Abstract
An efficient synthesis of β-keto-sulfones is described. The reaction of α-haloketones with sodium alkyl/aryl sulphinates in aqueous medium under microwave irradiation afforded the corresponding β-keto-sulfones in excellent yields.
Introduction
Organo sulfones are very important and belong to a fascinating branch of chemistry. The presence of a sulfone group, in an organic compound adds variety to its chemical architecture Citation1 Citation2 and also enhances the biological activity of the compound. Among organo sulfones, β-keto-sulfones are a very significant group of intermediates, as they are used as precursors in Michael and Knoevenagel reactions Citation3 Citation4, in the preparation of acetylenes, allenes, chalcones Citation5–10, vinylsulfones Citation11 and poly functionalized 4H-pyrans Citation3 Citation12. In addition, β-keto-sulfones are useful for the synthesis of ketones by facile reductive elimination of the sulfone group Citation13–17 and are useful for the synthesis of optically active β-hydroxy-sulfones Citation18–20 and α-halomethyl sulfones Citation21–24. This has led to the development of novel synthetic methodologies for these compounds. Several authors have been reported in literature for the synthesis of β-keto-sulfones, which includes alkylation of metallic arene sulphinates with either α-haloketone Citation25 or α-tosyloxy ketones Citation26–28 acylation of alkyl sulfones Citation29 Citation30, reactions of diazo sulfones with aldehydes catalyzed by SnCl2 Citation31 , reaction of an acid ester with α-sulfonyl carbanions Citation32, reaction of an acid anhydride with α-sulfonyl carbanions, addition of aldehydes to α-sulfonyl carbanions followed by oxidation of the resulting β-hydroxy-sulfones Citation33, oxidation of β-keto-sulphides, oxidation of β-keto-sulfoxides Citation34, condensation of an α-haloketone with thiolate anion followed by oxidation Citation35. However, most of them suffer from one or more limitations such as unavailability of starting materials, long reaction times, harsh conditions with low yields and use of toxic organic solvents. The direct and straightforward method is the treatment of metallic arene sulphinates with α-haloketone (25–27). However, the low solubility of metal sulphinate salts in organic solvents is the inadequacy. An efficient and eco-friendly method for the synthesis of β-keto-sulfones is highly desirable.
The driving force for microwave developments in organic synthesis Citation36–43 has many benfits. The increasing requirement for environmentally clean technology that minimizes the production of waste at source, microwave may offer cleaner reactions by improving product yields and selectivities, enhancing the product recovery. In recent years, organic reactions carried out in the absence of solvent have been attracting the attention of chemists due to ease of processing and eco-friendly in nature. Herein, we report a microwave assisted an efficient synthesis of β-keto-sulfones in aqueous medium.
Results and discussion
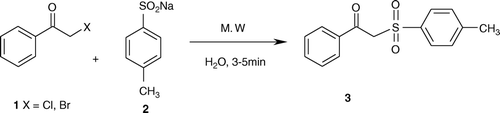
In this report (), we described an efficient method for the synthesis of sulfones using sodium alkyl/aryl sulphinate Citation44 in aqueous medium under microwave irradiation. The reaction of sodium p-toluenesulphinate with phenacyl bromide/chloride in aqueous medium under microwave irradiation produced the corresponding β-keto-sulfone in greater than 95%. The reaction of different α-haloketones with alkyl/aryl sulphinates proceeded efficiently and smoothly and the products were obtained in excellent yields. Various β-keto-sulfones have been synthesized in facile manner under microwave irradiation in aqueous medium (Scheme 1, ). Furthermore, we carried out the above reaction in conventional method in acetonitrile at room temperature, a small amount of product (<30%) formation was observed in 6 h. However, using catalytic amount of Tetrabutyl ammonium bromide (TBAB) as phase transfer catalyst gave the interesting results (∼90). Obviously, the phase transfer catalyst increases the solubility of sodium sulphinate salt in organic solvent and enhances the product conversion. The formation of products was characterized by their spectral data.
Table 1. Synthesis of β-keto-sulfones in aqueous medium under microwave irradiation.
Conclusion
In conclusion we described that microwave promoted an efficient synthesis of β-keto-sulfones in aqueous medium. The present procedure for the synthesis of β-keto-sulfones has the advantage of no use of toxic organic solvents, short reaction time, high yields of the products and simple work-up procedure which makes it a useful and important addition to the present existing methods.
Experimental section
General
The microwave reactions were carried out on ETHOS 1600, made: Milestone SRL Italy, 220 V/50 Hz; power supply equipped with Temperature and pressure control. Spectra were recorded with the following instruments: IR, Perkin Elmer spectrophotometer; 1H-NMR Varian Gemini 200 MHz and LCMS and Micromass VG 7070H (70 eV). Column chromatography was performed over silica gel (Achme 60–120 mesh or >300 mesh flash chromatography) and TLC (thin layer chromatography) with silica gel MERCK GF254 (pre-coated). The visualization of the spots in TLC plats was carried out either in UV light (short wave 250nm) or exposing the plates to iodine vapors or spraying with 10% sulfuric acid in methanol and subsequently heating on hot plate.
Typical experimental procedure (microwave)
A mixture of α-haloketone (10 mmol) and sodium alky/aryl sulphinate (11 mmol) was suspended in water (5 mL) in a reaction vessel, sealed without degassing and was subjected to microwave irradiation pre-set temperature at 100°C for an appropriate time (). After completion of the reaction, as monitored by TLC, the product was washed with ice-cold water and filtered to give crude product, which was purified by silica column chromatography.
Entry 1. 1H NMR (CDCl3, 300 MHz), δ=2.49 (s, 3H, Ar–CH3), 4.62 (s, 2H, CH2), 7.34 (d, 2H, J=8.5 Hz, Ar–H), 7.50 (t, 2H, J=8.0 Hz, Ar–H), 7.60 (t, 1H, J=3.5 Hz, Ar–H), 7.75 (d, 2H, J=8.0 Hz, Ar–H), 8.00 (d, 2H, J=8.5 Hz, Ar–H); EIMS: 274 (M• + ).
Entry 3. 1H NMR (CDCl3, 300 MHz), δ=4.50 (s, 2H, CH2), 7.34 (d, 2H, J=8.5 Hz, Ar–H), 7.49–7.55 (m, 5H, Ar–H), 7.60 (t, 1H, J=3.5 Hz, Ar–H), 8.00 (d, 2H, J=8.5 Hz, Ar–H); EIMS: 260 (M• + ).
Entry 5. 1H NMR (CDCl3, 300 MHz), δ=3.14 (s, 3H, CH3), 4.59 (s, 2H, CH2), 7.49 (t, 2H, J=8.25 Hz, Ar–H), 7.62 (t, 1H, J=3.4 Hz, Ar–H), 8.00 (d, 2H, J=8.25 Hz, Ar–H); EIMS: 198 (M• + ).
Entry 7. 1H NMR (CDCl3, 300 MHz), δ=2.42 (s, 3H, Ar–CH3), 2.49 (s, 3H, Ar–H), 4.62 (s, 2H, CH2), 7.34 (d, 2H, J=8.5 Hz, Ar–H), 7.51 (d, 2H, J=8.0 Hz, Ar–H), 7.80 (d, 2H, J=8.0 Hz, Ar–H), 8.00 (d, 2H, J=8.5 Hz, Ar–H); EIMS: 288 (M• + ).
Typical experimental procedure (conventional)
To a solution of α-haloketone (1 mmol) and sodium alky/aryl sulphinate (1.1 mmol) in acetonitrile (5 mL), was added TBAB. The mixture was stirred at room temperature for 1h. After completion of the reaction, as monitored by TLC, the solvent was evaporated and the product was extracted into ethyl acetate (3×15 mL). The combined organic extracts were dried over anhydrous sodium sulfate, evaporated under reduced pressure to give crude product, which was purified by silica column chromatography.
Acknowledgements
The authors are thankful to Dr. J. S. Yadav, Director of IICT for his constant encouragement and CSIR, UGC New Delhi for financial assistance.
References
- Simpkins , N.S. Sulfones in Organic Synthesis ; Baldwin , J.E. ; Oxford : Pergamon press , 1993 .
- Trost , B.M. 1991 . Comprehensive Organic Chemistry , Oxford : Pergamon Press .
- Marco , J.L. , Fernandez , I. , Khiar , N. , Fernandez , P. and Romero , A. 1995 . J. Org. Chem. , 60 : 6678 – 6679 .
- Reddy , M.V.R. and Reddy , S. 1984 . Acta Chim. Hung. , 115 : 269 – 271 .
- Ihara , M. , Suzuki , S. , Taniguchi , T. , Tokunaga , Y. and Fukumoto , K. 1995 . Tetrahedron , 51 : 9873 – 9890 .
- Baldwin , J.E. , Adlington , R.M. , Crouch , N.P. , Hill , R.L. and Laffeg , T.G. 1995 . Tetrahedron Lett. , 36 : 7925 – 7928 .
- Reddy , M.V.R. and Reddy , S. 1985 . Acta Chim. Hung. , 120 : 275 – 280 .
- Looker , J.J. 1966 . J. Org. Chem. , 31 : 2714 – 2715 .
- Sengupta , S. , Sarma , D.S. and Mondal , S. 1998 . Tetrahedron , 54 : 9791 – 9798 .
- Nenajdenko , V.G. , Krasovskiy , A.L. and Balenkova , E.S. 2007 . Tetrahedron , 63 : 12481 – 12539 .
- Sengupta , S. , Sarma , D.S. and Mondal , S. 1998 . Tetrahedron Asymmery , 9 : 2311 – 2316 .
- Marco , J.L. 1997 . J. Org. Chem. , 62 : 6575 – 6581 .
- Corey , E.J. and Chaykosky , M. 1964 . J. Am. Chem. Soc. , 86 : 1639 – 1640 .
- Trost , B.M. , Arndt , H.C. , Strege , P.E. and Verhoeven , T.R. 1976 . Tetrahedron Lett. , 27 : 3477 – 3478 .
- Kurth , M.J. and O'Brien , M.J. 1985 . J. Org. Chem. , 50 : 3846 – 3848 .
- Fujii , M. , Nakamura , K. , Mekata , H. , Oka , S. and Ohno , A. 1988 . Bull. Chem. Soc. Jpn. , 61 : 495 – 500 .
- Guo , H. and Zhang , Y. 2000 . Synth. Commun. , 30 : 2559 – 2564 .
- Svatos , A. , Hunkova , Z. , Kren , V. , Hoskovec , M. , Saman , D. , Valterova , I. , Vrkoc , J. and Koutek , B. 1996 . Tetrahedron Asymmetry , 7 : 1285 – 1294 .
- Bertus , P. ; Phansavath , P. ; Ratovelomanana-Vidal , V. ; Genêt , J.P. ; Touati , A.R. ; Homri , T. ; Hassine , B.B. Tetrahedron. Asymmetry 1999 , 10 , 1369 – 1380 .
- Gotor , V. , Rebolledo , F. and Liz , R. 2001 . Tetrahedron Asymmetry , 12 : 513 – 515 .
- Baker , F.C. ; Li , J.P.N. United States Patent . US 4247559 (C07D 207/452; A61K 031/40), January 27, 1981 .
- Eckstein , Z. , Zawistowska , M. , Palut , D. and Polubiec , E. 1966 . Pol. J. Chem. , 45 : 314 – 320 .
- Ejmocki , Z. ; Krassowska , B.K. ; Olezak , I. ; Eckstein , Z. J. Chem. Pol. . 1980 , 54 , 11–12 and 2153–2159 .
- Antane , S. ; Bernotas , R. ; Li , Y. ; McDevitt , R. ; Yan , Y. Synth. Commun . 2004 , 34 , 2443 – 2449 .
- Vennstra , G.E. ; Zwaneburg , B. Synthesis 1975 , 519 – 520 .
- Wildeman , J. ; Van Leusen , A.M. Synthesis 1979 , 733 – 734 .
- Xie , Y-Y. and Chen , Z-C. 2001 . Synth. Commun. , 31 : 3145 – 3149 .
- Kumar , D. , Sundaree , S. , Rao , V.S. and Rajender , S.V. 2006 . Tetrahedron Lett. , 47 : 4197 – 4199 .
- Katrizky , A.R. , Abdel-Fattah , A.A. and Wang , M.Y. 2003 . J. Org. Chem. , 68 : 1443 – 1446 .
- Truce , W.E. and Knospe , R.H. 1955 . J. Am. Chem. Soc. , 77 : 5063 – 5067 .
- Holmquist , C.R. and Roskamp , E.J. 1992 . Tetrahedron Lett. , 33 : 1131 – 1134 .
- Schank , K. ; Weber , A. Synthesis 1970 , 367 .
- Julia , M. and Paris , J.M. 1973 . Tetrahedron Lett. , 14 : 4833 – 4836 .
- Durst , T. Comprehensive Organic Chemistry ; Barton D.H.R. ; Ollis , W.D. ; Oxford, , UK : Peragmon Press , 1979 ; 4, Chapter 11.8 , p 174 .
- Trost , B.M. 1978 . Chem. Rev. , 78 : 363 – 382 .
- Lupy , A. 1999 . Top. Curr. Chem. , 206 : 153 – 207 .
- Villemin , D. , Labiad , B. and Loupy , A. 1993 . Synth. Commun. , 23 : 419 – 424 .
- Larhed , M. and Halberg , A. 1996 . J. Org. Chem. , 51 : 9582
- Wang , C.D. , Shi , X.-Z. and Xie , R.J. 1997 . Synth. Commun. , 27 : 2517 – 2520 .
- Varma , R.S. and Dahiya , R. 1997 . Tetrahedron Assymet. , 38 : 2039 – 2042 .
- Hosseini Sarvari , M. and Sharghi , H. 1997 . J. Org. Chem. , 69 : 6953 – 6956 .
- Bosch , A.I. ; Cruz , P. ; Eiez-Barra , E. ; Loupy , A. ; Langa , F. Synlett 1995 , 1259 – 1260 .
- Khosropour , A.R. ; Khodaei , M.M. ; Moghannian , H. Synlett 2005 , 955 – 958 .
- Vogel , A.I. 1999 . Vogel's Text Book of Practical Organic Chemistry , 5th ed. , 888 USA : Prentice Hall .