Abstract
An efficient eco-friendly synthesis of flavones and 2-styrylchromones via cyclodehydration of corresponding 1-(2-hydroxyaryl)-3-aryl/styryl-1,3-propanediones is described under solvent-free conditions using grinding technique.
Introduction
Flavones (2-phenylchromones) and 2-styrylchromones constitute an important class of naturally occurring compounds belonging to flavonoid group Citation1–4. These compounds have been reported to exhibit a variety of pharmacological properties Citation5–10 including anticancer Citation1 and anti HIV Citation11.
There are a number of methods available for the synthesis of chromone derivatives including the Allan–Robinson synthesis Citation12 and the oxidative cyclization of 2′-hydroxychalcones/2-hydroxycinnamylideneacetophenones Citation13. However, the cyclodehydration of 1-(2-hydroxyaryl)-3-aryl/styryl-1,3-propanediones obtained by the Baker–Venkataraman rearrangement of 2-aryloxy/cinammoyloxy acetophenones remains the most practical method for their preparation Citation14. The cyclodehydration of the ensuing 1,3-propanediones requires heating under strongly acidic conditions using acetic acid Citation15, hydrochloric acid Citation16, sulfuric acid Citation17, and p-toulenesulphonic acid Citation18. Recently, the cyclodehydration of these 1,3-propanediones has been reported with CuCl2 in ethanol Citation19 under microwave irradiation.
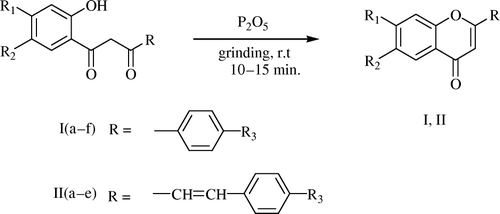
Today much emphasis is on the development of synthetic procedures which avoid toxic and hazardous chemicals and solvents Citation20. In continuation of our work to develop simple and eco-friendly procedures for the synthesis of organic compounds, we wish to report a high yield synthesis of flavones and 2-styrylchromones which involves the grinding of the intermediate 1,3-propanediones with phosphorous pentoxide in a mortar and pestle at room temperature in the absence of any solvent (). The moisture absorbed by the reaction mixture during the grinding seems to be sufficient for the formation of a homogeneous mixture and the product is isolated by diluting the reaction mixture with ice cold water.
The possibility of the cyclodehydration of 1,3-propanediones occurring by phosphoric acid which could have formed during the grinding by absorption of water by phosphorous pentoxide, was investigated by grinding the 1,3-propanediones with phosphoric acid alone. No reaction was found to have taken place even after grinding for 30 minutes.
The validity of the procedure was checked by preparing differently substituted flavones and 2-styrylchromones. The identity of the compounds () was confirmed from their IR, H1-NMR spectra and melting point comparison with literature value.
Table 1. Physical data of the compounds synthesized.
Experimental
All the chemicals were purchased from Aldrich and Fluka. Melting points were determined in open capillary tubes. IR (KBr) spectra were recorded in a Perkin–Elmer spectrum BX series FT-IR spectrophotometer and 1H NMR on Bruker Avance II 400 MHz instrument using tetramethyl-silane as an internal standard.
General procedure
Synthesis of flavones/2-styrylchromones
The substituted 1-(2-hydroxyphenyl)-3-phenyl/styryl-1,3-propanedione (2.1 mmol) was ground with phosphorous pentoxide (2.1 mmol) by a pestle in a mortar for 10–15 minutes. The completion of the reaction was checked by thin layer chromatography (benzene). The reaction mixture was diluted with ice-cold water and the solid that separated out was filtered at vacuum, washed with water, and recrystallized from methanol to afford flavones/2-styrylchromones.
4′-Methoxyflavone (, Compound Ic)
IR (KBr) 1646 (C = O); 1H NMR (CDCl3) δ 3.90 (s, 3H, OCH3); 6.75 (s, 1H, H-3); 7.0 (d, J=9.0 Hz, 2H, H-3′, H-5′); 7.25–7.55 (m, 3H, H-6, H-7, H-8); 7.80 (d, J=9.0 Hz, 2H, H-2′, H-6′); 8.20 (d, J=9.0Hz, 1H, H-5).
4′-Methoxy-6-methylflavone (, Compound Id)
IR (KBr) 1640 (C = O); 1H NMR (CDCl3) δ 2.30 (s, 3H, CH3); 3.75 (s, 3H, OCH3); 6.60 (s, 1H, H-3); 6.70 (d, J=9.0 Hz, 2H, H-3′, H-5′); 7.30 (s, 2H, H-7, H-8); 7.75 (d, J=9.0 Hz, 2H, H-2′, H-6′); 7.90 (s, 1H, H-5).
6-Methyl-2-styrylchromone (, Compound IIb)
IR (KBr) 1629 (C = O); 1H NMR (CDCl3) δ 2.45 (s, 3H, CH3); 6.30 (s, 1H, H-3); 6.78 (d, J=16.0 Hz, 1H, H-α); 6.82–7.42 (m, 8H, C6H5, H-β, H-7, H-8); 7.90 (s, 1H, H-5).
6-Methyl-2-(4-methoxystyryl)chromone (, Compound IId)
IR (KBr) 1640 (C = O); 1H NMR (CDCl3) δ 2.40 (s, 3H, CH3); 3.80 (s, 3H, OCH3); 6.20 (s, 1H, H-3); 6.50 (d, J=16.0 Hz, 1H, H-α); 6.70–7.80 (m, 8H, C6H5, H-β, H-7, H-8); 7.90 (s, 1H, H-5).
Conclusion
In conclusion, it can be said that the present method developed for the synthesis of flavones and 2-styrylchromones is simple, highly efficient and eco-friendly, and avoids the use of organic solvent during the reaction and working up of the reaction mixture.
Acknowledgements
The authors are grateful to M.D. University, Rohtak for financial assistance to provide research fellowship to Dinesh Sharma.
References
- Martens S. Mithofer A. Phytochemistry 2005 66 2399 2407
- Malikov , V.M. ; Yuldashev , M.P . Chem. Nat. Compd . 2002 , 38 , 358 – 406 .
- Huck , C.W. ; Huber , C.G. ; Ongania , K.H. ; Bonn , G.K. Chromatogr. A 2000 , 870 , 453 – 462 .
- Nagao , T. ; Abe , F. ; Kinjo , J. ; Okabe , H. Biol. Pharm. Bull . 2002 , 25 , 875 – 879 .
- Welton , A.F. ; Tobias , L.D. ; Fiedler-Nagy , C. ; Anderson , W. ; Hope , K. ; Meyers , K. ; Coffey , J.W. In Plant Flavonoids in Biology and Medicine: biochemical, pharmacological, and structure-activity relationships , Proceedings of a symposium held in Buffalo, New York, July 22–26, 1985; Cody , V. , Middleton , E. , Harborne J.B. , A.R. Liss : New York 1986 ; 231 – 242 .
- Xu , H.X. ; Lee , S.F. Phytother. Res. 2001 , 15 , 39 – 43 .
- Goker , H. ; Boykin , D. ; Yildiz , S. Bioorg. Med. Chem . 2005 , 13 , 1707 – 1714 .
- Kim , H.P. ; Son , K.H. ; Chang , H.W. ; Kang , S.S. J. Pharmacol. Sci. 2004 , 96 , 229 – 245 .
- Chu , H. ; Wu , H. ; Lee , Y. Tetrahedron 2004 , 60 , 2647 – 2655 .
- Silva , A.M.S. , Pinto , D.C.G.A. , Cavaleiro , J.A.S. , Levai , A. and Patonay , T. 2004 . ARKIVOC 2004 106 – 123 .
- Wu , J. ; Wang , X. ; Yi , Y. ; Lee , K. Bioorg. Med. Chem. Lett . 2003 , 13 , 1813 – 1815 .
- Allan , J. ; Robinson , R.J. J. Chem. Soc. 1924 , 125 , 2192 – 2195 .
- Linuma , M. ; Iwashima , K. ; Matsuura , S. Chem. Pharm. Bull. 1984 , 32 , 4935 – 4941 .
- Varma , R.S. ; Saini , R.K. ; Kumar , D. J. Chem. Res.(S) 1998 , 1 , 348 – 349 .
- Kumar , P.E. ; Prashad , K.J.R. Ind. J. Chem . 1999 , 38B , 1277 – 1279 .
- Jung , J.C. ; Min , J.P. ; Park , O.S. Synth. Commun. 2001 , 31 , 1837 – 1845 .
- Ares , J.J. ; Outt , P.E. ; Kakodkar , S.V. ; Buss , R.C. ; Geiger , J.C. J. Org. Chem . 1993 , 58 , 7903 – 7905 .
- Jain , P.K. ; Makrandi , J.K. ; Grover , S.K. Curr. Sci. 1981 , 50 , 857 – 858 .
- Kabalka , G.W. ; Mereddy , A.R. Tetrahedron Lett . 2005 , 46 , 6315 – 6317 .
- Makrandi , J.K. ; Lamba , M.S. ; Kumar , S. Green Chem. Lett. Rev. 2008 , 1 , 123 – 125 .
- Dann , O. ; Mylius , G. Libigs Ann. Chem . 1954 , 587 , 1 – 15 .
- Swapnil , S.R. , Pathan , M.Y. , Paike , V.V. , Pachmase , P.R. , Jadhav , W.N. and Pawar , R.P. 2006 . ARKIVOC 2006 43 – 48 .
- Fitzgerald , D.M. ; Sullivan , J.F. ; Philbin , E.M. ; Wheeler , T.S. J. Chem. Soc. 1955 , 1 , 860 – 862 .
- Banerji , A. and Goomer , N. 1980 . Synthesis 1980 874 – 875 .
- Lee , J.I. ; Son , H.S. ; Park , H. Bull. Korean Chem. Soc . 2004 , 25 , 1945 – 1947 .
- Cheema , U.S. ; Gulati , K.C. ; Venkataraman , K. J. Chem. Soc . 1932 , 1 , 925 – 933 .
- Gaggad , H.L. ; Wadodkar , K.N. ; Ghiya , B.J. Indian J. Chem . 1984 , 24B , 1244 – 1247 .
- Gulati , K.C. ; Seth , S.R. ; Venkataraman , K. J. Chem. Soc . 1934 , 1 , 1765 – 1767 .