Abstract
The rapid and very simple conjugate addition of thiols to α,β-unsaturated carbonyl compounds under solvent-free conditions in the presence of catalytic amount of lithium hydroxide at room temperature is reported. The reaction of aryl, alkyl, aliphatic, and hindered thiols with chalcone, enone, and nitrostyrene gave the corresponding Michael adducts with significant advantages, such as high conversions, short reaction time, mild reaction conditions, low cost, simple catalyst, and high to quantitative yields with excellent chemoselectivity.
Introduction
Due to the growing concern for the impact of organic solvents on the environment as well as on the human body, organic reactions without use of conventional organic solvents have attracted the attention of synthetic organic chemists. Thus, the development of simple and clean reactions without using organic solvents is one of the important topics in current chemistry Citation1.
Sulfur-containing motifs are ubiquitous in natural products Citation2 Citation3 and biologically active molecules (2a, 4), including calcium antagonist dilthiazem Citation5. Given the widespread availability of sulfur nucleophiles and α,β-unsaturated alkenes, there is substantial interest in developing efficient C–S bond-forming reactions via thia-Michael addition from these simple starting materials. Thus, several efforts have been made to develop newer and simple methodologies for thia-Michael addition that lead to the development of various base Citation6 and acid Citation7 catalysts. Furthermore, catalyst free and highly efficient conjugate addition of thiols to α,β-unsaturated carbonyl compounds in water and ionic liquids at room temperature and quaternary ammonium salts at high temperature have been reported Citation8.
However, there are various limitations such as long reaction times, use of organic solvents, high temperatures, moderate yields, and limited substrate. Additionally, the range of sulfur-centered nucleophiles and Michael acceptors well suited for both catalytic and stoichiometric methodologies are generally restricted to simple thiols and enones, and there have been few straightforward syntheses of these uncomplicated enones, e.g., chalcone, which cannot react in water even under forcible conditions.
In regard to the above, there is a constant need to develop a suitable alternative reaction methodology in which uses the hindered thiol and α,β-unsaturated ketones still remains an important reaction methodology challenge for the thia-Michael addition reaction.
Results and discussion
As a part of our research aimed at developing green chemistry by using water as the reaction medium or by performing organic transformations under solvent-free conditions Citation9, herein, we describe a simple, highly efficient, and eco-friendly method for the synthesis of β-sulfido carbonyl compounds from aromatic and aliphatic thiols and α,β-unsaturated Michael acceptors at room temperature under solvent-free conditions.
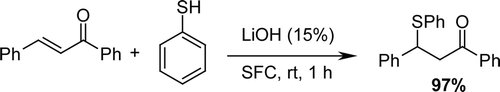
In order to find the best reaction conditions, we first studied the reaction of thiophenol and chalcone with different loading of starting materials and solvent. It was found that the reaction of chalcone (3 mmol) and thiophenole (3 mmol) in the presence of commercially available lithium hydroxide (15 mol%) under solvent-free conditions proceeded smoothly within 30 min to afford the conjugate addition products exclusively and in quantitative yields. Furthermore, in organic solvent such as CH2Cl2 and CH3CN, the Michael addition reaction proceeded at longer reaction times to give the desired product in low yields ().
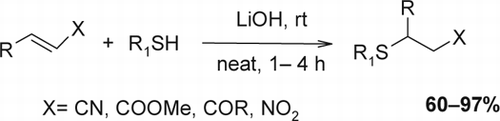
Having established the optimal conditions, subsequent studies were carried out under the optimized conditions with 15 mol% of LiOH at room temperature under solvent-free conditions to determine its scope with respect to the thiols and Michael acceptor. Thus, a series of thiols and unsaturated compounds were subjected to this simple procedure, and the results are summarized in . In all cases, lithium hydroxide-catalyzed reactions proceeded smoothly and gave the corresponding products in good to excellent yield.
Table 1. Range of alkyl halides and thiols.
Both aliphatic and aromatic thiols react with a variety of conjugated alkenes by this procedure to produce the corresponding adducts in high yields. As evident from the results, thiophenols bearing either electron-donating or electron-withdrawing groups did not make any difference in this reaction. Similarly, the corresponding products from the reaction of aliphatic thiols with α,β-unsaturated alkenes were obtained with high yields. In general, the reaction rates are faster with aromatic thiols compared to those of aliphatic thiols and very poor thiols such as 4-nitrothiophenol did not react due to the decrease in nucleophilicity of the sulfur atom of 4-nitrothiophenol compared to that of thiophenol. Under further observation, we examined the reactivity of heterocyclic thiols in the presence of lithium hydroxide. However, the reactions gave the products in moderate yields under solvent-free conditions due to poor nucleophilicity of heterocyclic thiols.
Similarly, various α,β-unsaturated compounds such as methyl acrylate, acrylonitrile, acrylamide, β-nitrostyrene, chalcone, benzylidenacetone, and cinnamonitrile underwent 1,4-addition with a variety of thiols to furnish the corresponding addition products. Furthermore, a reactive Michael acceptor such as methyl vinyl ketone was used to give Michael adducts under catalyst free conditions at 0°C with short reaction time. The Michael addition reaction was also attempted with β-nitrostyrene. Nitrostyrene derivatives are important intermediates not only for the synthesis of drugs, pigments, and pharmaceuticals, but also for the development of functional organic materials. In general, β-nitrostyrenes have good electrophilicity compared to enones and show a higher reactivity under solvent-free condition. When β-nitrostyrenes were treated with thiophenols in the absence of any catalyst at room temperature, the reaction afforded the corresponding compound in high yields at short reaction times (). In addition, chemoselective thia-Michael addition over aza-Michael addition during intra-molecular competition has been achieved in the reaction of 2-aminothiophenole with various Michael acceptor.
The reactions are relatively faster with aromatic thiols compared to aliphatic thiols. In general, the reactions are completed in a short period (1–4 h) and the products are obtained in high yields ranging from 60 to 97%. In the absence of catalyst, the reactions are slow and the products are obtained in low yields. Products of all known compounds gave acceptable 1H NMR and 13C NMR spectra that matched the data reported in the cited references.
Experimental
General methods
1H NMR spectra were recorded on 500 MHz NMR spectrometer and 13C NMR spectra were recorded on 125 MHz NMR spectrometer, respectively, using CDCl3 or DMSO, as a solvent, Chemical shifts have been expressed in (ppm) downfield from tetramethylsilane. All amines, Michael acceptor are commercially available and were purchased and used without further purification. Water and other solvents were distilled before used.
General procedure of Michael addition of enones and nitrostyrene with thiols catalyzed by LiOH
To a stirred solution of Michael acceptor (3 mmol) and LiOH (15 mol%) in the test tube, thiols (3.1 mmol) were added and the resulting mixture was stirred at room temperature for 60–240 min. After completion of reaction in most cases, pure products were obtained upon washing with water. In a few cases, the crude product was further purified by flash column chromatography to provide the corresponding product. All compounds were characterized on the basis of their spectroscopic data (IR, NMR) and by comparison with those reported in the literature Citation6 Citation7.
Conclusion
In summary, an operationally simple and straightforward thia-Michael addition of aromatic and aliphatic thiols to electron-deficient olefins under solvent-free conditions with good to excellent yields has been developed. The efficacy of this method is due to its operational simplicity which only requires stirring the reaction mixture at ambient temperature without the use of any additional energy source like heating or sonication. The procedure generality is evident from its success with varied kinds of Michael acceptors and thiols, and very importantly its economic viability due to the use of inexpensive and commercially available chemicals to affect this C–S bond formation.
Acknowledgements
Financial support of this work by Chemistry and Chemical Research Center of Iran is gratefully appreciated.
References
- (a) Toda , F. Acc. Chem. Res . 1995 , 28 , 480 – 486 ; (b) Metzger , J.O . Angew. Chem. Int. Ed. 1998 , 37 , 2975 ; (c) Tanaka , K. ; Toda , F. Chem. Rev . 2000 , 100 , 1025 – 1074 ; (d) Chakraborti , A.K. ; Shivani , G. J. Org. Chem . 2006 , 71 , 5785 – 5788 ; (e) Mihara , M. ; Nakai , T. ; Iwai , T. ; Ito , T. ; Mizuno , T. Synlett. 2007 , 13 , 2124 – 2126 .
- (a) Perlmutter , P. Conjugated Addition Reactions in Organic Synthesis ; Paragon : Oxford , 1992 ; p 114 ; (b) Sheldon , R.A. Chirotechnologies, Industrial Synthesis of Optically Active Compounds ; Dekker : New York , 1993 ; pp 39 – 72 .
- (a) Bakuzis , P. ; Bakuzis , M.L.F. J. Org. Chem . 1981 , 46 , 235 ; (b) Cherkauskas , J.P. ; Cohen , T. J. Org. Chem . 1992 , 57 , 6 – 8 ; (c) Fluharty , A.L . In The Chemistry of the Thiol Group ; Patai, S., Ed.; Wiley: New York, 1974; Part 2; p 589; (d) Yadav , J.S. ; Reddy , B.V.S. ; Baishya , G. J. Org. Chem . 2003 , 68 , 7098 – 7100 ; (e) Clark , J.H. Chem. Rev . 1980 , 80 , 429 .
- Fujita , E. and Nagao , Y.J. 1977 . Bioorg. Chem. , 6 : 287 – 309 .
- (a) Budriesi , R. ; Carosati , E. ; Chiarini , A. ; Cosimelli , B. ; Cruciani , G. ; Ioan , P. ; Spinelli , D. ; Spisani , R. J. Med. Chem . 2005 , 48 , 2445 – 2456 ; (b) Trost , B.M. ; Keeley , D.E. J. Org. Chem. 1975 , 40 , 2013 ; (c) Shono , T. ; Matsumura , Y. ; Kashimura , S. ; Hatanaka , K. J. Am. Chem. Soc . 1979 , 101 , 4752 – 4753 .
- (a) Perlmutter , P. Conjugated Addition Reactions in Organic Synthesis ; Pergamon: Oxford , 1992 ; pp 1 – 394 ; (b) Ranu , B.C. ; Bhar , S. ; Sarkar , D.C. Tetrahedron Lett . 1991 , 32 , 2811 – 2812 ; (c) Srivastava , N. ; Banik , B.K . J. Org. Chem . 2003 , 68 , 2109 – 2114 ; (d) Sreekumar , R. ; Rugmimi , P. ; Padmakumar , R. Tetrahedron Lett . 1997 , 38 , 6557 – 6560 ; (e) Sebti , S. ; Saber , A. ; Rhihil , A. Tetrahedron Lett . 1994 , 35 , 9399 – 9400 ; (f) Laszlo , P. ; Montaufier , M-T. ; Randriamahefa , S.L. Tetrahedron Lett. 1990 , 31 , 4867 – 4870 .
- (a) De Silva , F.M. ; Gomes , A.K. ; Jones Jr , J. Can. J. Chem . 1999 , 77 , 624 – 627 ; (b) Alam , M.M. ; Varala , R. ; Adapa , S.R. Tetrahedron Lett . 2003 , 44 , 5115 – 5119 ; (c) Wabnitz , T.C. ; Yu , J-Q. ; Spencer , J.B. Synlett. 2003 , 1070 – 1072 ; (d) Cheng , S. ; Comer , D.D. Tetrahedron Lett. 2002 , 43 , 1179 – 1181 ; (e) Zahouily , M. ; Abrouki , Y. ; Rayadh , A. ; Sebti , S. ; Dhimane , H. ; David , M. Tetrahedron Lett. 2003 , 44 , 2463 – 2465 ; (f) Bandini , M. ; Cozzi , P.G. ; Giacomini , M. ; Melchiorre , P. ; Selva , S. ; Ronchi , A.U. J. Org. Chem . 2002 , 67 , 3700 – 3704 ; (g) Zahouily , M. ; Abrouki , Y. ; Rayadh , A. Tetrahedron Lett . 2002 , 43 , 7729 – 7730 ; (h) Abrouki , Y. ; Zahouily , M. ; Rayadh , A. ; Bahlaouan , B. ; Sebti , S. Tetrahedron Lett . 2002 , 43 , 8951 – 8953 and references therein; (i) Kamimura , A. ; Murakami , N. ; Yokota , K. ; Shirai , M. ; Okamoto , H. Tetrahedron Lett . 2002 , 43 , 7521 – 7523 ; (j) Kangasabapathi , S. ; Sudalai , A. ; Benicewicz , B.C. Tetrahedron Lett . 2001 , 42 , 3791 – 3794 ; (k) Emori , E. ; Arai , T. ; Sasai , H. ; Shibasaki , M. J. Am. Chem. Soc . 1998 , 120 , 4043 – 4044 and references therein; (l) Ahuja , P.R. ; Natu , A.A. ; Gogte , V.N. Tetrahedron Lett. 1980 , 21 , 4743 – 4744 ; (m) Khatik , G.L. ; Sharma , G. ; Raj Kumar , G.S. ; Chakraborti , A.K. Tetrahedron 2007 , 63 , 1200 – 1210 .
- (a) Khatik , G.L. ; Kumar , R. ; Chakraborti , A.K. Org. Lett . 2006 , 8 , 2433 – 2436 ; (b) Ranu , B.C. ; Dey , S.S. ; Hajra , A. Tetrahedron 2003 , 59 , 2417 – 2421 ; (c) Yadav , J.S. ; Reddy , B.V.S. ; Baishya , G. J. Org. Chem . 2003 , 68 , 7098 – 7100 ; (d) Ranu , B.C. ; Dey , S.S. Tetrahedron 2004 , 60 , 4183 – 4188 ; (e) Zhang , H. ; Zhang , Y. ; Liu , L. ; Xu , H. ; Wang , Y. Synthesis 2005 , 2129 – 2136 ; (f) Bartoli , G. ; Bartolacci , M. ; Giuliani , A. ; Marcantoni , E. ; Massaccesi , M. ; Torregiani , E. J. Org. Chem . 2005 , 70 , 169 – 174 ; (g) Rajesh Gulhane , S. ; Chakraborti , A.K. J. Mol. Catal. A: Chem . 2007 , 263 , 137 – 142 ; (h) Rajesh Gulhane , S. ; Chakraborti , A.K. J. Mol. Catal. A: Chem . 2007 , 263 , 143 – 148 ; (i) Sharma , G. ; Kumar , R. ; Chakraborti , A.K. Tetrahedron Lett . 2008 , 49 , 4272 – 4275 ; (j) Chu , C-M. ; Tu , Z. ; Wu , P. ; Wang , C.C. ; Liu , J-T. ; Kuo , C-W. ; Shin , Y-H. ; Yao , C-F. Tetrahedron 2009 , 65 , 3878 – 3885 .
- (a) Azizi , N. ; Saidi , M.R. Org. Lett . 2005 , 7 , 3649 – 3651 ; (b) Azizi , N. ; Aryanasab , F. ; Torkiyan , L. ; Ziyaei , A. ; Saidi , M.R. J. Org. Chem . 2006 , 71 , 3634 – 3635 ; (c) Azizi , N. ; Torkiyan , L. ; Saidi , M.R. Org. Lett . 2006 , 8 , 2079 – 2082 ; (d) Azizi , N. ; Saidi , M.R. Tetrahedron 2007 , 63 , 888 – 891 ; (e) Azizi , N. ; Arynasab , F. ; Saidi , M.R. Org. & Bio. Chem. 2006 , 4 , 4275 – 4279 .