,
and
.
Next, we consider conclusion (b). The characteristic equation of the linearization system of model (Equation1(1)
(1) ) at the equilibrium
is
where
where
If
, then we have a positive root
. Therefore, when
,
is unstable for any
and
. This completes the proof.
Remark 3.8
Theorem 3.7 shows that delays and
do not impact the stability of
Biologically, we see that, when
, for any
and
as long as
,
,
then the CTL immune response can be determined, but the virus loads are so small that it can not activate the antibody immune responses.
Theorem 3.9
If
and
then the infection equilibrium
with CTL and antibody responses is globally asymptotically stable.
Proof.
Define a Lyapunov functional
Calculating the derivative of
along the solution of model (Equation1
(1)
(1) ), it follows that
Notice that
Hence,
and
if and only if
From the LaSalle's invariance principle [Citation6], we finally have that the equilibrium
is globally asymptotically stable when
,
,
,
and
. This completes the proof.
Remark 3.10
From Theorem 3.9 we see that delays and
do not impact the stability of
when delay
. Biologically, Theorem 3.9 implies that, when
, for any
and
as long as
,
,
and
then the susceptible cells, infected cells, free virus, CTL immune responses and antibody immune responses can coexist in vivo.
4. Hopf bifurcation analysis
We first discuss Hopf bifurcation at the equilibrium . By Theorem 3.7, we obtain the globally asymptotically stability of the equilibrium
when
and
. However, what kind of complicated dynamic behaviour will appear at the equilibrium
when
? By computing the characteristic equation for the corresponding linearized system of model (Equation1
(1)
(1) ) at the equilibrium
is given by
(3)
(3) where
However, when
or
Equation (Equation3
(3)
(3) ) is too complicated so that it can not make a good conclusion. Therefore, in the following discussions, we assume
For Equation (Equation3
(3)
(3) ), simple manipulation leads to
(4)
(4) where
Let
be a purely imaginary root of (Equation4
(4)
(4) ). Separating real and imaginary parts, it follows that
(5)
(5) Squaring and adding the two equations of (Equation5
(5)
(5) ), it follows that
(6)
(6) where
Let
then Equation (Equation6
(6)
(6) ) becomes
(7)
(7) Denote
Applying the results given in [Citation22] on the distribution of roots for five degree polynomial equation, we have the following results.
Lemma 4.1
For the polynomial equation (Equation7(7)
(7) ), the following results are true:
If
then Equation (Equation7
(7)
(7) ) has at least one positive root;
Assume that
and
If
then Equation (Equation7
(7)
(7) ) has no positive real root;
If
and
then Equation (Equation7
(7)
(7) ) has no positive real root;
If (a) and (b) are not satisfied, then Equation (Equation7
(7)
(7) ) has positive real root if and only if there exists at least one
such that
and
where
and
Assume that
and
Assume that
and
and
then Equation (Equation7
(7)
(7) ) has positive real root if and only if
and
and
where
Without loss of generality, we assume that Equation (Equation7(7)
(7) ) has m positive roots with
, denoted by
Then Equation (Equation6
(6)
(6) ) has m positive roots, say
From Equation (Equation5(5)
(5) ) we have
(8)
(8) Let
, we solve
from Equation (Equation8
(8)
(8) ) to obtain that
where
. Therefore, when
,
is a pair of purely imaginary roots of Equation (Equation4
(4)
(4) ). Clearly, for every
is monotonically increasing for
and
Therefore, there is a
and
such that
Define
(9)
(9)
Let be a root of Equation (Equation4
(4)
(4) ) satisfying
Differentiating Equation (Equation4
(4)
(4) ) with respect to
we get
This gives
We have
From Equation (Equation6
(6)
(6) ), we get
It follows that
We conclude that
and
have the same sign. Sum up the above discussion, we get the following conclusion.
Theorem 4.2
Let and
be defined by Equation (Equation9
(9)
(9) ), respectively.
If the following conditions are all not satisfied: (a)
(b)
,
and
or
and there exists
such that
and
(c)
,
and
or
and there exists
such that
and
; (d)
,
,
,
and
where
then equilibrium
is locally asymptotically stable for any
If one of the conditions given in (i) is satisfied, then equilibrium
is locally asymptotically stable for
.
If one of the conditions given in (i) holds and
then model (Equation1
(1)
(1) ) undergoes Hopf bifurcation from equilibrium
as
passes through the critical value
Next, we discuss Hopf bifurcation of the equilibrium . By Theorem 3.9, we only obtain the global asymptotic stability of the equilibrium
when
and
. When
, by the following theoretical analysis we will see that the Hopf bifurcation occurs at the equilibrium
. Since when
or
the discussions are very complicated, we here only discuss the case
and
.
At the equilibrium , the characteristic equation for the linearized system of model (Equation1
(1)
(1) ) is given by
(10)
(10) where
Let
be a purely imaginary root of (Equation10
(10)
(10) ). Separating real and imaginary parts, it follows that
(11)
(11) Squaring and adding the two equations of (Equation11
(11)
(11) ), it follows that
(12)
(12) where
Let
then Equation (Equation12
(12)
(12) ) becomes
(13)
(13) Denote
A similar argument as in Lemma 4.1 we can define for the polynomial equation (Equation13
(13)
(13) ), and using the same method as in Equation (Equation9
(9)
(9) ) we can further define
,
and
. Therefore, we have the following results.
Theorem 4.3
If the following conditions are all not satisfied: (a)
(b)
or
and there exists
such that
and
(c)
or
and there exists
such that
and
(d)
and
where
then
is locally asymptotically stable for any
If one of the conditions given in (i) is satisfied, then
is locally asymptotically stable for
.
If one of the conditions given in (i) holds and
then model (Equation1
(1)
(1) ) undergoes Hopf bifurcation from
as
passes through critical value
Remark 4.4
We here only establish the criteria on the existence of Hopf bifurcations at equilibria and
for model (Equation1
(1)
(1) ) in the case of delays
and
. However, when
or
whether we also can obtain similar results still is a very interesting and estimable problem. In the following section, we will give a discussion by means of the numerical simulations.
5. Numerical simulations
In the above sections, we establish the global asymptotic stability of equilibria and
when
and
, and by using the theory of bifurcation, we obtain the existence of the Hopf bifurcation and stability switches at equilibria
and
when
and
. However, aim at the case:
,
and
, the theoretical analysis is very complicated. In this section, by using the numerical simulation, it is shown that the Hopf bifurcation and stability switches occur at these equilibria as
increases. In model (Equation1
(1)
(1) ), we choose a, α, β,
and
as free parameters and fix all other parameters as displayed in Table .
Table 1. List of parameters.
Example 5.1
Take a=0, we have that Beddington–DeAngelis incidence
is simplified to saturation incidence
. Let
and
. All other parameter values are the same as in Table . From Figures we see that as
increases the dynamical behaviours of equilibrium
will occur: locally asymptotically stable → unstable and Hopf bifurcation appears → locally asymptotically stable → unstable and Hopf bifurcation appears.
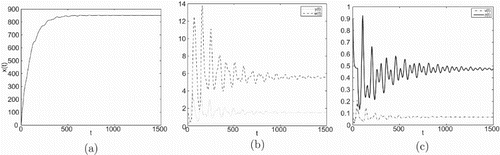
Figure 1. Taking , we have
and
, infection equilibrium with only CTL response
is asymptotically stable.
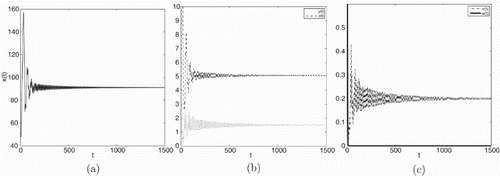
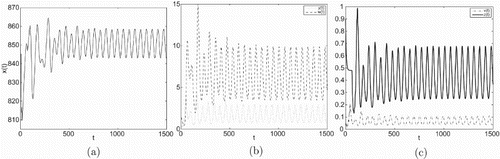
Figure 2. Taking we have
and
, infection equilibrium with only CTL response
occurs Hopf bifurcation.
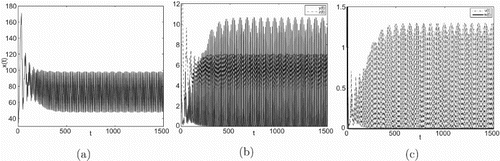
Figure 3. Taking , we have
and
, infection equilibrium with only CTL response
is asymptotically stable.
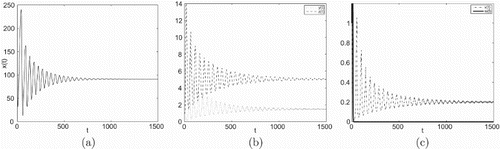
Figure 4. Taking , we have
and
, infection equilibrium with only CTL response
occurs Hopf bifurcation.
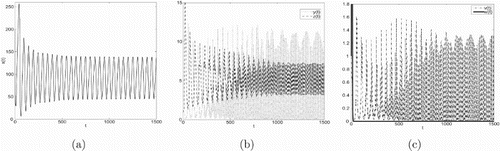
In Figures , (a), (b) and (c) are denoted time-series figures of ,
,
,
and
.
Example 5.2
Consider the Beddington–DeAngelis incidence . Let
and
All other parameter values are the same as in Table . From Figures we see that as
increases from zero the dynamical behaviours of equilibrium
will occur: locally asymptotically stable → unstable and Hopf bifurcation appears → locally asymptotically stable → unstable and Hopf bifurcation appears.
Figure 5. Taking , we have
and
, infection equilibrium with both antibody and CTL responses
is asymptotically stable.
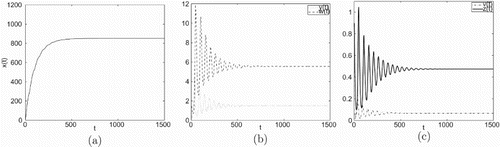
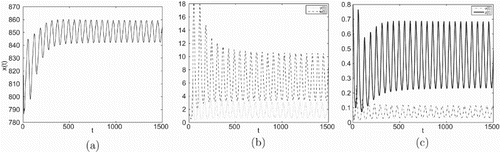
Figure 6. Taking , we have
and
, infection equilibrium with both antibody and CTL responses
occurs Hopf bifurcation.
6. Discussion
In this paper, we have investigated a virus infection model (Equation1(1)
(1) ) with intracellular delay
, virus replication delay
and immune response delay
. We assume that the production of CTL immune response depends on the infected cells and CTL cells based above important biological meaning. We see that similar assumption also is given in [Citation1,Citation9,Citation11,Citation12,Citation16,Citation18,Citation24]. Similarly, the production of antibody response depends on the virus and antibody (see [Citation1,Citation13,Citation14,Citation16]). Dynamical analysis shows that
,
and
play different roles in the stability of the model.
By the analysis, model (Equation1(1)
(1) ) has five possible equilibria, an infection-free equilibrium
immune-free equilibrium
, infection equilibrium
with only antibody response, infection equilibrium
with only CTL response and infection equilibrium
with both CTL and antibody responses. A combination of basic reproductive ratio of viral infection
for antibody response
for CTL immune response
for CTL immune response competitive
and for antibody response competitive
determines the existence of these equilibria. Furthermore, they also determine the global properties of the model. We have shown that when
,
is globally asymptotically stable, which means that the viruses are cleared and immune is not active. When
,
and
,
is globally asymptotically stable, which means that the infection becomes chronic but with no persistent CTL immune responses and antibody responses. When
and
,
is globally asymptotically stable, which means that the infection becomes chronic with persistent antibody responses, but the infected cells can not stimulate and activate CTL immune responses. As respect to the analysis of
, we consider special case
,
and
, when
and
,
is globally asymptotically stable, which means that the infection becomes chronic with persistent CTL immune responses, but the virus loads can not activate the antibody responses. About the stability of
, we have obtained that for special case
,
and
, when
and
,
is globally asymptotically stable, that is, susceptible cells, infected cells, free virus, antibody responses and CTL responses coexist in vivo. We see that
and
do not affect the stability of the equilibria.
When , by using the bifurcation theory, we obtain the sufficient conditions on the existence of Hopf bifurcation at
and
. Meanwhile, by means of numerical simulations, it is shown that the Hopf bifurcation and stability switches occur at
and
as
increases. Figures indicate that
remains stable as
is small, and along with the increase of
,
becomes unstable and periodic oscillations appear. It shows that stability switches occur as
increases. Similarly, from Figures , we see that along with the increase of
the dynamical behaviours of model (Equation1
(1)
(1) ) at
appear very large diversification. Particularly, when
is small enough,
is asymptotically stable and when
is increasing, the stability switches occur at
, and when
is unstable, Hopf bifurcation occurs. Finally, when
is large enough,
is always unstable. Summarizing these discussions, we point out that
affects markedly the stability of
and
This illustrates the fact that
plays a negative part in the disease prevalence and control. Motivated by the above discussion, one can realize that the emergence in the broadly neutralizing antibodies reacted to change in infected cells that could kill viruses. On the whole, this paper somehow provides better understanding about neutralizing antibodies and might help to design a powerful vaccine, which prevents at least uninfected peoples from ever becoming infected with virus. However, by considering some other factors such as the antibody delay, diffusion and a time-varying drug concentration, whether we also can obtain that the global asymptotic stability of equilibria will also be a very estimable and significative subject. This is left to the future research.
Disclosure statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Funding
References
- P. Balasubramaniam, P. Tamilalagan, and M. Prakash, Bifurcation analysis of HIV infection model with antibody and cytotoxic T-lymphocyte immune responses and Beddington–DeAngelis functional response, Math. Methods Appl. Sci. 38 (2015), pp. 1330–1341. doi: 10.1002/mma.3148
- K. Hattaf, N. Yousfi, and A. Tridane, Mathematical analysis of a virus dynamics model with general incidence rate and cure rate, Nonlinear Anal. Real World Appl. 13 (2012), pp. 1866–1872. doi: 10.1016/j.nonrwa.2011.12.015
- K. Hattaf, N. Yousfi, and A. Tridane, Stability analysis of a virus dynamics model with general incidence rate and two delays, Appl. Math. Comput. 221 (2013), pp. 514–521.
- G. Huang, W. Ma, and Y. Takeuchi, Global analysis for delay virus dynamics model with Beddington–DeAngelis function response, Appl. Math. Lett. 24 (2011), pp. 1199–1203. doi: 10.1016/j.aml.2011.02.007
- Y. Ji, Global stability of a multiple delayed viral infection model with general incidence rate and an application to HIV infection, Math. Biosci. Eng. 12 (2015), pp. 525–536. doi: 10.3934/mbe.2015.12.525
- Y. Kuang, Delay Differential Equations with Applications in Population Dynamics, Academic Press, San Diego, 1993.
- M.Y. Li and H. Shu, Global dynamics of an in-host viral model with intracellular delay, Bull. Math. Biol. 72 (2010), pp. 1492–1505. doi: 10.1007/s11538-010-9503-x
- K.A. Pawelek, S. Liu, F. Pahlevani, and L. Rong, A model of HIV-1 infection with two time delays: Mathematical analysis and comparison with patient data, Math. Biosci. 235 (2012), pp. 98–109. doi: 10.1016/j.mbs.2011.11.002
- H. Shu, L. Wang, and J. Watmoughs, Global stability of a nonlinear viral infection model with infinitely distributed intracellular delays and CTL immune responses, SIAM J. Appl. Math. 73 (2013), pp. 1280–1302. doi: 10.1137/120896463
- X. Song, X. Zhou, and X. Zhao, Properties of stability and Hopf bifurcation for a HIV infection model with time delay, Appl. Math. Model. 34 (2010), pp. 1511–1523. doi: 10.1016/j.apm.2009.09.006
- X. Tian and R. Xu, Global stability and Hopf bifurcation of an HIV-1 infection model with saturation incidence and delayed CTL immune response, Appl. Math. Comput. 237 (2014), pp. 146–154.
- X. Wang, A. Elaiw, and X. Song, Global properties of a delayed HIV infection model with CTL immune response, Appl. Math. Comput. 218 (2012), pp. 9405–9414.
- T. Wang, Z. Hu, and F. Liao, Stability and Hopf bifurcation for a virus infection model with delayed humoral immunity response, J. Math. Anal. Appl. 411 (2014), pp. 63–74. doi: 10.1016/j.jmaa.2013.09.035
- J. Wang, J. Pang, T. Kuniya, and Y. Enatsu, Global threshold dynamics in a five-dimensional virus model with cell-mediated, humoral immune responses and distributed delays, Appl. Math. Comput. 241 (2014), pp. 298–316.
- Z. Wang and R. Xu, Stability and Hopf bifurcation in a viral infection model with nonlinear incidence rate and delayed immune response, Commun. Nonlinear Sci. Numer. Simul. 17 (2012), pp. 964–978. doi: 10.1016/j.cnsns.2011.06.024
- Y. Wang, Y. Zhou, F. Brauer, and J.M. Heffernan, Viral dynamics model with CTL immune response incorporating antiretroviral therapy, J. Math. Biol. 67 (2013), pp. 901–934. doi: 10.1007/s00285-012-0580-3
- D. Wodarz, Hepatitis C virus dynamics and pathology: The role of CTL and antibody responses, J. Gen. Virol. 84 (2003), pp. 1743–1750. doi: 10.1099/vir.0.19118-0
- H. Xiang, L. Feng, and H. Huo, Stability of the virus dynamics model with Beddington–DeAngelis functional response and delays, Appl. Math. Model. 37 (2013), pp. 5414–5423. doi: 10.1016/j.apm.2012.10.033
- Y. Yan and W. Wang, Global stability of a five-dimensional model with immune responses and delay, Discrete Contin. Dyn. Syst. B 17 (2012), pp. 401–416. doi: 10.3934/dcdsb.2012.17.401
- N. Yousfi, K. Hattaf, and M. Rachik, Analysis of a HCV model with CTL and antibody responses, Appl. Math. Sci. 57 (2009), pp. 2835–2845.
- Z. Yuan and X. Zou, Global threshold dynamics in an HIV virus model with nonlinear infection rate and distributed invasion and production delays, Math. Biosci. Eng. 10 (2013), pp. 483–498. doi: 10.3934/mbe.2013.10.483
- T. Zhang, H. Jiang, and Z. Teng, On the distribution of the roots of a fifth degree exponential polynomial with application to a delayed neural network model, Neurocomputing 72 (2009), pp. 1098–1104. doi: 10.1016/j.neucom.2008.03.003
- H. Zhu, Y. Luo, and M. Chen, Stability and Hopf bifurcation of a HIV infection model with CTL-response delay, Comput. Math. Appl. 62 (2011), pp. 3091–3102. doi: 10.1016/j.camwa.2011.08.022
- H. Zhu and X. Zou, Dynamics of a HIV-1 infection model with cell-mediated immune response and intracellular delay, Discrete Contin. Dyn. Syst. Ser. B 12 (2009), pp. 511–524. doi: 10.3934/dcdsb.2009.12.511