Abstract
The green, mild, and efficient synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-diones by Knoevenagel condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with Meldrum's acid in presence of 1-benzyl-3-methylimidazolium chloride ((bnmim)(Cl)) ionic liquid at room temperature is reported. This method gives remarkable advantages such as a simple procedure, mild conditions, faster (10–20 min) reactions, and excellent yields. Additionally, the (bnmim)(Cl) was successfully recycled at least four times without significant loss of activity.
Introduction
In recent years, the application of ionic liquids in organic synthesis have attracted considerable attention due to their special properties such as good solvating capability, wide liquid range, non-inflammability, negligible vapor pressure, easy recycling, high-thermal stability, and rate enhancers Citation1. Nowadays, much attention has been focused on organic reactions catalyzed by ionic liquids Citation2. Particularly, imidazolium-based ionic liquids have been successfully used in many organic transformations such as Diels–Alder (Citation3a), Wittig (3b), and Suzuki cross-coupling (3c).
The Knoevenagel condensation is one of the most important methods for the preparation of substituted alkenes by the reaction of carbonyl compounds with active methylene compounds Citation4.
Meldrum's acid (2,2-dimethyl-1,3-dioxane-4,6-dione) is an active methylene compound having rigid cyclic structure with high acidity (pKa = 4.9) which undergoes hydrolysis very easily Citation5. Recently, there have been several methods reported in the literature for the Knoevenagel condensation of aldehydes with Meldrum's acid Citation6.
Compounds having a chromone moiety are synthetically versatile molecules with a reactive carbonyl group. They have considerable significance for their biological activities Citation7 and for their reactivity toward nucleophiles, which allow the synthesis of a wide variety of heterocycles. The substrate, 4-oxo-(4H)-1-benzopyran-3-carbaldehyde has three active sites such as, α, β-unsaturated carbonyl group, a carbon–carbon double bond and a formyl group. Of these, the formyl group has the highest reactivity toward active methylene compounds. The condensation reactions of 4-oxo-(4H)-1-benzopyran-3-carbaldehyde with active methylene compounds are well known Citation8. It is well known that 2,2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-diones are generally synthesized by condensation of 4-oxo-4H-benzopyran-3-carbaldehyde with Meldrum's acid in presence of alumina under microwave irradiation Citation9.
Results and discussion
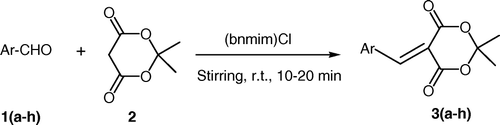
In continuation of our work on Knoevenagel condensations Citation9 Citation10 and the development of novel synthetic methodologies Citation11, herein, we would like to report a simple, efficient, and green methodology for the synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-diones. The synthetic route has been shown in .
In search of an efficient ionic liquid and the best experimental conditions, the reaction of 4-oxo-4H-benzopyran-3-carbaldehyde 1a and Meldrum's acid 2 at room temperature has been considered as the model reaction. We screened different ionic liquids such as, 1-hexyl-2,3-dimethylimidazolium chloride ((hdmim)(Cl)), 1-hexyl-3-methylimidazolium chloride ((hmim)(Cl)), 1-butyl-3-methylimidazolium chloride ((bmim)(Cl)), 1-butyl-3-methylimidazolum tetrafluroborate ((bmim)(BF4)), 1-butyl-3-methylimidazolium hexaflurophosphate ((bmim)(PF6)), and 1-benzyl-3-methylimidazolium chloride ((bnmim)(Cl)) for the model reaction. All the results are shown in . In ionic liquids such as (bmim)(BF4), (bmim)(PF6), and (bnmim)(Cl), the desired product was obtained with satisfactory yield. Considering the reaction time and yield of product, (bnmim)(Cl) () was selected as the optimum ionic liquid to promote the synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-diones (, entry 6).
Table 1. Effect of different ionic liquids for the synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-dione 3a.a

Table 2. Effect of concentration of (bnmim)(Cl) for the synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-dione 3a.a
To determine the appropriate concentration of the (bnmim)(Cl), we investigated the model reaction at different concentrations including 5, 10, 15, and 20 mol%. The product formed in 86%, 89%, 93%, and 93% yield, respectively, indicating that 15 mol% of (bnmim)(Cl) is sufficient (, entry 3).
We have developed a newer route for the condensation of various 4-oxo-(4H)-1-benzopyran-3-carbaldehyde with Meldrum's acid in the presence of (bnmim)(Cl) at room temperature (). For 4-oxo-(4H)-1-benzopyran-3-carbaldehyde, which has three active sites, the reactions selectively occurred at the formyl group. The reaction does not require any additional catalyst because the ionic liquid acts as a catalyst as well as solvent (10a, 10b). The liberated water during the reaction was adsorbed by the ionic liquid and hence the reactions proceeded well. All the reactions were carried out using mild reaction conditions at room temperature with constant stirring. Using this methodology, condensation reactions were completed in shorter reaction times (10–20 min) with excellent yield (90–96%).
Table 3. Knoevenagel condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with Meldrum's acid in presence of (bnmim)(Cl) at room temperature.a
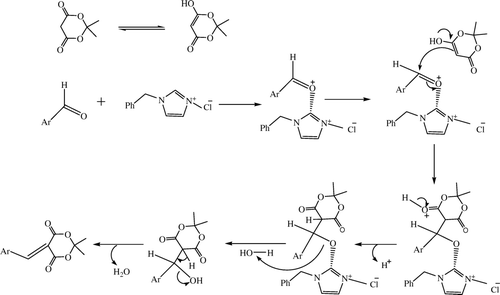
We have successfully used recovered (bnmim)(Cl) for the model reaction and the results of recycling experiments are shown in . These results clearly indicate that the recovered (bnmim)(Cl) can be recycled successfully without significant loss of activity. The possible mechanism of this reaction is shown in .
Table 4. Recycling of (bnmim)(Cl) for the synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-dione 3a.a
Experimental section
All chemicals were purchased from Merck, Aldrich, and Rankem chemical companies and used without further purification. The uncorrected melting points of compounds were taken in an open capillary in a paraffin bath. The progress of the reactions was monitored by Thin Layer Chromatography (TLC). IR spectra were recorded on Perkin-Elmer FTIR spectrophotometer in KBr disc. 1H NMR spectra were recorded on an 300 MHz FT-NMR spectrometer in CDCl3 as a solvent and chemical shift values are recorded in units ▵ (ppm) relative to tetramethylsilane (Me4Si) as an internal standard.
The required 4-oxo-4H-benzopyran-3-carbaldehydes was prepared by Vilsmeir–Haack reaction Citation12.
Synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1,3-dioxane-4, 6-dione 3(a–h)
A mixture of 4-oxo-4H-benzopyran-3-carbaldehyde (1 mmol), Meldrum's acid (1 mmol), and (bnmim)(Cl) (15 mol%) were taken in a single neck round-bottom flask. The contents of the flask were stirred at room temperature for the appropriate time given in . When TLC showed complete disappearance of starting material, the mixture was poured over ice-water and extracted with diethyl ether (3×10 mL). The organic layer was dried over anhydrous Na2SO4. The solvent evaporated under reduced pressure and solid compound was recrystallized from ethyl acetate to afford the corresponding 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-dione 3a in excellent yield. Furthermore, the aqueous layer was distilled at 80C under vacuum to remove water, leaving behind the ionic liquid (bnmim)(Cl). The structures of the products were confirmed by IR, 1H NMR, and mass spectral data.
Spectral data of compounds
3a IR (KBr, cm−1): 3062, 2996, 1732, 1670, 1396, 1251. 1H NMR (300 MHz, CDCL3) ▵ (ppm): 1.8 (6H, s, 2×CH3), 7.2–8.1 (4H, m, aromatic), 8.7 (1H, s, olefinic), 9.6 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=301 [M + 1].
3b IR (KBr, cm−1): 3061, 2992, 1730, 1669, 1372, 1296, 797. 1H NMR (300 MHz, CDCl3) ▵ (ppm) = 1.9 (6H, s, 2×CH3), 7.2–8.2 (3H, m, aromatic), 8.6 (1H, s, olefinic), 9.6 (1H, s, C2–H of chromone moiety). EIMS (electron ionization mass spectroscopy) (m/z,%):=335 [M + 1].
3c IR (KBr, cm−1): 3055, 2990, 1710, 1650, 1390, 1280. 1H NMR (300 MHz, CDCl3) ▵ (ppm): 2.5 (3H, s, Ar–CH3), 1.9 (6H, s, 2×CH3), 7.2–8.2 (3H, m, aromatic), 8.7 (1H, s, olefinic), 9.6 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=315 [M + 1].
3d IR (KBr, cm−1): 3065, 2989, 1729, 1674, 1392, 1293, 791. 1H NMR (300 MHz, CDCl3) ▵ (ppm): 1.9 (6H, s, 2×CH3), 7.2–8.2 (3H, m, aromatic), 8.6 (1H, s, olefinic), 9.5 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=370 [M + 1].
3e IR (KBr, cm−1): 3060, 2996, 1718, 1649, 1396, 1283, 796. 1H NMR (300 MHz, CDCl3) ▵ (ppm): 2.5 (3H, s, Ar–CH3), 1.9 (6H, s, 2–CH3), 7.2–7.5 (2H, s, aromatic), 8.6 (1H, s, olefinic), 9.5 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=349 [M + 1].
3f IR (KBr, cm−1): 3084, 3018, 1714, 1662, 1392, 1280, 798. 1H NMR (300 MHz, CDCl3) ▵ (ppm): 1.8 (6H, s, 2×CH3), 7.2–8.3 (2H, s, aromatic), 8.6 (1H, s, olefinic), 9.5 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=370 [M + 1].
3g IR (KBr, cm−1): 3063, 2993, 1735, 1664, 1395, 1280, 805. 1H NMR (300 MHz, CDCl3) ▵ (ppm): 1.8 (6H, s, 2×CH3), 7.2–8.2 (3H, m, aromatic), 8.6 (1H, s, olefinic), 9.6 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=380 [M + 1].
3h IR (KBr, cm−1): 3061, 2992, 1730, 1669, 1372, 1296, 797. 1H NMR (300 MHz, CDCl3) ▵ (ppm) = 1.9 (6H, s, 2×CH3), 7.2–8.2 (3H, m, aromatic), 8.6 (1H, s, olefinic), 9.6 (1H, s, C2–H of chromone moiety). EIMS (m/z,%):=319 [M + 1].
Conclusion
In conclusion, we developed a simple, safe, efficient, and green methodology for the synthesis of 2, 2-dimethyl-5-[(4-oxo-4H-chromen-3-yl) methylene]-1, 3-dioxane-4, 6-dione from the condensation of substituted 4-oxo-4H-benzopyran-3-carbaldehyde with Meldrum's acid in the presence of (bnmim)(Cl) at room temperature. The notable merits offered by this methodology are mild reaction conditions, simple procedures, cleaner reaction, short reaction time and excellent yield of products. Additionally, the (bnmim)(Cl) can be recycled at least four times without significant loss of activity, which makes the present protocol more convenient and environmentally benign.
Acknowledgements
We are grateful to the Head Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431 004 (MS), for providing the laboratory facilities.
References
- (a) Thomas , W Chem. Rev . 1999 , 99 , 2071 – 2084 ; (b) Sheldon , R Chem. Commun . 2001 , 23 , 2399 – 2407 ; (c) Zhao , D ; Wu , M ; Kou , Y ; Min , K Catal. Today . 2002 , 2654 , 1 – 33 ; (c) Sarda , S.R ; Pathan , M.Y ; Paike , V.V ; Pachmase , P.R ; Jadhav , W.N ; Pawar , R.P Arkivoc . 2006 , 16 , 43 – 48 .
- (a) Peng , J ; Deng , Y Tetrahedron Lett . 2001 , 42 , 403 – 405 ; (b) Ji , S-J ; Jiang , Z-Q ; Lu , J ; Loh , T-P Synlett . 2004 , 5 , 831 – 835 ; (c) Gong , K ; He , Z-W ; Xu , Y ; Fang , D ; Liu , Z-L Monatsh. Chem . 2008 , 139 , 913 – 915 .
- (a) Fischer , T ; Sethi , A ; Welton , T ; Woolf , J Tetrahedron Lett . 1999 , 40 , 793 – 796 ; (b) Le Boulaire , V.R Chem. Commun . 2000 , 22 , 2195 – 2196 ; (c) Mathews , C.J ; Smith , P.J ; Welton , T Chem. Commun . 2000 , 14 , 1249 – 1250 .
- (a) Cao , Y-Q ; Dai , Z ; Zhang , R Synth. Commun . 2004 , 34 , 2965 – 2971 ; (b) Sachan , N ; Kadam , S.S ; Kulkarni , V.M Ind. J. Hetero. Chem . 2007 , 17 , 57 – 62 ; (c) Mahalle , R.S ; Netankar , P.D ; Bondge , S.P ; Mane , R.A Green Chem. Lett. Rev . 2008 , 1 , 103 – 106 ; (d) Saha , M ; Roy , S ; Chaudhuri , S ; Bhar , S Green Chem. Lett. Rev . 2008 , 1 , 113 – 121 .
- McNab , H. 1978 . Chem. Soc . Rev. , 7 : 345 – 358 .
- (a) Daqing , S ; Yucheng , W ; Zaisheng , L ; Guiyuan , D Synth. Commun . 2000 , 30 , 713 – 718 ; (b) Ren , Z ; Cao , W ; Tong , W ; Jing , X Synth. Commun . 2002 , 32 , 1947 – 1952 ; (c) Aimin , S ; Xiaobing , W ; Kit , S.L Tetrahedron Lett . 2003 , 44 , 1755 – 1758 ; (d) Desai , U.V ; Pore , D.M ; Mane , R.B ; Solabannavao , S.B ; Wadgaonkar , P.P Synth. Commun . 2004 , 34 , 25 – 32 .
- Gerwick , W.H. , Lopez , A. , Van Duyne , G.D. , Clardy , J. , Ortiz , W. and Buez , A. 1979 . Tetrahedron Lett. , 270 : 1986 – 1990 .
- (a) Polykov , V.K ; Shevtsova , R.G Ukr. Khim. Zh . 1981 , 47 , 85 – 87 ; (b) Hass , G ; Stanton , J.L ; Vonsprecher , A ; Paul , W J. Hetero.Chem . 1981 , 18 , 607 – 610 ; (c) Treibs , A ; William , R ; Grimm , D Liebigs Ann. Chem . 1981 , 3 , 306 – 401 ; (d) Shkumat , A.P ; Babich , Y.P ; Pivenko , N.S ; Polyakov , V.K Zh.Obshch. Khim . 1989 , 59 , 1116 – 1122 ; (d) Prousek , J Colt. Czech. Chem. Commun . 1993 , 58 , 3014 – 3016 ; (e) Rama Sarma , G.V.S ; Reddy , V.M Ind. J. Hetero. Chem . 1993 , 3 , 111 – 114 .
- Shindalkar , S.S. , Madje , B.R. and Shingare , M.S. 2006 . Ind. J. Chem . Sec. B. , 45 : 2571 – 2573 .
- (a) Hangarge , R.V ; Sonwane , S.A ; Jarikote , D.V ; Shingare , M.S Green Chem . 2001 , 3 , 310 – 312 ; (b) Hangarge , R.V ; Jarikote , D.V ; Shingare , M.S Green Chem . 2002 , 4 , 266 – 268 ; (c) Shindhalkar , S.S ; Madje , B.R ; Shingare , M.S Ind. J. Chem . 2005 , 44B , 1519 – 1521 ; (d) Shindalkar , S.S ; Madje , B.R ; Shingare , M.S J. Korean Chem. Soc . 2005 , 49 , 377 – 380 ; (e) Madje , B.R ; Shindalkar , S.S ; Ware , M.N ; Shingare , M.S Arkivoc . 2005 , 14 , 82 – 86 .
- (a) Madje , B.R .; Patil , P.T ; Shindhalkar , S.S .; Benjamin , S.B .; Shingare , M.S .; Dongare , M.K . Catalysis Commun . 2004 , 5 , 353 – 357 ; (b) Pokalwar , R.U .; Hangarge , R.V ; Maske , P.V ; Shingare , M.S Arkivoc . 2006 , 11 , 196 – 204 ; (c) Shindarlkar , S.S ; Madje , B.R ; Shingare , M.S Mendeleev Commun . 2007 , 17 , 43 – 44 ; (d) Shelke , K F ; Markhele , V.M ; Kategaonkar , A.H ; Shingare , M.S Bull. Cata. Soc. Ind . 2007 , 6 , 136 – 139 ; (e) Shelke , K.F ; Madje , B.R ; Sadhaphal , S.A ; Shitole , N.V ; Shingare , M.S Chem. Ind. J . 2008 , 4 , 277 – 280 ; (f) Shelke , K.F ; Kakade , G.K Shingate , B.B ; Shingare , M.S Rasayan J. Chem . 2008 , 1 , 489 – 494 ; (g) Shelke , K.F ; Sapkal , S.B ; Shingare , M.S Chine. Chem. Lett . in press .
- Nohara , A. , Umetani , T. and Sanno , Y. 1974 . Tetrahedron , 30 : 3553 – 3561 .
![Figure 1. 1-benzyl-3-methylimidazolium chloride [(bnmim)(Cl)].](/cms/asset/299ff83a-3cfc-47fa-b91b-3e1f8fff5794/tgcl_a_376480_o_f0002g.gif)