 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this paper, we use an adaptive modeling framework to model and study how nutritional status (measured by the protein to carbohydrate ratio) may regulate population dynamics and foraging task allocation of social insect colonies. Mathematical analysis of our model shows that both investment to brood rearing and brood nutrition are important for colony survival and dynamics. When division of labour and/or nutrition are in an intermediate value range, the model undergoes a backward bifurcation and creates multiple attractors due to bistability. This bistability implies that there is a threshold population size required for colony survival. When the investment in brood is large enough or nutritional requirements are less strict, the colony tends to survive, otherwise the colony faces collapse. Our model suggests that the needs of colony survival are shaped by the brood survival probability, which requires good nutritional status. As a consequence, better nutritional status can lead to a better survival rate of larvae and thus a larger worker population.
1. Introduction
In social insect colonies such as ants, bees and wasps, all members of the colony work collectively to ensure colony survival. Colonies act as a single common organism capable of making decisions and forming complex behavioural connections between its members [Citation17,Citation18]. They exhibit a decentralized system with a sophisticated division of labour resulting from interactions among members of the colony and the environment [Citation1,Citation3,Citation18]. In addition to the reproductive division of labour between the queen and the workers, workers also have a division of labour between foragers which leave the nest to search for food and non-foragers that carry out tasks within the nest [Citation9]. In social insect societies, foraging responsibilities are assigned to a subset of adult colony members [Citation8]. Internal and external factors happening at both the individual and colony level shape the foragers' decision to bring back a certain type of food [Citation8].
Currently, there are few studies that have focused on the outcome of nutrient regulation in social insects at the colony level [Citation8] (but see [Citation11,Citation12,Citation32]). Many of these studies lack focus on the overall outcome of colony population dynamics, and how nutrient regulation among foragers affects the number of reared brood, mortality of adult workers, and in general colony survival. In this study, we focus on the mechanisms that regulate foraging behaviour of eusocial workers and the outcomes of these mechanisms on colony performance, including but not limited to the number of brood raised and worker mortality. The collection of food resources by an individual forager is based not only on the colony's current nutritional status but also on the worker's physical caste, age, and prior experience [Citation26,Citation33]. The nutritional needs of the colony are shaped by the differing needs of larvae and workers in the colony [Citation8,Citation10,Citation26]. For instance, the growth of larvae relies heavily on protein, while worker ants require primarily carbohydrates as a source of energy [Citation4,Citation5,Citation10,Citation13,Citation24,Citation34]. Many studies have shown that the ratio of protein to carbohydrates in the diet of a range of insect species is crucial for performance [Citation8,Citation10,Citation21,Citation28,Citation30], though, in general, carbohydrates are often more attractive to foragers than protein [Citation11,Citation26]. However, the protein required for growth may be in greater demand when a queen is laying eggs [Citation26].
In order for social insect foragers to compensate for potential nutrient restrictions in the food available to the colony [Citation6,Citation7,Citation26,Citation29], foragers adjust their collection in favour of food sources containing limiting nutrients [Citation7,Citation11,Citation26]. This guarantees that the colony meets its longer term objectives and thus promotes colony growth and reproduction [Citation26]. According to Dussutour et al. [Citation11], within a colony, workers recruit nestmates for food collection at different rates depending upon food type [Citation5,Citation27], food concentration, and hunger level [Citation11,Citation23]. At the individual level, when workers are starved, recruitment will be stronger to carbohydrate-rich food sources than to sources high in protein [Citation11]. At a collective level, deployment of foragers to carbohydrate-rich or highly proteinaceous material increases in the presence of larvae, resulting in an increase in the collection of carbohydrates and protein [Citation2,Citation11,Citation27].
There are several empirical studies that have studied how a colony is affected by the availability of required nutrients for colony growth and reproduction, and how workers regulate collection of these nutrients to meet individual and collective demands [Citation8,Citation11–13,Citation22,Citation26]. However, currently there are no mathematical models to our knowledge that have attempted to study these mechanisms dynamically. The main goal of this paper is to propose and study an adaptive modeling framework to further understand how nutritional status may regulate population dynamics and foraging task allocation of social insect colonies. The proposed model contains three compartments that allow us to analyse and measure the impacts of nutritional status that can benefit colony growth and survival. Our model assumes that (1) nutritional status is measured by the protein to carbohydrate ratio, which reflects the ratio of workers foraging for protein to workers foraging for carbohydrates; (2) brood are able to survive if the protein to carbohydrate ratio falls into a certain range; and (3) the colony recruits workers to forage for protein or carbohydrate in order to maximize the brood survival rate. In addition, our proposed model includes division of labour implicitly. Also, by considering the basic mechanisms affecting colony growth such as cooperative effort for reproductive division of labour, successful brood maturation/survival, and recruitment of workers to collect different nutrients based on specific colony nutritional demands, our model could help us understand how other life history factors affect the performance (number of brood raised and mortality of workers) of the colony.
The rest of this article is organized as follows: In Section 2, we describe the detailed derivation of our proposed model. In Section 3, we provide the mathematical analysis of our model including lemmas, propositions, and theorems, the proofs of which can be found in Section 6. In Section 4, we provide numerical simulations illustrating the equilibrium dynamics of the model to further obtain biological insights of some life history parameters of the colony. Lastly, the conclusion of this paper is found in Section 5.
2. Derivation of the mathematical model
Let represent the brood population;
be the portion of foragers collecting proteinaceous material, called the protein forager;
be the portion of foragers collecting carbohydrates, called the carbohydrate forager. The total forager population is denoted as
. The following ecological assumptions determine the population of
and
:
Brood population
: The brood population L increases with the average egg-laying rate of the queen(s) given by γ, which is discounted by two factors:
(a) The survival rate function of eggs is determined by the cooperative efforts of workers A in the colony. We adopt the modeling approach from Kang et al. [Citation15,Citation25], where the cooperative efforts that lead to the eggs' survival is measured by a Holling type-III function
, where b is a half-saturation constant and a is the portion of the division of labour invested towards the successful development of the larvae.
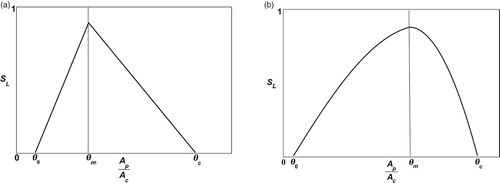
(b) The survival rate of larvae to workers is determined by the available nutrients in the colony which is reflected through the protein to carbohydrate ratio of worker collectors
. Examples of
could be
with
as a scaling factor of nutrient collection,
representing the optimal nutrient ratio, and
representing the maximal nutrient ratio that brood can survive (see Figure (a)), or general functions such as the normal biological performance curve (see Figure (b)). Notice that
can be negative, thus we define
such that
is a survival probability.
The brood population decreases by a maturation rate
, which describes the rate at which brood matures into the adult class A. Thus, we have following equation:
When
is less than
, the brood survival rate increases, and decreases when
is greater than
. This phenomenon has been supported by the work of [Citation8,Citation12,Citation20,Citation21], in which it is explained that worker survivability decreases as a probable side effect of an excess ingestion of proteins and of carbohydrate limitation. Figure (a) shows a general case of
with different
and
. The partial derivative of
reveals constant rates, showing a linear relation between the brood survival rate and nutritional status. In this study, we assume that when the nutrition level hits or exceeds the critical value
, i.e.
, the nutrient becomes toxic such that no brood can survive. Lastly, Figure (b) shows how the survival rate grows with respect to the collection of nutrients until it reaches
.
In general, we expect the protein to carbohydrate ratio
of the colony to fall in a certain range in order for the colony to survive and grow, say,
, where
is the minimum nutrient ratio necessary for brood survival. This is supported by [Citation8,Citation12]. Thus it is reasonable to assume that
has the following simple form:
(1)
(1) subject to
and
In particular, we have
Then we have
(2)
(2) In the symmetric case, i.e.
, then we have
with
,
and
. Thus,
if
and
if
. In addition, we have the following:
(3)
(3) The special case of the symmetric scenario above is
, i.e.
. In this case, we have
with
in our proposed model (Equation4
(4)
(4) ). In the following section, we will provide mathematical analysis of the general case of
shown in (Equation1
(1)
(1) ) and the related results can be applied to the symmetric case and its special case directly.
The total forager population
: The population A increases by the maturation rate of brood and decreases with a density-dependent death rate
. The density-dependent mortality rate follows the approach of [Citation15], thus we have following equation:
The protein forager population
: The ratio
measures the nutritional status of the colony. Assume that the brood can survive in a range of nutrient ratio, i.e.
, and any ratio greater than
can be toxic to brood. In addition, there is an optimal nutritional ratio
, denoted by
, such that brood could have the optimum survival rate at this ratio. More specifically, the brood survival rate increases with respect to the value of
when
, and passing this optimal ratio
, the brood survival rate decreases with
. The survival rate of brood is zero when
or
. Thus,
• The portion of the successful brood developed into adults which enter into the protein forager pool can be modeled by the term:
where
represents the nutritional requirements of the colony measured by the ratio
and a nutrient collection factor.
• Based on the nutritional requirements of the colony and other related stimuli, a protein forager can become a carbohydrate forager, and vice versa. This task switching rate depends upon different factors such as the nutritional status of the colony, presence of larvae, individual preference, food type, food concentration and hunger level [Citation5,Citation7,Citation11,Citation23,Citation26,Citation27]. In this paper, we assume that the task switching rate of protein foragers to carbohydrate foragers depends on the brood population L, the nutritional requirement of the colony
, and the available carbohydrate forager
, thus its switching rate is
. Similarly, the switching rate of carbohydrate foragers to protein foragers is termed as
. This gives the net task switching rate of the protein forager:
. For instance, if the ratio
is less than the optimal nutrient ratio
(where the maximum brood survival rate occurs), then we expect
and
, thus this indicates that carbohydrate foragers will switch tasks to forage for protein, i.e.
. In a similar fashion, if the ratio
, protein foragers will switch to forage for carbohydrates, that is,
.
• The total forager population
decreases with a density-dependent death rate
, then the protein forager population decreases with the density-dependent mortality rate
.
Considering the factors above, we derive the population dynamics of the protein forager as follows:
Figure 1. Examples of a survival rate function: (a) A general case of with different
and
; (b) The normal biological performance curve
.
Based on the ecological assumptions above, the population dynamics of a social insect colony with nutrient regulating foraging activities is described as follows:
(4)
(4)
The biological meaning of the parameters and the related values are listed in Table .
Table 1. Parameter description and interval values of Model (Equation4 (4)
(4)  (4) ).
(4) ).
3. Mathematical analysis
The state space of the proposed ecological model (Equation4(4)
(4) ) is
. All parameters
,
are assumed to be strictly positive based on their biological meaning. We focus on the proposed function
shown in Equation (Equation1
(1)
(1) ) and Figure (a). The related mathematical results should be easily adopted to the symmetric case (Equation3
(3)
(3) ). Under such conditions, we first show that Model (Equation4
(4)
(4) ) is biologically well-defined, i.e. it is positively invariant and bounded in
in the following lemma:
Lemma 3.1
Model (Equation4(4)
(4) ) is positively invariant and bounded in
. In particular, if
and
then
and
for all t>0.
The extinction equilibrium of Model (Equation4
(4)
(4) ) always exists. The local stability of the trivial equilibrium
cannot be analysed directly for our model (Equation4
(4)
(4) ). However, from the first two equations of Model (Equation4
(4)
(4) ), we have
Note that Model (Equation4
(4)
(4) ) is positively invariant and bounded from Lemma 3.1, thus we can conclude that if
, then the inequality above implies that
converges to a nonnegative constant. In addition, we have
,
and
. Therefore, if
, then for some initial conditions around
, Model (Equation4
(4)
(4) ) converges to the extinction equilibrium
in
. Thus, we have the following proposition:
Proposition 3.1
If then for some initial condition around the extinction equilibrium point
taken in
the trajectory of Model (Equation4
(4)
(4) ) converges to
.
Remark 3.1
Note that the inequality implies that
. Proposition 3.1 implies that if a is not large enough (i.e. the investment to the brood growth is small), or the death rate of adults is too large, then the brood population and the total forager population approaches the extinction equilibrium point
. In the symmetric case, we have
, then the inequality becomes
with
.
Assume that is an interior equilibrium of Model (Equation4
(4)
(4) ) with the general case of
. Then based on the equation of
shown in (Equation4
(4)
(4) ), we can conclude that
can exist only if
as it requires
. Biologically, this implies that the colony survival requires the nutritional needs of brood being on the positive gradient of the brood survival probability
. Thus, we have
, and therefore
and
To solve for
, we set
, which implies the following equations
which gives
Therefore,
of an interior equilibrium
satisfies the following equation:
(5)
(5) Recall that
. Depending on the exact values of
, the Equation (Equation5
(5)
(5) ) can have zero, one, or two positive roots.
Let
where
be the possible positive roots of Equation (Equation5
(5)
(5) ). Let us denote
as follows:
(6)
(6)
which is an increasing function of
.
In the symmetric case, we have , then
shown in (Equation6
(6)
(6) ) can be rewritten as
(7)
(7) Also note that
. Then the following theorem provide conditions for existence of equilibrium solutions of Model (Equation4
(4)
(4) ):
Theorem 3.1
Existence of Equilibria
For Model (Equation4(4)
(4) ),
| 1. | If | ||||
| 2. | If | ||||
| 3. | If | ||||
| 4. | If | ||||
Remark 3.2
The detailed proof of Theorem 3.1 is shown in the last section. The number of equilibria of Model (Equation4(4)
(4) ) is determined by the positive root(s) of Equation (Equation5
(5)
(5) ). Theorem 3.1 implies that the value of the division of labour invested on larvae a and the minimal protein to carbohydrate ratio
determine the existence of the interior equilibrium
.
Our simulations (see Section 4) suggest that Model (Equation4(4)
(4) ) has simple dynamics: no limit cycle and only equilibrium dynamics. At the stable equilibrium, the ratio describing the nutritional level of the colony is
(8)
(8) Equation (Equation8
(8)
(8) ) suggests that the larger the total population of workers investing in nutrient collection is, the higher the ratio of protein to carbohydrates will be, i.e. better nutrient status of the colony. Notice that
depends on
, so
does as well.
Now we discuss stability of the interior equilibrium for Model (Equation4(4)
(4) ). Let
be an arbitrary positive interior equilibrium of Model (Equation4
(4)
(4) ). The Jacobian matrix associated to Model (Equation4
(4)
(4) ) at equilibrium is:
(9)
(9)
Then the characteristic equation of
is
(10)
(10) where
(11)
(11)
The stability of the steady state
can be determined by the distribution of the roots of Equation (Equation10
(10)
(10) ). That is, if all the roots of Equation (Equation10
(10)
(10) ) have negative real parts, then
is locally asymptotically stable; if at least one root of Equation (Equation10
(10)
(10) ) has positive real parts, then
is unstable; if any root has zero real part and other roots all have negative real parts, then the stability of
cannot be determined by the linearized system directly.
The following theorem provides a global result on dynamics of the proposed model (Equation4(4)
(4) ) regarding when a colony will collapse.
Theorem 3.2
Extinction of species
If and
then Model (Equation4
(4)
(4) ) has global stability at
.
Biological implications: Theorem 3.2 has stronger result than results stated in Proposition 3.1 and indicates that the portion of the division of labour invested on larvae a and the nutrient are important factors determining whether larvae and adult worker ants can survive. This theorem provides a sufficient condition leading to the collapse of the colony.
Theorem 3.3
Stability Conditions
For Model (Equation4(4)
(4) ),
Assume that
and
, then Model (Equation4
(4)
(4) ) has a unique interior equilibrium
. If it satisfies
, then
is locally asymptotically stable.
Assume that
and
, Model (Equation4
(4)
(4) ) has two interior equilibria
, where
, if
but
, then the interior equilibrium
is locally asymptotically stable while
is unstable.
Biological Implications: The results in Lemma 3.1, Theorems 3.1 and 3.3, imply that the division of labour invested on larvae a decreases past the critical point shown in (Equation6
(6)
(6) ) and the first dotted line in Figure . Model (Equation4
(4)
(4) ) exhibits a backward bifurcation shown in Figure where
. In Figure (a), we set
and in Figure (b), we set
. Based on the expression of the critical value
shown in (Equation6
(6)
(6) ),
is an increasing function of
, which is reflected in the difference between Figure (a) and Figure (b). The value of
measures the minimum ratio of protein to carbohydrates that can allow the survival of larvae. Simulations shown in Figure (a, b) and suggest that the larger value of
, the more likely the colony can survive with a larger population of workers A and thus the higher nutrient ratio
. In summary, our theoretical work combined with the related simulations suggest that the division of labour invested on larvae a and the minimal nutrient ratio
can affect colony survival, the distribution of the brood and the total forager population affect the protein forager population. For instance, the larger
, the more division of labour invested on larvae is required to ensure survival of the colony. Also, under this scenario, the population distribution of brood and workers is smaller. Moreover, Figure shows the bifurcation diagrams of the ratio of
versus the division of labour invested on larvae a with different values of
, other parameters values are taken as those in Figure .
Figure 2. Backward bifurcation diagram of the division of labour invested on brood a v.s. the total forager population A. Other parameters values are . The solid line indicates that the equilibrium is locally asymptotically stable while the dashed line indicates that the equilibrium is unstable. The first vertical dotted line is the critical point
for saddle node bifurcation and the second dotted line is the transition point when the system has two interior equilibrium to one interior equilibrium. The blue colour indicates
which is always stable; the green colour indicates
which is always unstable; and the red colour is the extinction equilibrium
. (a)
. (b)
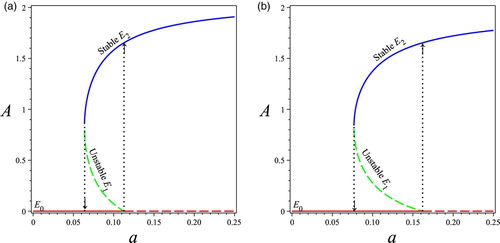
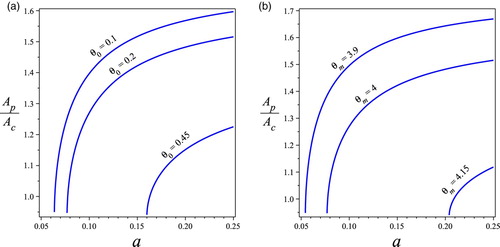
Figure 3. Bifurcation diagrams of the ratio of v.s. the division of labour invested on larvae a with: (a) different values of nutrient threshold
and (b) different values of optimal nutrient ratio
when
. Other parameters values are
. (a) Effects on
. (b) Effects on
.
All our theoretical results can apply to the symmetric case when and
. Now we focus on the special case of the symmetric case
. For convenience, let
(12)
(12) Let
and
Define
and M as follows:
(13)
(13)
Theorem 3.4
Dynamics of the Special Symmetric Case
Model (Equation4(4)
(4) ) is positive invariant in
and every trajectory attracts to a compact set
. In addition,
| 1. | If | ||||
| 2. | If | ||||
| 3. | If | ||||
| 4. | If | ||||
Remark 3.3
Theoretical results and numerical simulations (see Section 4) confirm that the special symmetric case of Model (Equation4(4)
(4) ), i.e.
and
, undergoes a backward bifurcation as a decreases past the critical value
defined in (Equation12
(12)
(12) ). Conditions shown in Theorem 3.4 suggest the importance of the protein to carbohydrate ratio, i.e.
, in determining the colony population dynamics.
Summary of Dynamics: According to our analytical results shown in this section, we can conclude that Model (Equation4(4)
(4) ) undergoes a backward bifurcation as a decreases past
(or
in the case of
and
). More specifically, it exhibits the following global dynamics:
| 1. | If | ||||
| 2. | If | ||||
| 3. | If | ||||
Our theoretical results suggest that the survival rate of larva to worker plays critical roles in determining colony population dynamics. We assume that
takes the form of (Equation1
(1)
(1) ) based on relevant biological studies. Our analysis implies that the values of parameters
and
in
have pronounced impacts on dynamical outcomes. In the next section, we use bifurcation diagrams to explore detailed impacts.
4. Numerical simulations
In this section, we use numerical simulations to illustrate equilibrium dynamics of the proposed model and obtain further biological insights on the dynamical outcomes of certain life history parameters of the colony.
For the general case of , the dynamics of Model (Equation4
(4)
(4) ) depends on the division of labour a, egg laying rate γ, the scaling factor on the brood survival rate due to the nutritional status
, the minimal nutrition ratio
, the maturation rate β, and the natural mortality d. To explore the effects of a and
, we perform bifurcation diagrams in Figures and by setting
Figures and suggest that (1) small values of division of labour a can lead to colony collapse; (2) intermediate values of a can make the system go through saddle node bifurcation; and (3) large values of a can insure colony survival. This implies that Model (Equation4
(4)
(4) ) goes through backward bifurcation on a. We can see that the larger value of a can lead to the larger population A (see Figure ) and the larger nutrient ratio
(see Figure ). Figures and also show the effects of the minimal nutrient requirement for brood survival
: The larger value of
, (1) the larger critical threshold
; (2) the smaller population A; and (3) the smaller nutrient status, i.e. the smaller value of
.
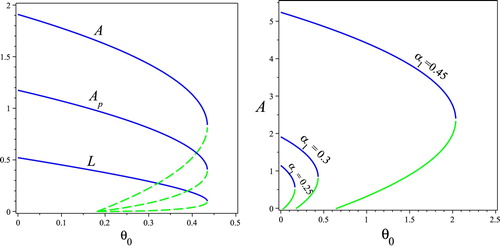
Next, we perform bifurcation diagrams of Model (Equation4(4)
(4) ) regarding how the minimum nutritional requirement
and the scaling factor of survival probability of brood
affect population dynamics of the colony in Figure . Figure (a) shows that Model (Equation4
(4)
(4) ) exhibits reversed backward bifurcation on
. Figure (b) suggests that the larger value of
, the larger population of worker A and the better probability of colony survival.
Figure 4. (a) The bifurcation diagrams of and
v.s. the nutrient threshold
with
; and (b) the bifurcation diagram of A v.s. the nutrient threshold
with different values of
. Other parameters values are taken as
. (a) Effects of
on population dynamics. (b) Effects of
on A
In the remaining of this section, we focus on the symmetric case of shown in (Equation3
(3)
(3) ) where
and
.
Special symmetric case (i.e.
): Figure (a) provides an example of bifurcation diagram on division of labour invested on larvae a of Model (Equation4
(4)
(4) ) by choosing the following parameters values:
Figure (a) shows that Model (Equation4
(4)
(4) ) goes through backward bifurcation at
. If
, then colony collapses; if
, Model (Equation4
(4)
(4) ) has two positive interior equilibria
and
with
being locally stable; and if
, then the colony survives.
Figure 5. Bifurcation diagrams of the division of labour invested on larvae a for Model (Equation4(4)
(4) ). Backward bifurcation occurs at
for the case of
(a); and at
for the case of
(b). Other parameters values are taken as
. The solid line indicates that the equilibrium is locally asymptotically stable while the dotted line indicates that the equilibrium is unstable. The blue colour indicates
which is always stable; the green colour indicates
which is always unstable; and the red colour is the extinction equilibrium
. (a)
. (b)
![Figure 5. Bifurcation diagrams of the division of labour invested on larvae a for Model (Equation4(4) L′=γSLmaxaA2b+aA2−βL,A′=βL−dA2,Ap′=βL⋅max{0,∂SL∂ApAc}+(max{0,∂SL∂ApAc}Ac−max{0,∂SL∂AcAp}Ap)L−dAAp.(4) ). Backward bifurcation occurs at a∗≜4bd2β2(1−α)2α2γ[α2γ+4dβ2(1−α)] for the case of 2θm=θc=8 (a); and at a~∗=4bd2β2(1−α)2α4γ2+4dαβ2γ(1−α)(α−(1−α)(2θm−θc)) for the case of 2θm>θc=7.8 (b). Other parameters values are taken as b=0.1,d=0.1,α=0.3,β=0.7,γ=0.9,θm=4. The solid line indicates that the equilibrium is locally asymptotically stable while the dotted line indicates that the equilibrium is unstable. The blue colour indicates E2 which is always stable; the green colour indicates E1 which is always unstable; and the red colour is the extinction equilibrium E0. (a) 2θm=θc=8. (b) 2θm>θc=7.8](/cms/asset/918f6db9-017c-4c9f-a621-0010c61610c4/tjbd_a_1786859_f0005_oc.jpg)
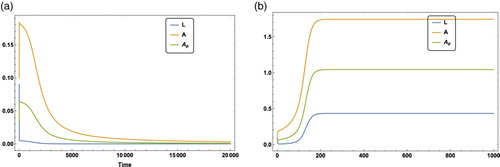
Figure provides two examples of population dynamics of Model (Equation4(4)
(4) ) to show the effects of a. The initial condition is
and other parameters values are the same as in Figure (a). According to Theorem 3.4, Model (Equation4
(4)
(4) ) has global stability at
(i.e. colony collapses) if
(see Figure (a)) and Model (Equation4
(4)
(4) ) has two positive interior equilibria with
being a locally asymptotically stable interior equilibrium if
(see Figure (b)). It indicates that larvae L, worker ants A, and the ants collecting proteinaceous material
can coexist with proper initial conditions if a is in the intermediate range.
Figure 6. The time series of Model (Equation4(4)
(4) ) when
with an initial value
and other parametric values being
which is the same set of parameters values in Figure (a). Figure (a) is the case when a = 0.07 where population goes extinct over time. Figure (b) is the case when a = 0.1 where the colony has a locally asymptotically stable interior equilibrium
. (a) Time series of Model (Equation4
(4)
(4) ) when
when the portion of the division of labour invested on larvae is a = 0.07. (b) The time series of Model (Equation4
(4)
(4) ) when
when the portion of the division of labour invested on larvae is a = 0.1.
Symmetrical case (i.e.
): Figure (b) provides an example of bifurcation diagram on division of labour invested on larvae (a) of Model (Equation4
(4)
(4) ) by taking same parameter values in Figure (a) except that
. Figure (b) shows that Model (Equation4
(4)
(4) ) undergoes a bifurcation as the portion of the division of labour invested on larvae a decreasing past
. Notice that the symmetrical case has
. Thus, the comparisons between Figure (a) and Figure (b) can provide insights on the effect of a and
(or
because we set
and
): (1) The smaller value of
, the larger critical threshold
; (2) The smaller value of
, the smaller population A. The dynamical outcomes of symmetrical cases are similar to the general case shown in Figures and .
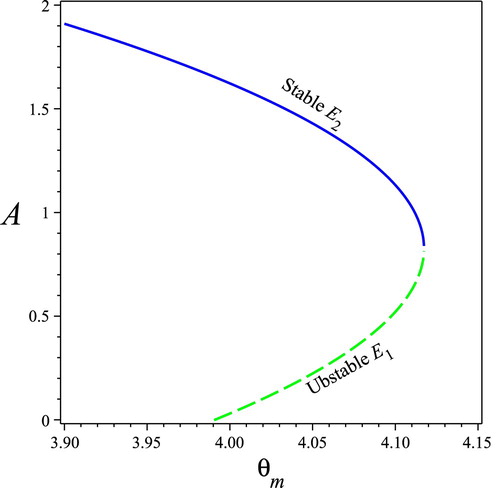
To understand the effects of the optimal nutrient ratio (or
), we perform a bifurcation diagram on
shown in Figure by setting
Notice that
, thus the value of
in Figure starts with
. Figure shows that Model (Equation4
(4)
(4) ) exhibits reversed backward bifurcation on
(or
): (1) small values of the optimal nutrient ratio
can insecure the persistence of the colony; (2) intermediate values of
can go through saddle node bifurcation; and (3) large values of
can lead to colony collapse.
Figure 7. Bifurcation diagram for Model (Equation4(4)
(4) ) on the optimal nutrient ratio
. An unstable interior equilibrium (green dotted) bifurcates
. The solid line indicates that the equilibrium is stable, while the dotted line indicates that the equilibrium is unstable. Parameters values:
.
5. Conclusion
Variation in nutrient consumption among individuals is considered a conserved mechanism regulating castes and division of labour in social insects colonies. In eusocial insects, foragers, who perform food collection tasks, need to satisfy their own nutrient requirements in addition to those of the non-foraging workers, as well as the larvae and queen(s), which have significantly higher protein needs [Citation14]. In this paper, we propose and study a nonlinear differential equations system to explore how nutritional status may regulate population dynamics and foraging task allocation of social insect colonies by applying adaptive modeling framework. Our model assumes that foragers adjust their preferences in favour of food sources containing limiting nutrients to maintain colony growth and reproduction [Citation7,Citation11,Citation26].
Our proposed model consists of a population of larvae L, foragers collecting carbohydrate , and foragers collecting protein
. We assume that the survival rate of larvae is determined by the available nutrition in the colony, which is reflected through the ratio of workers collecting protein to those collecting carbohydrates
. Our formulation of
is based on biological studies (see [Citation11,Citation26]) and embeds with an adaptive modeling approach adopted from [Citation15,Citation16,Citation19]. Our theoretical results and bifurcation analysis conclude that our proposed model exhibits backward bifurcations that generate bistability (see examples in Figure ). The bistability of the colony implies that initial conditions are important for colony survival under certain ranges of life history parameters. More specifically, the dynamical features and the related biological implications of Model (Equation4
(4)
(4) ) can be summarized as follows:
The nutrition status measured by
is an increasing function of the total population of workers A, or vice versa. This result may stem from the assumption that larvae (or brood in general) have higher nutritional needs to ensure survival. The biological implications of this are that higher nutritional status
can lead to a better survival rate of larvae, thus the colony can grow with larger worker population A.
The survival probability of brood is an increasing function of the following important life history parameters of colony:
The division of labour invested on brood measured by a can have huge impacts on dynamical outcomes of the colony. From Lemma 3.1, Theorems 3.1 and 3.3, Model (Equation4
(4)
(4) ) exhibits a backward bifurcation (shown in Figure ) as a decreasing past its critical point
which is an increasing function of the maturation rate β, the minimal nutrient ratio
; and a decreasing function of queen(s) laying egg rate γ. The larger the value of a, the more likely it is that the colony survives and grows.
Effects of nutrient thresholds
: Figures , and suggest that Model (Equation4
(4)
(4) ) exhibits reversed backward bifurcation on
(or
): (1) small values of the optimal nutrient ratio
can insecure the persistence of the colony; (2) intermediate values of
can go through saddle node bifurcation that leads to bistability; and (3) large values of
can lead to colony collapsing.
In addition, the larger value of the optimal nutrient ratio
(or
) leads to (1) larger critical threshold
; (2) smaller population A; and (3) smaller nutrient status, i.e. smaller value of
.
Effects of the brood survival rate
: Figure (a) shows that Model (Equation4
(4)
(4) ) exhibits reversed backward bifurcation on
. Figure (b) suggests that the larger value of
, the larger the population of workers A will be, which increases the probability of colony survival.
Our proposed model and study provide new insights into the strategies used by social insects (such as harvesting ants) facing nutritional challenges, and our results deepen our understanding of their nutritional ecology. Task allocation has been studied in social insects, that is, how colonies change the allocation of tasks in response to changing colony needs. One of future directions would be extending our current model to include more tasks such as brood care, foraging, and study how different tasks are related to colony needs including nutritional requirement. An another future direction is to extend our current model to include an additional level such as food resource of the colony. For example, leaf-cutter ants collect leaves as food resource to cultivate fungi and harvest the fruits of fungi as their food. The nutrient requirement of the leaf-cutter ants colony has two levels: one is the needs of the colony itself such as brood and the other one is the needs of the fungi. It would be interesting to explore how nutrient needs of the colony and fungus garden affect the foraging behaviour of leaf-cutter ants during its ontology.
6. Proofs
Proof of Lemma 3.1
Proof.
For any , from Model (Equation4
(4)
(4) ), we obtain
and
for all
. Since
, then
for all
. Moreover, if
and
, then
for all
. If
and
, then by continuity arguments, it is impossible for either
or
or
to drop below 0. Hence, for any
and
, we obtain
and
for all
.
Now assume and
, then since the function of
exists maximum when
and according to the expression of
, we have
for all
when
. Thus, a standard comparison theorem shows that
. This indicates that for any
, there exists T large enough, such that
Therefore, from the expression of
, we have
Since ε can be arbitrarily small, thus
. Thus, we have shown that the Model (Equation4
(4)
(4) ) is positively invariant and bounded in
. More specifically, the compact set
attracts all points in
. Due to
and the boundedness of
, hence we can obtain
is bounded for all
.
Moreover, if and
, then we have follows:
Therefore, if
and
, then
and
for all t>0.
Proof of Theorem 3.1
Proof.
It is easy to see that is always an equilibrium of Model (Equation4
(4)
(4) ). The nullclines of (Equation4
(4)
(4) ) can be found as
By solving
for
, we have
and substitute it to
, which results in the following equation:
(14)
(14) The roots of (Equation14
(14)
(14) ) are given by
where
.
Thus, we have the following three cases:
Let .
If
and
, then there is only one trivial equilibrium:
and no other positive interior equilibrium.
If
and
, then Model (Equation4
(4)
(4) ) has two positive equilibria which collapse into one equilibrium
as
Or if
and
, then Model (Equation4
(4)
(4) ) has only one positive equilibrium
If
and
, then Model (Equation4
(4)
(4) ) has two positive equilibria in the following form:
Proof of Theorem 3.2
Proof.
From Proposition 3.1, we know that for some initial condition taken in and if
, the trajectory of Model (Equation4
(4)
(4) ) is converging to the origin
. And according to Theorem 3.1, if
and
, then there is only one trivial equilibrium
and no other positive equilibrium. Therefore, we can conclude that Model (Equation4
(4)
(4) ) has global stability at
when
and
.
Proof of Theorem 3.3
Proof.
The local stability of equilibria is determined by computing the eigenvalues of the Jacobian matrix about each equilibrium.
Let be an arbitrary positive equilibrium of Model (Equation4
(4)
(4) ). The Jacobian matrix at this equilibrium is
(15)
(15) where
Then we have the characteristic equation of
is
(16)
(16) where
This indicates the following two cases:
If Model (Equation4
(4)
(4) ) has a unique interior equilibrium
, then under the conditions
and
and
, thus, by applying the Routh-Hurwitz criterion, we can obtain that the interior equilibrium
of Model (Equation4
(4)
(4) ) is locally asymptotically stable.
If Model (Equation4
(4)
(4) ) has two interior equilibria
where
, then under the conditions
,
, and
but
, we can obtain that the interior equilibrium
is locally asymptotically stable while
is unstable.
Proof of Theorem 3.4
Proof.
For any , note that
thus according to Theorem A.4 (p. 423) of [Citation31], we can conclude that the model (Equation4
(4)
(4) ) is positive invariant in
. Now we can proceed to show the boundedness of the system. First, assume
and
, then since the function of
exists maximum when
and according to the expression of
, we have the following inequalities due to the property of positive invariance:
which implies that
This suggests that there exists
such that the following inequalities hold as time t is large enough,
which indicates that
Then, we also have the following inequalities hold as time t is large enough,
which shows that
Therefore, every trajectory starting from
converges to the compact set
Let
be an interior equilibrium of Model (Equation4
(4)
(4) ). Then its stability is determined by the eigenvalues
of its associated Jacobian matrix as follows:
since
, and
. Therefore, we have
and the characteristic equation of
is
where
This indicates the following two cases:
If Model (Equation4
(4)
(4) ) has a unique interior equilibrium
, then under the conditions
,
and
where
(17)
(17) we get
. And we can verify that
. Thus, we can conclude that the interior equilibrium
is locally stable by applying the Routh-Hurwitz criterion.
If Model (Equation4
(4)
(4) ) has two interior equilibria
where
, then under the conditions
,
, and
we obtain
and
but
. We also can verify that
. Therefore, the interior equilibrium
is locally asymptotically stable while
is unstable.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- S.N. Beshers and J.H. Fewell, Models of division of labor in social insects, Ann. Rev. Entomol. 46 (2001), pp. 413–440. doi: 10.1146/annurev.ento.46.1.413
- M.V. Brian, Population turnover in wild colonies of the ant Myrmica, Ekologia Polska 20 (1973), pp. 43–53.
- S. Camazine, J.L. Deneubourg, N.R. Franks, J. Sneyd, G. Theraulaz, and E. Bonabeau, Self-organization in Biological Systems, Princeton University Press, Princeton, NJ, 2001.
- D.L. Cassill, A. Stuy, and R.G. Buck, Emergent properties of food distribution among fire ant larvae, J. Theor. Biol. 195 (1998), pp. 371–381. doi: 10.1006/jtbi.1998.0802
- D.L. Cassill and W.R. Tschinkel, Regulation of diet in the fire ant, Solenopsis invicta, J. Insect Behav. 12 (1999), pp. 307–328. doi: 10.1023/A:1020835304713
- D.L. Cassill and W.R. Tschinkel, Information flow during social feeding in ant societies, Inf. Process. Soc. Insects (1999), pp. 69–81. doi: 10.1007/978-3-0348-8739-7_4
- R.M. Clark, Behavioral and nutritional regulation of colony growth in the desert leafcutter ant Acromyrmex versicolor. PhD thesis, Arizona State University, 2011.
- S.C. Cook, M.D. Eubanks, R.E. Gold, and S.T. Behmer, Colony-level macronutrient regulation in ants: mechanisms, hoarding and associated costs, Anim. Behav. 79 (2010), pp. 429–437. doi: 10.1016/j.anbehav.2009.11.022
- T.H.F. Daugherty, A.L. Toth, and G.E. Robinson, Nutrition and division of labor: effects on foraging and brain gene expression in the paper wasp Polistes metricus, Mol. Ecol. 20 (2011), pp. 5337–5347. doi: 10.1111/j.1365-294X.2011.05344.x
- A. Dussutour and S.J. Simpson, Description of a simple synthetic diet for studying nutritional responses in ants, Insectes Soc. 55 (2008), pp. 329–333. doi: 10.1007/s00040-008-1008-3
- A. Dussutour and S.J. Simpson, Carbohydrate regulation in relation to colony growth in ants, J. Exp. Biol. 211 (2008), pp. 2224–2232. doi: 10.1242/jeb.017509
- A. Dussutour and S.J. Simpson, Communal nutrition in ants, Curr. Biol. 19 (2009), pp. 740–744. doi: 10.1016/j.cub.2009.03.015
- A. Dussutour and S.J. Simpson, Ant workers die young and colonies collapse when fed a high-protein diet, Proc. Biol. Sci. 279 (2012), pp. 2402–2408. doi: 10.1098/rspb.2012.0051
- B. Holldobler and E.O. Wilson, The Superorganism, 1st ed., W.W. Norton., New York, 2009.
- Y. Kang, R. Clark, M. Makiyama, and J. Fewell, Mathematical modeling on obligate mutualism: interactions between leaf-cutter ants and their fungus garden, J. Theor. Biol. 289 (2011), pp. 116–127. doi: 10.1016/j.jtbi.2011.08.027
- Y. Kang and J. Fewell, Coevolutionary dynamics of a host-parasite interaction model: obligatory v.s. facultative parasitism, Nat. Resour. Model. 28 (2015), pp. 398–455. doi: 10.1111/nrm.12078
- Y. Kang, M. Rodriguez-Rodriguez, and S. Evilsizor, Ecological and evolutionary dynamics of two-stage models of social insects with egg cannibalism, J. Math. Anal. Appl. 430 (2015), pp. 324–353. doi: 10.1016/j.jmaa.2015.04.079
- Y. Kang and G. Theraulaz, Dynamical models of task organization in social insect colonies, Bull. Math. Biol. 78 (2016), pp. 879–915. doi: 10.1007/s11538-016-0165-1
- Y. Kang and O. Udiani, Dynamics of a single species evolutionary model with allee effects, J. Math. Anal. Appl. 418 (2014), pp. 492–515. doi: 10.1016/j.jmaa.2014.03.083
- A.D. Kay, S. Rostampour, and R. Sterner, Ant stoichiometry: elemental homeostasis in stage-structured colonies, Funct. Ecol. 20 (2006), pp. 1037–1044. doi: 10.1111/j.1365-2435.2006.01187.x
- K.P. Lee, S.J. Simpson, F.J. Clissold, R. Brooks, J.W.O. Ballard, P.W. Taylor, N. Soran, and D. Raubenheimer, Lifespan and reproduction in Drosophila: new insights from nutritional geometry, Proc. Natl. Acad. Sci. 105 (2008), pp. 2498–2503. doi: 10.1073/pnas.0710787105
- M. Lihoreau, J. Buhl, M.A. Charleston, G.A. Sword, D. Raubenheimer, and S.J. Simpson, Nutritional ecology beyond the individual: a conceptual framework for integrating nutrition and social interactions, Ecol. Lett. 18 (2015), pp. 273–286. doi: 10.1111/ele.12406
- A.C. Mailleux, C. Detrain, and J.L. Deneubourg, Starvation drives a threshold triggering communication, J. Exp. Biol. 209 (2006), pp. 4224–4229. doi: 10.1242/jeb.02461
- G.P. Markin, Food distribution within laboratory colonies of the argentine ant, Tridomyrmex humilis (Mayr), Insectes Soc. 17 (1970), pp. 127–157. doi: 10.1007/BF02223074
- K. Messan, G. DeGrandi-Hoffman, C. Castillo-Chavez, and Y. Kang, Migration effects on population dynamics of the honybee-mite interactions, Math. Model. Nat. Phenom. 12 (2017), pp. 84–115. doi: 10.1051/mmnp/201712206
- S. Pohl, M.E. Frederickson, M.A. Elgar, and N.E. Pierce, Colony diet influences ant worker foraging and attendance of Myrmecophilous Lycaenid Caterpillars, Front. Ecol. Evol. 4 (2016), pp. 114. doi: 10.3389/fevo.2016.00114
- S. Portha, J.L. Deneubourg, and C. Detrain, Self-organized asymmetries in ant foraging: a functional response to food type and colony needs, Behav. Ecol. 13 (2002), pp. 776–781. doi: 10.1093/beheco/13.6.776
- D. Raubenheimer and S.J. Simpson, Integrating nutrition: a geometrical approach. Proceedings of the 10th International Symposium on Insect-Plant Relationships, Springer, Dordrecht, 1999, pp. 67–82.
- T.D. Seeley, Social foraging in honey bees: how nectar foragers assess their colony's nutritional status, Behav. Ecol. Sociobiol. 24 (1989), pp. 181–199. doi: 10.1007/BF00292101
- S.J. Simpson, R.M. Sibly, K.P. Lee, S.T. Behmer, and D. Raubenheimer, Optimal foraging when regulating intake of multiple nutrients, Anim. Behav. 68 (2004), pp. 1299–1311. doi: 10.1016/j.anbehav.2004.03.003
- H.R. Thieme, Mathematics in Population Biology, Princeton University Press, Princeton, NJ, 2003.
- A.L. Toth, S. Kantarovich, A.F. Meisel, and G.E. Robinson, Nutritional status influences socially regulated foraging ontogeny in honey bees, J. Exp. Biol. 208 (2005), pp. 4641–4649. doi: 10.1242/jeb.01956
- J.F.A. Traniello, Foraging strategies of ants, Ann. Rev. Entomol. 34 (1989), pp. 191–210. doi: 10.1146/annurev.en.34.010189.001203
- E.O. Wilson and T. Eisner, Quantitative studies of liquid food transmission in ants, Insectes Soc. 4 (1957), pp. 157–166. doi: 10.1007/BF02224149
