Abstract
Baker–Venkataraman rearrangement of 2-aryloxyacetophenones and 2-cinnamoyloxyacetophenones to 2-hydroxydibenzoylmethanes and 2-hydroxybenzoylcinnamoylmethanes is the key step in the synthesis of flavones and 2-styrylchromones, a class of naturally occurring compounds and several other benzofuranone derivatives. A very simple and highly efficient eco-friendly procedure for Baker–Venkataraman rearrangement has now been developed which involves the grinding of 2-aryloxyacetophenones/2-cinnamoyloxyacetophenones with pulverized potassium hydroxide in a mortar by a pestle and avoids the use of organic solvents at any stage of the reaction.
Introduction
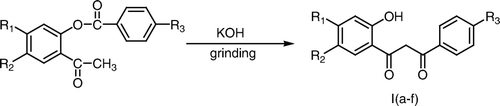
Baker–Venkataraman rearrangement of 2-aryloxyacetophenones to 1-(2-hydroxyphenyl)-3-phenyl propane-1,3-diones, commonly known as 2-hydroxydibenzoylmethanes is considered to be an important reaction as these β-diketones are the required intermediates for the synthesis of various naturally occurring compounds Citation1 such as flavones Citation2, coumaran-3-ones Citation3, isoxazoles Citation4, pyramidines Citation5, and pyrazolines Citation6 which posses a broad spectrum of pharmacological activities Citation7.
The reaction is generally carried out by heating 2-aryloxyacetophenones with pulverized potassium hydroxide in pyridine medium Citation8 or by heating with barium hydroxide in dimethyl sulfoxide medium Citation9. The above transformation has also been carried out in aqueous–benzene biphase medium using phase transfer catalysis Citation10. Other bases which have been used for this rearrangement include sodamide Citation11 and sodium hydride Citation12.
Today much emphasis is being placed on the development of the procedures which avoid the use of hazardous and toxic chemicals. In continuation of our work to develop the green procedures for the organic reactions under solvent-free conditions Citation13, we wish to report herein a simple and efficient procedure for the Baker–Venkataraman rearrangement which involves the grinding of 2-aryloxyacetophenones with pulverized potassium hydroxide under grinding conditions in a mortar and pestle at room temperature in the absence of any solvent (Scheme ). The moisture absorbed by the potassium hydroxide appears to be sufficient for the formation of a homogeneous mixture and the reaction requires a very short duration (15 min.) for completion. The product is also recovered simply by acidification of the reaction mixture in cold water and avoids the need for organic solvent extraction of the compound. An attempt was also made using other bases such as barium hydroxide, calcium hydroxide, and calcium oxide which proved to be futile.
Table 1. Physical data of the compounds synthesized.
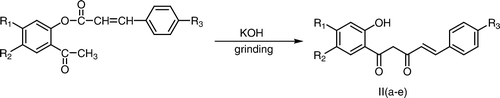
The present method was found to be extended successfully for the preparation of 2-hydroxybenzoylcinnamoylmethanes from 2-cinnamoyloxyacetophenones by grinding the 2-cinnamoyloxyacetophenones with pulverized potassium hydroxide under similar conditions (Scheme ).
Result and discussion
An efficient procedure for the Baker–Venkataraman rearrangement under solvent-free conditions has been described which involves the grinding of 2-aryloxyacetophenones/2-cinnamoyloxyacetophenones with pulverized potassium hydroxide in a mortar by a pestle to give corresponding 2-hydroxydibenzoylmethanes/2-hydroxybenzoylcinnamoylmethanes. The product is separated out by acidification of the reaction mixture in cold water and also avoids the use of organic solvent during the isolation step of the product.
Experimental section
General procedure
All the compounds were characterized from their spectral data (IR and 1H NMR) and compared with authentic samples (CO-TLC and CO-IR). A mortar and pestle of porcelain was used for all the experiments.
Synthesis of 2-hydroxydibenzoylmethanes/2-hydroxybenzoylcinnamoylmethanes
The substituted 2-aryloxyacetophenones/2-cinnamoyloxyacetophenone (2.07 mmol) was ground with pulverized potassium hydroxide (2.07 mmol) by a pestle in a mortar for 3–5 minutes and the reaction mixture was left at room temperature for 10 minutes. The completion of the reaction was checked by thin layer chromatography. The reaction mixture was diluted with ice cold water and acidified with conc. HCl (pH 5.5–6.0). The solid that separated out was filtered, washed with water, and recrystallized from aqueous ethanol to afford 2-hydroxydibenzoylmethanes/2-hydroxybenzoylcinnamoylmethanes.
Conclusion
In conclusion, it can be said that the present method developed for the synthesis of 2-hydroxydibenzoylmethanes and 2-hydroxybenzoylcinnamoylmethanes is simple, highly efficient, and eco-friendly. This is a clean, mild, and superior method which may be used as an alternative to the known literature methods.
Acknowledgements
The authors are thankful to M.D.University, Rohtak for financial assistance to provide research fellowship to Dinesh Sharma.
References
- Roitman , J.N. , Mann , K. and Wolenweber , E. 1992 . Phytochemistry , 31 : 985 – 987 .
- Staunton , J. , Barton , H.D. and Ollis , W.D. 1979 . Comprehensive Organic Chemistry , 659 – 692 . London : Pergamon Press .
- Prakash , O. , Goyal , S. , Pahuja , S. and Singh , S.P. 1990 . Synth. Commun. , 20 : 1409 – 1415 .
- Chincholkar , M.M. and Jamode , V.S. 1979 . Indian J. Chem. , 17B : 510 – 511 .
- Thool , A.N. and Ghiya , B.J. 1988 . J. Indian Chem. Soc. , 65 : 522 – 524 .
- Joshi , M.G. and Wadodkar , K.N. 1982 . Indian J. Chem. , 21B : 689 – 690 .
- (a) Cassady , J.M. ; Baird , W.H. ; Chang , C. J. Nat. Prod. 1990 , 53 , 23 – 41 ; (b) Havsteen , B. Biochem. Pharmac . 1983 , 32 , 1141 – 1148 .
- Mahal , H.S. and Venkataraman , K. 1933 . Curr. Sci. , 2 : 214 – 215 .
- Makrandi , J.K. ; Lamba , M.S. ; Kumar , S. J. Chem. Res . 2006 , 133 – 134 .
- Grover , S.K. ; Makrandi , J.K. ; Saxena , S. Synthesis . 1985 , 697 – 698 .
- Kostanecki , S.V. ; Tambor , J. Chem. Ber . 1900 , 33 , 330 – 334 .
- Hirao , I. ; Yamaguchi , M. ; Hamada , M. Synthesis 1984 , 1076 – 1079 .
- Makrandi , J.K. , Lamba , M.S. and Kumar , S. 2008 . Green Chem. Lett. Rev. , 1 : 123 – 125 .
- Bansal , V. , Singh , P.K. and Khanna , R.N. 1996 . Ind. J. Chem , 35B : 586 – 587 .
- Baker , W. ; Glocking , F. J. Chem. Soc. 1950 , 2759 – 2764 .
- Fitzgerald , D.M. ; Sullivan , J.F. ; Philbin , E.M. ; Wheeler , T.S. J. Chem. Soc . 1955 , 860 – 862 .
- Banerji , A. ; Goomer , N. Synthesis 1980 , 874 – 875 .
- Schmid , H. and Banholzer , K. 1954 . Helv. Chim. Acta. , 37 : 1706 – 1716 .
- Gaggad , H.L. , Wadodkar , K.N. and Ghiya , B.J. 1985 . Indian. J. Chem. , 24B : 1244 – 1247 .
- Goel , S. ; Ritu, Makrandi , J.K. Indian. J. Chem . 2006 , 45B , 1278 – 1281 .