 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this paper, we propose an SIVS epidemic model with continuous age structures in both infected and vaccinated classes and with a general nonlinear incidence. Firstly, we provide some basic properties of the system including the existence, uniqueness and positivity of solutions. Furthermore, we show that the solution semiflow is asymptotic smooth. Secondly, we calculate the basic reproduction number by employing the classical renewal process, which determines whether the disease persists or not. In the main part, we investigate the global stability of the equilibria by the approach of Lyanpunov functionals. Some numerical simulations are conducted to illustrate the theoretical results and to show the effect of the transmission rate and immunity waning rate on the disease prevalence.
1. Introduction
Vaccination is one of the most effective methods of preventing infectious diseases. Indeed, for some children diseases like Measles, Rubella, and Chicken pox, preventive vaccines may provide a permanent immunity against the diseases. However, life-long immunity cannot be offered by preventive vaccines for some diseases such as Hepatitis B, Influenza, and Mumps. The immunity of vaccinated persons will wane and they will become vulnerable to the diseases again. It is necessary to design a framework to study the effect of waning immunity on the spread of an epidemic. Compartmental models have been developed to provide deep insights on the dynamics of an epidemic with non-permanent immunity (see, for example, [Citation5,Citation19,Citation29]).
It is strongly supported by data that a vaccine usually wanes with respect to the vaccinated time. Many scholars have successfully addressed this by adding a vaccinated compartment to classic epidemic models [Citation6,Citation10,Citation15,Citation18,Citation30,Citation31]. Obtained results include threshold dynamics [Citation6,Citation30,Citation31] and backward bifurcation [Citation18]. In this regard, the immunity duration has been becoming an important issue for the evolution and efficacy of the vaccine. Recently, we proposed an SIVS-type epidemic model with vaccination age in [Citation31] to explore the non-fixed immunity duration,
(1)
(1) where
and
denote the population sizes of the susceptible and infected at time t, respectively;
denotes the population density of the vaccinated at time t with vaccination age a. The parameters have the following meanings: Λ is the input rate of the new members, μ denotes the natural death rate, φ represents the vaccinated rate for the susceptible, γ denotes the cure rate for infected individuals, δ represents the disease-caused death rate of infected individuals,
denotes the immunity waning rate at age a,
represents the incidence rate and satisfies the following property:
We showed that system (Equation1
(1)
(1) ) exhibits a threshold dynamics by constructing appropriate Lyapunov functionals. In this sense, the basic reproduction number
is a key value determining whether the disease dies out or persists.
As we know, the incidence rate is an important factor affecting the disease dynamics. In the above mentioned works, the incidences used include the bilinear-type () [Citation5,Citation6,Citation18,Citation19], the standard-type (
) [Citation15], the saturated-type
[Citation30,Citation31], and the nonlinear-type
[Citation29]. In the literature, Feng and Thieme firstly proposed a nonlinear general incidence of the form
[Citation8]. Thereafter, Huang et al. [Citation13] and Korobeinikov [Citation17] studied some epidemic models with incidence rates of the form
. Furthermore, Korobeinikov [Citation16] obtained the global stability of basic SIR and SIRS epidemic models with the incidence rate of the form
.
Most classical epidemic models are compartmental models described by ordinary differential equations, where all infectious individuals are assumed to be homogeneous during their infectious period. This assumption has been proved to be reasonable in the study of the dynamics of communicable diseases such as influenza as well as in the study of sexually transmitted diseases. However, infectivity experiments on HIV/AIDS indicate that the transmission style follows an early infectivity peak (a few weeks after exposure) and a late infectivity plateau [Citation9]. To describe such a phenomenon, the concept of infection age (the time that has passed since infection) has been introduced into classical models. Therefore, epidemic models with infection age have been extensively studied in the literature. To name a few, see [Citation3,Citation4,Citation22,Citation25,Citation28,Citation32], where the incidence is bilinear in most of the models. Recently, epidemic models with infection age and nonlinear incidence have been extensively studied (see, for instance, [Citation4,Citation25,Citation28]).
To the best of our knowledge, not much has been done for epidemic models with two age structures [Citation7,Citation23]. In [Citation23], McCluskey considered an SEI model with continuous age structures in both the exposed and infectious classes and a threshold dynamics was established by using the approach of Lyapunov functionals while in [Citation7], Duan et al. studied an SVEIR epidemic model with ages of vaccination and latency and also obtained a threshold dynamics. Magal and McCluskey in [Citation21] proposed a two group SI epidemic model with age of infection and discussed the global stability of steady states.
Motivated by the above discussion, the purpose of this paper is to make further contribution to the study of epidemic models with two age structures and nonlinear incidence. Precisely, we introduce age structure into the infected individuals in (Equation1(1)
(1) ) and use a general incidence. Furthermore, let
denote the density of infected individuals at time t with infection age a. The model to be studied is as follows,
(2)
(2) with initial condition
Here Λ, μ, φ, and
have the same biological meanings as in those (Equation1
(1)
(1) ). For the other parameters,
is the recovery rate of the infected with infection age a,
is the disease-induced death rate with infection age a, and
is the transmission coefficient with infection age a. In epidemiology,
is called the force of infection, which justifies the form of incidence
. Note that the models in [Citation4,Citation6,Citation7,Citation22,Citation31] are just special cases of (Equation2
(2)
(2) ) and hence over results will cover those in the above-mentioned references.
Throughout this paper, we make the following assumptions on the parameter functions.
| (A1) | The functions ϵ, | ||||
| (A2) | The functions γ, | ||||
Moreover, we suppose that the nonlinear incidence f satisfies:
| (B1) | For S, | ||||
Assumption (B1) is a combination of those in [Citation17,Citation25]. It is easy to see that f is locally Lipschitz continuous on S and J, that is, for every C>0, there exists some such that
(3)
(3) whenever
,
,
,
.
The pahse space of (Equation2(2)
(2) ) is
. For
, we denote
In order to study the existence of solutions to (Equation2
(2)
(2) ), we will extend X.
Let ,
,
, and
. Define two linear operators
(j=1, 2) as follows.
(4)
(4) and
(5)
(5) For any
(
denotes the resolvent set of
) and
, if
then by simple calculation we obtain
(6)
(6) Similarly, for any
and
, if
with
, then
(7)
(7) Define a linear operator
with
. It is easy to see that
. For convenience, denote
. It follows from the definition of A that it has the following properties.
Lemma 1.1
If then
. More precisely, for any
with
any
we have
if and only if
(8)
(8)
(9)
(9)
(10)
(10) Moreover, A is a Hille-Yosida operator and
Proof.
By Equation (Equation6(6)
(6) ) and (Equation7
(7)
(7) ), we immediately obtain (Equation8
(8)
(8) ) and (Equation9
(9)
(9) ). It follows from the definition of A that
and hence
For any
with
, we have
The results immediately follow.
Let . Define a nonlinear operator
by
(11)
(11) where
. Then, setting
, we can rewrite (Equation2
(2)
(2) ) as an abstract Cauchy problem in
(12)
(12) It follows from Lemma 1.1 and (Equation3
(3)
(3) ), together with Lemma 3.1 [Citation20] that (Equation12
(12)
(12) ) has a unique continuous solution if the initial condition
satisfies the compatibility condition
Hence, for each
satisfying the above coupling condition, (Equation2
(2)
(2) ) has a unique solution in X. Then we can define a solution semiflow
of (Equation2
(2)
(2) ) by
where
is the unique solution of (Equation2
(2)
(2) ) with the initial condition
.
Let . Then it follows from (Equation2
(2)
(2) ) that
(13)
(13) From this, we can easily deduce that Γ is a positively invariant and attracting set of the semiflow Φ, where
The aim of this paper is to establish the global dynamics of (Equation2
(2)
(2) ). As a result, we only need to consider (Equation2
(2)
(2) ) with initial conditions in Γ. The following estimates are easy to obtain.
Theorem 1.2
Let Assumption (B1) hold. For all the following statements are true.
and
for all
and
where L is the Lipschitz coefficient.
Proof.
We readily obtain (i) by the positivity of the solution and Equation (Equation13(13)
(13) ). By Assumption (B1), we conclude that
Substituting this inequality into S equation of (Equation2
(2)
(2) ), we have
From Fluctuate Lemma, it follows that there exists a sequence
such that
and
Then
. Integrating the third equation of (Equation2
(2)
(2) ) along the characteristic line yields
(14)
(14) where
denotes the probability of a vaccinated individual having immunity until age a. Therefore,
(15)
(15) Taking limit inferior on both sides of (Equation15
(15)
(15) )
(16)
(16) On the other hand,
This completes the proof.
Corollary 1.3
Suppose Assumptions (A1) (A2) and hold. Then the semiflow Φ is point dissipative. In fact, there is a bounded set that attracts all points in X.
The rest of this paper is organized as follows. In Section 2, we establish the asymptotic smoothness of the semiflow Φ. Then we study the existence and local stability of equilibria in Section 3. Before obtaining the main result, a threshold dynamics of (Equation2(2)
(2) ), in Section 5, we show the uniform persistence in Section 4. In Section 6, we provide some numerical simulations to demonstrate the main results and to analyze the effect of the transmission rate and immunity waning rate on the disease prevalence. The paper concludes with a brief discussion.
2. Asymptotic smoothness
In this section, we establish the asymptotic smoothness of the solution semiflow Φ. For any closed, bounded, and positively invariant set , we need to show that there exists a compact set
such that
as
, where
is the Hausdorff semi-distance (see, for example, [Citation11]).
Before proceeding, we get the expressions of i and v as follows by integrating along the characteristic lines,
where
denotes the probability of an infected individual surviving to infection age a time units later.
Proposition 2.1
The function is uniformly continuous, that is, for any
there exists h>0 such that
Proof.
For and h>0, we have
The last integral is estimated by
Note that
for
. Now we are in position to estimate
as follows
Now the result follows immediately from Assumption (A1).
Proposition 2.2
The semiflow Φ is asymptotically smooth.
Proof.
Let be a bounded set with
for
For
and
, define
where
(17)
(17)
(18)
(18)
(19)
(19)
(20)
(20) Then
. It is easy to see that
,
,
, and
are nonnegative. It follows from (Equation18
(18)
(18) ) and (Equation20
(20)
(20) ) that
and thus Assumption (1) in Lemma 3.2.3 [Citation11] holds.
Next, we establish that is completely continuous. This means that for any fixed
and any bounded set
, the set
is precompact. It is enough to show that
is precompact. This can be obtained by Fréchet-Kolmogrov Theorem [Citation24]. Firstly, it follows from the definitions of
and
that
is bounded. This implies that the first condition of the Fréchet-Kolmogrov Theorem holds. Secondly, it is easy to see that
by (Equation19
(19)
(19) ) and this indicates that the third condition of the Fréchet-Kolmogrov Theorem is satisfied. Finally, to verify the second condition of the Fréchet-Kolmogrov Theorem, we need to show that
is uniformly continuous under
that is,
(21)
(21) and
(22)
(22) Equation (Equation22
(22)
(22) ) has been proved in Yang et al. [Citation31, Proposition 3.7] and hence we only need to prove (Equation21
(21)
(21) ). Obviously (Equation21
(21)
(21) ) holds when t=0 since
by (Equation19
(19)
(19) ). Now let t>0. Since we are concerned with the limit as h tends to
, we assume that
. Then
Here we have used
and
for
and
. By the first equation of (Equation2
(2)
(2) ),
It follows that
Then with the help of Proposition 2.1, we easily see that (Equation21
(21)
(21) ) holds.
The following result follows immediately from Proposition 1.2, Proposition 2.2, and Theorem 2.33 of [Citation24].
Theorem 2.3
Suppose that Assumptions (A1), (A2), and (B1) hold. Then the semiflow Φ has a compact attractor in Γ.
3. The existence and local stability of equilibria
In this section, we mainly focus on the calculation of the basic reproduction number and investigate the existence and local stability of equilibria of (Equation2
(2)
(2) ). Denote
Clearly,
. Note that
is the total transmission rate of an infectious individual in its infectious period.
Let be an equilibrium of (Equation2
(2)
(2) ). Then
(23)
(23) Obviously,
and
. It follows that
(24)
(24) Therefore,
where
is a nonnegative zero of g with
(25)
(25) Clearly,
as
. Note that
, which implies that (Equation2
(2)
(2) ) always has a disease-free equilibrium
Next, we calculate the basic reproduction number
. Linearizing (Equation2
(2)
(2) ) at the disease-free equilibrium
, we obtain the following linear system in the disease invasion phase:
(26)
(26) Define
and
. Borrowing the definition of
in (Equation4
(4)
(4) ), we obtain the following abstract Cauchy problem:
(27)
(27) Let
be the
-semigroup generated by
. From the variation of constant formula, we obtain
(28)
(28) Applying
on both sides of (Equation28
(28)
(28) ) yields
(29)
(29) where
denotes the density of newly infected,
and
. Then the next generator is defined by
where
. So the basic reproduction number
can be defined as follows:
(30)
(30) In epidemiology,
is the average number of cases produced by an infectious individual in the whole infectious period when introduced into a wholly susceptible population.
It is easy to see that an equilibrium must be endemic if it is not disease free. In the following, we discuss the existence of endemic equilibria. We firstly derive a necessary condition on the existence of endemic equilibria.
Suppose that there is an endemic equilibrium . Then from
, we know that there exists a
such that
(31)
(31) This, combined with (B1), implies that
Therefore, a necessary condition on the existence of endemic equilibria is
.
Now we show that is also a sufficient condition on the existence of endemic equilibria. In fact, suppose that
. Note that
and
. It follows that
for J>0 and sufficiently small. This, combined with the Intermediate Value Theorem and
, implies that
has a positive solution in
. Hence there exists at least one endemic equilibrium. Actually, there is only one endemic equilibrium. Otherwise, let
and
be two distinct endemic equilibria. Without loss of generality, we assume that
. Denote
. Then
, which implies that
. With the help of (B1), we get
a contradiction.
To summarize, we have the following result on the existence of equilibria.
Theorem 3.1
Let be defined as in (Equation30
(30)
(30) ).
If
then (Equation2
(2)
(2) ) has a unique equilibrium, which is the disease-free equilibrium
.
If
then, besides
(Equation2
(2)
(2) ) also has a unique endemic equilibrium,
where
with
being the unique positive zero of g
defined by (Equation31
(31)
(31) )) on
.
In the remaining of this section, we study the local stability of equilibria by linearization. Linearizing (Equation2(2)
(2) ) at an equilibrium
will produce the associated characteristic equation
where
are the Laplace transforms of
and
, respectively. The equilibrium
is locally (asymptotically) stable if all eigenvalues of the characteristic equation have negative real parts and it is unstable if at least one eigenvalue has a positive real part.
Theorem 3.2
Let be defined in (Equation30
(30)
(30) ).
The disease-free equilibrium
is locally asymptotically stable if
and it is unstable if
.
If
then the endemic equilibrium
is locally asymptotically stable.
Proof.
(i) The characteristic equation at is
where
We claim that all roots of
have negative real parts. In fact, if
is a root with nonnegative real part, then
a contradiction. Thus we have proved the claim.
First, suppose . Then
. This, combined with
and the Intermediate Value Theorem, tells us that
has a positive root and hence
is unstable if
.
Now, suppose . We claim that all roots of
have negative real parts. Otherwise, let
be a root of
with
. Then
implies that
a contradiction. This proves the claim and hence
is locally asymptotically stable if
.
(ii) The characteristic equation at is
(32)
(32) We claim that (Equation32
(32)
(32) ) has no root with a nonnegative real part. Otherwise, suppose (Equation32
(32)
(32) ) has a root
with
. Since
, we know
and hence
as
. On the other hand, from (B1) and the second equation of (Equation24
(24)
(24) ), we have
It follows that
a contradiction to the assumption that
is a root of (Equation32
(32)
(32) ). This completes the proof.
4. Uniform persistence
We start with the uniformly weak ρ-persistence.
Define by
Let
Obviously, if
, then
as
.
Definition 4.1
[Citation24, pp. 61]
System (Equation2(2)
(2) ) is said to be uniformly weakly ρ-persistent (respectively, uniformly strongly ρ-persistent) if there exists an
, independent of the initial conditions, such that
for
.
To show that (Equation2(2)
(2) ) is uniformly weakly ρ-persistent, we need the following Fluctuation Lemma. For a function
, we denote
Lemma 4.2
Fluctuation Lemma [Citation12]
Let be a bounded and continuously differentiable function. Then there exist sequences
and
such that
and
as
.
The next result will be helpful in the coming discussion.
Lemma 4.3
[Citation14]
Suppose is a bounded function and
. Then
Lemma 4.4
Let be a solution of (Equation2
(2)
(2) ). Then
.
Proof.
By Lemma 4.2, there exists such that
,
, and
as
. Then
Letting
and using Lemma 4.3, we have
or
as required.
Proposition 4.5
If then (Equation2
(2)
(2) ) is uniformly weakly ρ-persistent.
Proof.
By way of contradiction, for any , there exists an
such that
Since
, there exists an
such that
(33)
(33) where
and
is the Laplace transform of
as before. In particular, for this
, there exists an
such that
We will get a contradiction as follows.
Firstly, there exists such that
for
. Without loss of generality, we assume that
as we can replace
with
. Then
for
.
Secondly, we show . Using the Fluctuation Lemma, there exists a sequence
such that
,
,
as
. By Lemma 4.4, without loss of generality, we can assume that
for
. Then by (B1), we have
This, combined with (Equation14
(14)
(14) ), gives
Now, for any
, there exists
such that
for
. Then, for
,
Letting
gives
This implies that
. Since ξ is arbitrary, we immediately get
.
Finally, since , there exists
such that
for
. Again we can assume
. Then
Here we have used the Mean Value Theorem for
with respect to J,
, and assumption (B1). Taking Laplace transforms on both sides of the above inequality yields
Then
for all
since
for
. In particular,
, a contradiction. This completes the proof.
Any global attractor of Φ only contains points where a total trajectory passes through it. A total trajectory of Φ is a function such that
for all
and all
. For a total trajectory, for
and
,
(34)
(34) In what follows, we will show the semiflow
is uniformly strongly
persistent. For
we have
(35)
(35) where
Following the approach in [Citation24], we have the following proposition.
Proposition 4.6
Let be a total trajectory in Γ for all
Then
is positive and either J is identically zero or it is strictly positive.
Proof.
Define is a semi-trajectory of system (Equation2
(2)
(2) ) with initial condition
for
and
First, we show
for any
Suppose that it doesn't hold. Then for some
and
. By the continuity of
, there exists a sufficiently small
such that
, which is a contradiction with
Therefore,
is strictly positive for each
Secondly, we claim that is identically zero for all
if
. For
, we have
. It follows from Gronwall inequality that
. Besides, for
,
Thus
. So that for all
is identically zero.
Now we are going to assume that is non-zero for each
If there exists a
such that
for all
, then
for
Gronwall inequality ensures that
is identically zero, giving a contradiction. Thus, there exists a sequence
toward
as n goes to infinity such that
For each
let
Since
for each
Hence, there exists a positive value ξ such that
for each
Recalling Equation (Equation35
(35)
(35) ), we have
where
Hence,
for each
From Corollary B.6 in [Citation24], we conclude that there exists a constant b>0 such that
for all t>b. Letting
, we have
Therefore, for all
is strictly positive.
Proposition 4.5 implies that is invariant under Φ. By Theorem 2.3, Φ has a compact attractor
. Then
is a compact attractor of the restricted semiflow
. This, combined Propositions 4.5 and 4.6 with Theorem 3.2 in [Citation27], immediately yields the uniformly strong ρ-persistence.
Theorem 4.7
If system (Equation2
(2)
(2) ) is uniformly strongly ρ-persistent.
Corollary 4.8
Suppose . Let
be a total trajectory in
. Then there exists an
such that
and
for all
and
.
Proof.
Theorem 1.2 provides a positive lower bound such that
for all
It follows from Theorem 4.7 that there exists a positive number
such that
for all
. Thus,
. Hence,
for all
and
By the v equation in (Equation34
(34)
(34) ), we have
. Letting
completes the proof.
5. A threshold dynamics
The main result of this paper is a threshold dynamics determined by . We start with the global stability of the disease-free equilibrium
.
Theorem 5.1
If then the disease-free equilibrium
is globally asymptotically stable in X.
Proof.
By Theorem 3.2, it suffices to show that for
. Let
and
be a total trajectory in
. Then
for
.
Firstly,
Taking superior limit on both sides of the above inequality and applying Lemma 4.3, we have
Therefore,
as
.
Secondly, we show that . For any
, there exists a
such that
for
by Lemma 4.4. Then for
, it follows from (B1) and (Equation34
(34)
(34) ) that
Taking limit suprema and using Lemma 4.3 again, we obtain
As ξ is arbitrary, this immediately gives
. Since
, we have
.
Thirdly, we show . In fact, we use (Equation34
(34)
(34) ) again to get
With the help of Lemma 4.3, one has
which implies that
.
Fourthly, we show . It suffices to show
. We achieve this by using Lemma 4.2. There exists
such that
and
as
. With (B1) and (Equation34
(34)
(34) ), we have
Letting
immediately yields
Here we have used
as
. So we have
as sought.
Finally, we show . With (Equation14
(14)
(14) ), we have
Applying Lemma 4.3, we get
. Therefore,
and this completes the proof.
The following corollary guarantees the well-definition of the constructive Lyapunov functional.
Corollary 5.2
If the following statements hold. For all
and
where L is the Lipschitz coefficient and
is defined in Corollary 4.8.
To establish the global stability of the endemic equilibrium by constructing a suitable Lyapunov functional, we need an additional assumption on the incidence rate, that is,
| (B2) | For S>0,
| ||||
This condition holds automatically for most nonlinear incidences. For example, is one of such. Hence our results cover some existing ones. To build the Lyapunov functional, we introduce the function
defined by
It is well-known that
for
and it attains the global minimum 0 only at x=1.
Theorem 5.3
Suppose that and (B2) holds. Then the endemic equilibrium
is globally asymptotically stable in
.
Proof.
By Theorem 3.2, it suffices to show . Let
be a total trajectory in
. By Corollary 4.8, there exists
such that
with
,
, and
for any
and
.
Let
Then
Define
where
Then V is well-defined because of Corollary 5.2.
Now we show that the upper-right derivative along the solution is non-positive. We first have
Here we have used
. Noting
and
, we obtain
Similarly, noting
and
, we have
Therefore,
By Jensen's inequality and the concavity of ϕ,
Because of (B2) and the monotonicity of ϕ and f, we have
, that is, V is nonincreasing. Since V is bounded on
, the α-limit set of
must be contained in
, the largest invariant subset of
. It follows from
that
,
, and
. Consequently,
since
.
The above analysis indicates that the α-limit set of consists of just the endemic equilibrium
and hence
for all
. It follows that
for
. Thus
and the proof is complete.
6. Numerical simulations
In this section, we first perform numerical experiments to illustrate the theoretical results. It follows from Theorem 5.1 and Theorem 5.3 that the basic reproduction number is a key threshold to determine whether or not the disease persists. For convenience, we choose
We take the values of some parameters as in Table . Besides, we take the transmission rate
in the form of
to illustrate the theoretical results by changing
.
Table 1. List of parameter values.
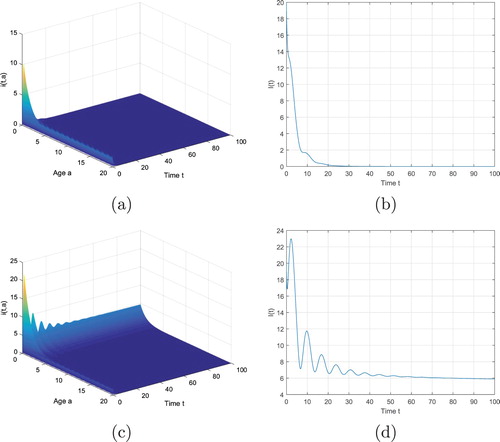
First, we fix . If we take
, then the basic reproduction number
. It follows from Theorem 5.1 that the disease-free equilibrium
is globally asymptotically stable. (a) and (b) of Figure show this fact. Then we enlarge the transmission rate to
and have
. (c) and (d) of Figure indicates that the endemic equilibrium
is asymptotically stable, which supports Theorem 5.3.
Figure 1. Time evolution of the infective population ,
,
for system (Equation2
(2)
(2) ) with initial value
and
. (a) and (b) with
, (c) and (d) with
.
Next we study the effect of some parameters on the disease spread.
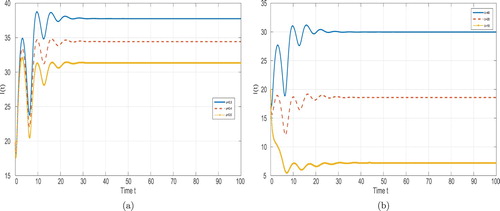
Vaccination plays an important role in controlling the disease prevalence. (a) of Figure indicates that improving the vaccine coverage for the susceptible decreases the final size of the disease prevalence. In fact, improving the vaccine rate can reduce the basic reproduction number. Theorem 5.1 implies that taking suitable vaccine measures can slow down the disease prevalence and even make the disease die out. (b) of Figure shows that taking vaccine on newborns has the familiar effect as increasing φ.
Figure 2. Time evolution of the infective population ,
,
for system (Equation2
(2)
(2) ) with different vaccinated rates and input rate. (a) with
(b) with
.
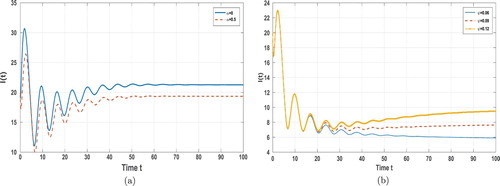
As for the transmission rate, we take α to be 0 and 0.5, respectively. Whenever we take any value for α, the value of the basic reproduction number doesn't change any more. Since , Theorem 5.3 implies that the disease must break out. From (a) of Figure , we see that enlarging α can lower the final size of the disease (the value of the component I of the endemic equilibrium). Furthermore, we readily see that enlarging the immunity waning rate can raise the value of the basic reproduction number. Hence this move increases the transmission risk. (b) of Figure shows that the final size of the disease increases as the immunity waning rate increases.
Figure 3. Time evolution of the infective population ,
,
for system (Equation2
(2)
(2) ) with different transmission rates and different immunity waning rates. (a) with different values
, (b) with different immunity waning rates
.
7. Conclusion and discussion
We proposed a general SIVS model with infection age and vaccinated age. It turned out that the dynamics of the model is determined by the basic reproduction number , which determines whether the disease dies out or persists. Our model generalizes many existing ones with some being listed in Table . Here f, F, G have familiar features as Assumption of (B1) in our paper. Moreover, the idea of this paper can be applied to some other structured epidemic models such as SVIR [Citation6], SEIR [Citation13,Citation19] models, and even if two-group model [Citation21].
Table 2. Incidence rates.
In this paper, we mainly focus on the global stability of equilibria. However, some complex phenomena such as multiple endemic equilibria (backward bifurcations) [Citation18,Citation29] and unstable endemic equilibrium [Citation1,Citation2,Citation26] have appeared in some epidemic models with vaccination. In the future, we shall address them with more age structures.
Disclosure statement
There is no any potential conflict of interest in this work.
Additional information
Funding
References
- V. Andreasen, Instability in an SIR-model with age-dependent susceptibility, in Mathematical Population Dynamics: Analysis of Heterogeneity, O. Arino, M. Kimmel and M. Langlais, eds., Wuerz Publ., Winnipeg, 1995, pp. 3–14.
- Y. Cha, M. Iannelli, and F.A. Milner, Stability change of an epidemic model, Dynam. Syst. Appl. 9 (2000), pp. 361–376.
- Y. Chen, J. Yang and F. Zhang, The global stability of an SIRS model with infection age, Math. Biosci. Eng. 11 (2014), pp. 449–469. doi: 10.3934/mbe.2014.11.449
- Y. Chen, S. Zou, and J. Yang, Global analysis of an SIR epidemic model with infection age and saturated incidence, Nonlinear Anal. Real World Appl. 30 (2016), pp. 16–31. doi: 10.1016/j.nonrwa.2015.11.001
- D. Ding and X. Ding, Global stability of multi-group vaccination epidemic models with delays, Nonlinear Anal. Real World Appl. 12 (2011), pp. 1991–1997. doi: 10.1016/j.nonrwa.2010.12.015
- X. Duan, S. Yuan, and X. Li, Global stability of an SVIR model with age of vaccination, Appl. Math. Comput. 226 (2014), pp. 528–540.
- X. Duan, S. Yuan, Z. Qiu, and J. Ma, Global stability of an SVEIR epidemic model with ages of vaccination and latency, Comput. Math. Appl. 68 (2014), pp. 288–308. doi: 10.1016/j.camwa.2014.06.002
- Z. Feng and H.R. Thieme, Endemic models with arbitrarily distributed periods of infection I: Fundamental properties of the model, SIAM J. Appl. Math. 61 (2000), pp. 803–833. doi: 10.1137/S0036139998347834
- D.P. Francis, P.M. Feorino, J.R. Broderson, H.M. Mcclure, J.P. Getchell, C.R. Mcgrath, B. Swenson, J.S. Mcdougal, E.L. Palmer, A.K. Harrison, F. Barre-Sinoussi, J.-C. Chermann, L.Montagnier, J.W. Curran, C.D. Cabradilla, and V.S. Kalyanaraman, Infection of chimpanzees with lymphadenopathy-associated virus, Lancet 2 (1984), pp. 1276–1277. doi: 10.1016/S0140-6736(84)92824-1
- H. Gulbudak and M. Martcheva, A Structured avian influenza model with imperfect vaccination and vaccine induced asymptomatic infection, Bull. Math. Biol. 76(10) (2014), pp. 2389–2425. doi: 10.1007/s11538-014-0012-1
- J.K. Hale, Asymptotic Behavior of Dissipative Systems, AMS, Providence, 1988.
- W.M. Hirsch, H. Hanisch, and J.P. Gabriel, Differential equation models of some parasitic infections: Methods for the study of asymptotic behavior, Comm. Pure Appl. Math. 38 (1985), pp. 733–753. doi: 10.1002/cpa.3160380607
- G. Huang, Y. Takeuchi, W. Ma, and D. Wei, Global stability for delay SIR and SEIR epidemic models with nonlinear incidence rate, Bull. Math. Biol. 72 (2010), pp. 1192–1207. doi: 10.1007/s11538-009-9487-6
- M. Iannelli, Mathematical Theory of Age-Structured Population Dynamics, Applied Mathematics Monographs, vol. 7, Giardini Editorie Stampatori, Pisa, 1995.
- M. Iannelli, M. Martcheva, and X.-Z. Li, Strain replacement in an epidemic model with super-infection and perfect vaccination, Math. Biosci. 195 (2005), pp. 23–46. doi: 10.1016/j.mbs.2005.01.004
- A. Korobeinikov, Lyapunov functions and global stability for SIR and SIRS epidemiological models with non-linear transmission, Bull. Math. Biol. 68 (2006), pp. 615–626. doi: 10.1007/s11538-005-9037-9
- A. Korobeinikov and P.K. Maini, Nonlinear incidence and stability of infectious disease models, Math. Med. Biol. 22 (2005), pp. 113–128. doi: 10.1093/imammb/dqi001
- X. Li, J. Wang, and M. Ghosh, Stability and bifurcation of an SIVS epidemic model with treatment and age of vaccination, Appl. Math. Model. 34 (2010), pp. 437–450. doi: 10.1016/j.apm.2009.06.002
- J. Li, Y. Yang, and Y. Zhou, Global stability of an epidemic model with latent stage and vaccination, Nonlinear Anal. Real World Appl. 12 (2011), pp. 2163–2173. doi: 10.1016/j.nonrwa.2010.12.030
- P. Magal, Compact attractors for time periodic age-structured population models, Electron. J. Differential Equations 65 (2001), pp. 1–35.
- P. Magal and C.C. McCluskey, Two group infection age model: An application to nosocomial infection, SIAM J. Appl. Math. 73(2) (2013), pp. 1058–1095. doi: 10.1137/120882056
- P. Magal, C.C. McCluskey, and G.F. Webb, Lyapunov functional and global asymptotic stability for an infection-age model, Appl. Anal. 89 (2010), pp. 1109–1140. doi: 10.1080/00036810903208122
- C.C. McCluskey, Global stability for an SEI epidemiological model with continuous age-structure in the exposed and infectious classes, Math. Biosci. Eng. 9 (2012), pp. 819–841. doi: 10.3934/mbe.2012.9.819
- H.L. Smith and H.R. Thieme, Dynamical Systems and Population Persistence, Grad. Stud. Math., vol. 118, AMS, Providence, RI, 2011.
- B. Soufiane and T.M. Touaoula, Global analysis of an infection age model with a class of nonlinear incidence rate, J. Math. Anal. Appl. 434 (2016), pp. 1211–1239. doi: 10.1016/j.jmaa.2015.09.066
- H.R. Thieme, Stability change of the endemic equilibrium in age-structured models for the spread of S-I-R type infectious diseases, in Differential Equations Models in Biology, Epidemiology and Ecology, S. Busenberg and M. Martelli, eds., Springer, Berlin, 92, 1991, 139–158.
- H.R. Thieme, Uniform persistence and permanence for non-autonomous semiflows in population biology, Math. Biosci. 166 (2000), pp. 173–201. doi: 10.1016/S0025-5564(00)00018-3
- J. Wang, R. Zhang, and T. Kuniya, Global dynamics for a class of age-infection HIV models with nonlinear infection rate, J. Math. Anal. Appl. 432 (2015), pp. 289–313. doi: 10.1016/j.jmaa.2015.06.040
- Y. Xiao and S. Tang, Dynamics of infection with nonlinear incidence in a simple vaccination model, Nonlinear Anal. Real World Appl. 11 (2010), pp. 4154–4163. doi: 10.1016/j.nonrwa.2010.05.002
- J. Xu and Y. Zhou, Global stability of a multi-group model with vaccination age, distributed delay and random perturbation, Math. Biosci. Eng. 12 (2015), pp. 1083–1106. doi: 10.3934/mbe.2015.12.1083
- J. Yang, M. Maia, and L. Wang, Global threshold dynamics of an SIVS model with waning vaccine-induced immunity and nonlinear incidence, Math. Biosci. 268 (2015), pp. 1–8. doi: 10.1016/j.mbs.2015.07.003
- J. Yang, X. Li, and F. Zhang, Global dynamics of a heroin epidemic model with age structure and nonlinear incidence, Int. J. Biomath. 9(3) (2016), p. 1650033. doi: 10.1142/S1793524516500339
