Abstract
2-Phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones, 12–22, were synthesized with good yields in a short reaction time by the “one-pot” multicomponent reaction of the appropriate 2-amino-4,6-diarylpyrimidines, benzaldehyde, and thioglycolic acid under microwave irradiation in the presence of activated fly ash catalyst. The characterization of these compounds was confirmed by melting point, elemental analysis, MS, FT-IR, and one-dimensional NMR (1H and 13C) spectroscopic data.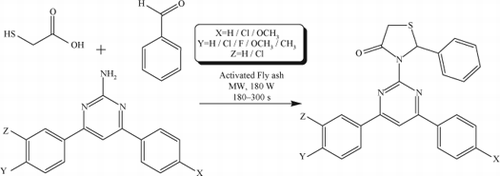
Introduction
Nowadays the pharmaceutical industries are in need of new innovative alternate synthetic routes for synthesizing therapeutic and pharmacologically important compounds. Microwave activation as a non-conventional energy source has become a very popular and useful technology in organic chemistry. Microwave-assisted organic synthesis (MAOS) serves the need for accelerated chemical synthesis remarkably well, reducing times for the optimization and performance of reactions from hours or days to minutes. The environmental impact of organic chemical syntheses can be significantly reduced by incorporating cleaner unit processes. Solvent-free synthesis of organic compounds involving easily separable solid catalysts has attracted notable interest and offers a clean, economical and environmentally safe protocol. During the initial stage, only pozzolanic activity of fly ash is paid attention Citation1 Citation2. Many researchers devoted themselves to the research of the potential activity of fly ash and the hydration process of fly ash cement Citation3. Recently, microwave-assisted synthesis of “one-pot” conversion of ketones into amides, Knoevenagel condensation, Schiff bases formation, Biginelli and Hantzsch reactions were carried out using activated fly ash as catalyst Citation4. In addition, activated fly ash is used for the “one-pot” synthesis of 1,2,4,5-tetrazines Citation5 and 1,2,3-selenadiazoles Citation6.
Various 4-thiazolidinones have attracted considerable attention as they are endowed with wide range of pharmacological activities. Peptidoglycan is an essential component of the cell wall of both gram-positive and gram-negative bacteria. 4-thiazolidinones have been reported as novel inhibitors of the bacterial enzyme Mur B which is a precursor, acting during the biosynthesis of peptidoglycan Citation7. A wide variety of biological properties such as hypolipidaemic Citation8, antidegenerative Citation9, muscarinic receptor 1 agonist Citation10, antiproteolytic Citation11, anti-inflammatory Citation12, antiviral Citation13, antifungal Citation14, antibacterial Citation15, antitubercular Citation16, anticonvulsant Citation17, respiratory Citation18, and hypnotic Citation19 activities have been reported for 4-thiazolidinones.
Aminopyrimidine nuclei are common in marketed drugs such as anti-atherosclerotic aronixil, anti-histaminic thonzylamine, anti-anxielytic buspirone, anti-psoriatic enazadrem, and other medicinally relevant compounds. Pyrimidines are the basic nucleus in nucleic acids and have been associated with a number of biological activities. Some notable biological activity of pyrimidine derivatives include adenosine receptor antagonists Citation20, kinase inhibitors Citation21, analgesic and anti-inflammatory Citation22, inhibitors of cyclin-dependent kinases 1 and 2 Citation23, calcium channel antagonist Citation24, antihistaminic Citation25, and antitubercular Citation26 activities.
In continuation of our interest in synthesizing pharmacologically important compounds in “dry media” Citation27 Citation28, we planned and succeeded to synthesize a system, which comprises both 4-thiazolidinones and 2-amino-4,6-diarylpyrimidine components together to give a compact heterocyclic structure like the title 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones in “one-pot” catalyzed by activated fly ash under microwave irradiation.
Results and discussion
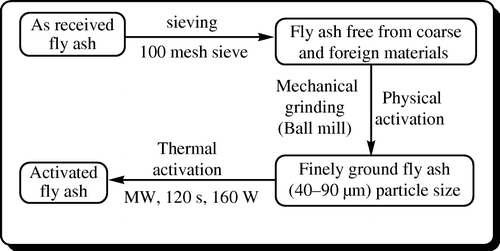
The fly ash collected from Neyveli Lignite Corporation, Neyveli, Tamil Nadu, India, was utilized for catalyzing the reactions. The physical properties, such as specific gravity and specific surface area, of fly ash used were 1.9 and 127 m2/g, respectively. The chemical compositions (%) of fly ash Citation3 used were SiO2, Fe2O3, Al2O3, CaO, MgO, loss of ignition, and insoluble residue in the ratio 64.03, 6.50, 15.50, 4.62, 3.00, 4.35, and 2.00, respectively. The purpose of the present investigation is to activate the as-received fly ash by physical method followed by thermal method ( ) and to study the influence of activated fly ash to catalyze the one-pot cyclization reaction for the formation of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones.
The classical method available for the synthesis of thiazolidin-4-ones was the conversion of appropriate Schiff bases of respective amines and aldehyde by thioglycolic acid in refluxing benzene/dioxane catalyzed by p-toluene sulfonic acid/ZnCl2 using a Dean-Stark apparatus for 12 h. Various problems were associated with the above synthesis such as severe reaction conditions using hazardous benzene as solvent, poor yields, difficulty in product isolation, and longer reaction times. In the present “one-pot” procedure, novel 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones 12–22 are synthesized by the addition of benzaldehyde and thioglycolic acid to 2-amino-4,6-diarylpyrimidines under microwave irradiation in the presence of catalytic amount of activated fly ash (50 mg) in high yields when compared with general conditions under microwave irradiation in solvent-free conditions. Initially, conversion of 2-amino-4,6-diarylpyrimidines 1–11 to 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones 12–22 was effected in the absence of activated fly ash. No yields were achieved. Instead, if activated fly ash was used as a dehydrating agent, the yield of the product has been improved significantly (i.e., about 95%) under microwave irradiation. The schematic representation and the analytical data of compounds 12–22 are given in and , respectively. The structure of the newly synthesized compounds 12–22 is confirmed by melting point, elemental analysis, mass spectroscopy (MS), Fourier transform infrared (FT-IR), and one-dimensional nuclear magnetic resonance (NMR) (1H and 13C) spectroscopic data.
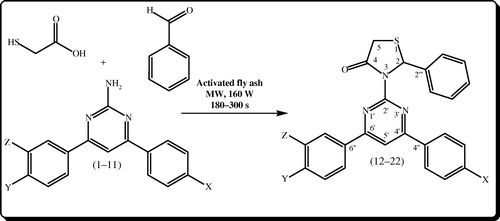
Table 1. Physical and analytical data of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones 12–22.
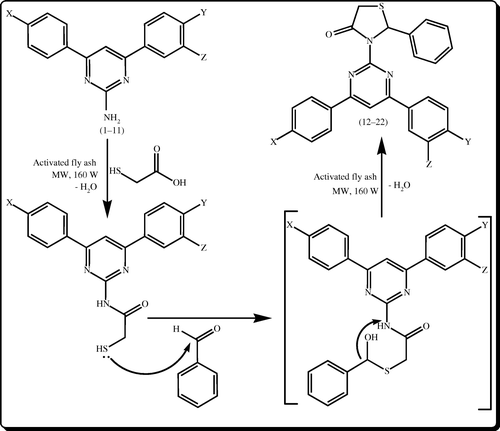
The conversion of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones 12–22 from 2-amino-4,6-diarylpyrimidines 1–11 by the present procedure was believed to be followed via 2-mercapto-N-(4,6-diarylpyrimidin-2-yl) acetamides and rapidly rearranged to give in the second step. The attempt to isolate the respective 2-mercapto-N-(4,6-diarylpyrimidin-2-yl) acetamides from the reaction mixture was unsuccessful. A plausible reaction mechanism () has been proposed for the conversion of 2-amino-4,6-diarylpyrimidines to the 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones catalyzed by activated fly ash under microwave irradiation.
Experimental
Performing TLC assessed the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. Infrared (IR) spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–330 FT-IR spectrophotometer and noteworthy absorption values (cm−1) alone are listed. 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively on Bruker AMX 400 NMR spectrometer using DMSO-d as solvent. The ESI +ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer. BIOTAGE Initiator microwave synthesizer, a Swedish scientific microwave oven, was used for the irradiation. By adopting the literature precedent 2-amino-4,6-diarylpyrimidines 1–11 Citation29, was synthesized.
Experimental method for the synthesis of 2-phenyl-3-(4,6-diphenylpyrimidin-2-yl) thiazolidin-4-one 12
A mixture containing 0.01 mole of 2-amino-4,6-diphenylpyrimidine 1, 0.01 mole of thioglycolic acid, 0.01 mole of benzaldehyde, and activated fly ash (50 mg) was added in an alumina bath and mixed properly with the aid of a glass rod (10 s) and then irradiated in a microwave oven for 180 s at 160 W (monitored by thin layer chromatography, TLC). After completion of the reaction, the reaction mixture was extracted with ethyl acetate (3×5 mL). The catalyst and other solid wastes were removed by filtration. The combined organic layer was washed with 10% sodium bicarbonate solution followed by water three times and then dried over anhydrous MgSO4. The organic layer was concentrated in vacuo to furnish the products, which were purified by column chromatography using silica gel (100–200 mesh), with ethyl acetate – petroleum ether (bp 40–60) in the ratio (2:8) as eluent. IR (KBr) (cm−1): 3125, 3033, 2927, 2851, 1716, 1627, 1576, 1350, 710, 698, 649; 1H NMR (δ ppm): 3.21 (d, 1H, CH2a at H5a, J=15.37 Hz), 3.38 (d, 1H, CH2e at H5e, J=15.37 Hz), 5.25 (s, 1H, CH at H2), 7.19–8.37 (m, 16H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.0 C-5, 62.5 C-2, 108.1 C-5′, 131.4 C-2′′′, 125.9–128.8 –Carom, 139.1 C-4′′, 139.1 C-6′′, 161.3 C-4′, 161.3 C-6′, 163.8 C-2′, 170.6 C-4.
The compounds 13–22 were synthesized correspondingly
3-(4′-(4′′-chlorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one
13 IR (KBr) (cm−1): 3120, 3033, 2927, 2851, 1696, 1627, 1575, 1310, 894, 710, 650, 647; 1H NMR (δ ppm): 3.22 (d, 1H, CH2a at H5a, J=15.37 Hz), 3.39 (d, 1H, CH2e at H5e, J=15.34 Hz), 5.27 (s, 1H, CH at H2), 7.31–8.44 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 33.9 C-5, 62.6 C-2, 108.8 C-5′, 127.5–133.1 –Carom, 131.4 C-2′′′, 135.9 ipso C, 139.1 C-4′′, 139.7 C-6′′, 164.9 C-4′, 165.0 C-6′, 162.9 C-2′, 170.6 C-4.
3-(4′-(3′′-chlorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one
14 IR (KBr) (cm−1): 3115, 3033, 2927, 2850, 1714, 1627, 1575, 1344, 894, 767, 690, 648; 1H NMR (δ ppm): 3.22 (d, 1H, CH2a at H5a, J=15.36 Hz), 3.39 (d, 1H, CH2e at H5e, J=15.37 Hz), 5.27 (s, 1H, CH at H2), 7.21–8.24 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 33.9 C-5, 62.4 C-2, 108.9 C-5′, 124.6–129.0 –Carom, 130.6 ipso C, 131.5 C-2′′′, 139.0 C-4′′, 141.8 C-6′′, 164.9 C-4′, 165.5 C-6′, 162.7 C-2′, 170.6 C-4.
3-(4′-(4′′-methoxyphenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one
15 IR (KBr) (cm−1): 3065, 3038, 2927, 2851, 1714, 1627, 1577, 1351, 700, 650, 649; 1H NMR (δ ppm): 3.23 (d, 1H, CH2a at H5a, J=15.35 Hz), 3.39 (d, 1H, CH2e at H5e, J=15.27 Hz), 3.84 (s, 3H, OCH3), 5.28 (s, 1H, CH at H2), 7.21–8.21 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 54.9 OCH3 on aryl ring, 62.5 C-2, 108.7 C-5′, 126.0–128.6 –Carom, 129.1 C-2′′′, 139.1 C-4′′, 141.5 C-6′′, 164.0 C-4′, 165.0 C-6′, 162.3 C-2′, 170.6 C-4.
3-(4′-(4′′-methylphenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one
16 IR (KBr) (cm−1): 3060, 3033, 2926, 2852, 1715, 1627, 1579, 1350, 714, 700, 643.; 1H NMR (δ ppm): 2.32 (s, 3H, CH3), 3.23 (d, 1H, CH2a at H5a, J=15.34 Hz), 3.40 (d, 1H, CH2e at H5e, J=15.36 Hz), 5.27 (s, 1H, CH at H2), 7.20–8.24 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 24.5 CH3 on aryl ring, 34.1 C-5, 62.6 C-2, 108.4 C-5′, 126.0–131.4 –Carom, 133.1 C-2′′′, 135.9 ipso C, 138.7 C-4′′, 139.1 C-6′′, 164.9 C-4′, 165.5 C-6′, 162.6 C-2′, 170.8 C-4.
3-(4′-(4′′-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one
17 IR (KBr) (cm−1): 3071, 3027, 2928, 2852, 1712, 1626, 1575, 1352, 836, 769, 698; 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J=15.24 Hz), 3.37 (d, 1H, CH2e at H5e, J=15.28 Hz), 5.26 (s, 1H, CH at H2), 6.64–8.19 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 62.9 C-2, 108.9 C-5′, 127.3–143.1 –Carom, 143.6 C-2′′′, 145.1 C-4′′, 146.1 C-6′′, 166.8 C-4′, 167.0 C-6′, 163.9 C-2′, 171.4 C-4.
3-4′-phenyl-(6′-(4′′-chlorophenyl) pyrimidin-2′-yl)-2-phenylthiazolidin-4-one
18 IR (KBr) (cm−1): 3071, 3027, 2926, 2852, 1721, 1627, 1576, 1398, 782, 730, 693, 582; 1H NMR (δ ppm): 3.21 (d, 1H, CH2a at H5a, J=15.33 Hz), 3.38 (d, 1H, CH2e at H5e, J=15.32 Hz), 5.25 (s, 1H, CH at H2), 7.15–7.93 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.1 C-5, 62.9 C-2, 108.9 C-5′, 126.5–128.6 –Carom, 129.2 C-2′′′, 139.1 C-4′′, 141.9 C-6′′, 164.4 C-4′, 165.3 C-6′, 162.5 C-2′, 170.8 C-4.
3-4′-phenyl-(6′-(4′′-methoxyphenyl) pyrimidin-2′-yl)-2-phenylthiazolidin-4-one
19 IR (KBr) (cm−1): 3065, 3033, 2928, 2851, 1715, 1627, 1590, 1370, 835, 770, 699, 656; 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J=15.08 Hz), 3.37 (d, 1H, CH2e at H5e, J=15.34 Hz), 3.86 (S, 3H, OCH3), 5.26 (s, 1H, CH at H2), 6.97–8.20 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.1 C-5, 55.2 OCH3 on aryl ring, 62.5 C-2, 108.6 C-5′, 114.1–129.1 –Carom, 129.5 C-2′′′, 130.2 ipso C, 139.2 C-6′′, 146.1 C-4′′, 163.8 C-4′, 164.4 C-6′, 161.2 C-2′, 170.7 C-4.
3-(4′-(4′′-chlorophenyl)-6′-(p-tolylpyrimidin-2′-yl))-2-phenylthiazolidin-4-one
20 IR (KBr) (cm−1): 3060, 3027, 2927, 2851, 1721, 1626, 1576, 1398, 781, 728, 694, 650; 1H NMR (δ ppm): 2.40 (s, 3H, CH3), 3.20 (d, 1H, CH2a at H5a, J=15.04 Hz), 3.37 (d, 1H, CH2e at H5e, J=15.11 Hz), 5.26 (s, 1H, CH at H2), 7.18–8.33 (m, 14H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 25.2 CH3 on aryl ring, 34.5 C-5, 62.9 C-2, 108.9 C-5′, 131.4 C-2′′′, 126.1–130.4 –Carom, 133.1 ipso C, 138.7 C-6′′, 139.1 C-4′′, 164.8 C-4′, 165.0 C-6′, 161.2 C-2′, 170.8 C-4.
3-(4′,6′-bis(p-chlorophenyl) pyrimidin-2′-yl)-2-phenylthiazolidin-4-one
21 IR (KBr) (cm−1): 3060, 3027, 2927, 2852, 1727, 1627, 1575, 1400, 897, 787, 730, 693; 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J=15.21 Hz), 3.36 (d, 1H, CH2e at H5e, J=15.23 Hz), 5.26 (s, 1H, CH at H2), 7.28–8.20 (m, 14H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 62.5 C-2, 108.7 C-5′, 129.1 C-2′′′, 126.0–128.6 –Carom, 139.1 C-6′′, 141.8 C-4′′, 164.1 C-4′, 161.3 C-6′, 165.3 C-2′, 170.9 C-4.
3-(4′-(p-chlorophenyl)-6′-(p-fluorophenyl) pyrimidin-2′-yl)-2-phenylthiazolidin-4-one
22 IR (KBr) (cm−1): 3065, 3027, 2926, 2853, 1719, 1627, 1576, 1394, 897, 833, 776, 728, 695; 1H NMR (δ ppm): 3.18 (d, 1H, CH2a at H5a, J=14.92 Hz), 3.35 (d, 1H, CH2e at H5e, J=14.92 Hz), 5.26 (s, 1H, CH at H2), 7.18–8.19 (m, 14H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.1 C-5, 62.6 C-2, 110.1 C-5′, 133.2 C-2′′′, 127.3–143.1 –Carom, 145.1 C-6′′, 146.8 C-4′′, 166.8 C-4′, 167.0 C-6′, 163.4 C-2′, 171.8 C-4.
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. One of the authors namely V. Kanagarajan is grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, Republic of India for providing financial support in the form of CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. J. Thanusu wishes to thank Annamalai University authorities for providing financial support in the form of Research Fellowship.
References
- Watt , J.D. ; Throne , D.J. J. Appl. Chem. 1965 , 15 , 595 – 604 .
- Throne , D.J. ; Watt , J.D. J. Appl. Chem. 1966 , 16 , 33 – 39 .
- Saraswathy , V. ; Muralidharan , S. ; Thangavel , K. ; Srinivasan , S. Cement Concr. Res. 2003 , 25 , 673 – 680 .
- Gopalakrishnan , M. ; Sureshkumar , P. ; Kanagarajan , V. ; Thanusu , J. ; Govindaraju , R . Arkivoc 2006 , 13 , 130 – 141 .
- Gopalakrishnan , M. ; Thanusu , J. ; Kanagarajan , V. J. Korean Chem. Soc . 2007 , 51 , 520 – 525 .
- Gopalakrishnan , M. ; Thanusu , J. ; Kanagarajan , V. J. Korean Chem. Soc. 2007 , 52 , 47 – 51 .
- Andres , C.J. ; Bronson , J.J. ; D'Andrea , S.V. ; Deshpande , M.S. ; Falk , P.J. ; Grant-Young , K.A. ; Harte , W.E. ; Ho , H-T. ; Misco , P.F. ; Robertson , J.G. ; Stock , D. ; Sun , Y. ; Walsh , A.W. Bioorg. Med. Chem. Lett. 2000 , 10 , 715 – 717 .
- Nampurath , G.K. ; Mathew , S.P. ; Khanna , V. ; Zachariah , R.T. ; Kanji , S. ; Chamallamudi , M.R. Chem. Biol. Interact . 2008 , 171 , 363 – 368 .
- Ottanà , R. ; Maccari , R. ; Ciurleo , R. ; Gabriella Vigorita , M. ; Maria Panico , A. ; Cardile , V. ; Garufi , F. ; Ronsisvalle , S. Bioorg. Med. Chem . 2007 , 15 , 7618 – 7625 .
- Narendra Sharath Chandra , J.N. ; Malviya , M. ; Sadashiva , C.T. ; Subhash , M.N. ; Rangappa , K.S. Neurochem. Int. 2008 , 52 , 376 – 383 .
- Kishore , V. ; Narain , N.K. ; Kumar , S. ; Parmar , S.S. Pharmacol. Res. Commun. 1976 , 8 , 43 – 51 .
- Newbould , B.B. Br. J. Pharmacol. Chemother. 1965 , 24 , 632 – 640 .
- Schauer , P. ; Likar , M. ; Tisler , M. ; Krbavcic , A. ; Pollak , A. Path. Microbiol. 1965 , 28 , 382 – 387 .
- Chaubey , V.N. ; Singh , H. Bull. Chem. Soc. Jpn. 1970 , 43 , 2233 – 2236 .
- Akerblom , E.B. J. Med. Chem. 1974 , 17 , 609 – 615 .
- Litvinchuk , M.D. Farmakol Toksikol. 1963 , 26 , 725 – 728 .
- Dimri , A.K. ; Parmar , S.S. J. Heterocycl. Chem. 1978 , 15 , 335 – 336 .
- Parmar , S.S. ; Dwivedi , C. ; Chaudhari , A. ; Gupta , T.K. J. Med. Chem. 1972 , 15 , 99 – 101 .
- Singh , S.P. ; Parmar , S.S. ; Raman , K. ; Stenberg , V. Chem. Rev. 1981 , 81 , 175 – 203 .
- Van Veldhoven , J.P.D. ; Chang , L.C.W. ; Künzel , J. ; Mulder-Krieger , T. ; Struensee-Link , R. ; Beukers , M.W. ; Brussee , J. ; Ijzerman , A.P. Bioorg. Med. Chem. 2008 , 16 , 2741 – 2752 .
- Hughes , T.V. ; Emanuel , S.L. ; Beck , A.K. ; Wetter , S.K. ; Connolly , P.J. ; Karnachi , P. ; Reuman , M. ; Seraj , J. ; Fuentes-Pesquera , A.R. ; Gruninger , R.H. ; Middleton , S.A. ; Lin , R. ; Davis , J.M. ; Moffat , D.F.C. Bioorg. Med. Chem. Lett. 2007 , 17 , 3266 – 3270 .
- Chhabria , M.T. ; Bhatt , H.G. ; Raval , H.G. ; Oza , P.M. Bioorg. Med. Chem. Lett. 2007 , 17 , 1022 – 1024 .
- Sayle , K.L. ; Bentley , J.F. ; Boyle , T. ; Calvert , A.H. ; Cheng , Y. ; Curtin , N.J. ; Endicott , J.A. ; Golding , B.T. ; Hardcastle , I.R. ; Jewsbury , P. ; Mesguiche , V. ; Newell , D.R. ; Noble , M.E.M. ; Parsons , R.J. ; Pratt , D.J. ; Wang , L.Z. ; Griffin , R.J. Bioorg. Med. Chem. Lett. 2003 , 13 , 3079 – 3082 .
- Pastor , A. ; Alajarin , R. ; Vaquero , J.J. ; Alvarez-Builla , J. ; de Casa-Juana , M.F. ; Sunkel , C. ; Priego , J.G. ; Fonseca , I. ; Sanz-Aparicio , J. Tetrahedron 1994 , 50 , 8085 – 8098 .
- Youssouf , M.S. ; Kaiser , P. ; Singh , G.D. ; Singh , S. ; Bani , S. ; Gupta , V.K. ; Satti , N.K. ; Suri , K.A. ; Johri , R.K. Int. Immunopharmacol. 2008 , 8 , 1049 – 1055 .
- Gasse , C. ; Douguet , D. ; Huteau , V. ; Marchal , G. ; Munier-Lehmann , H. ; Pochet , S. Bioorg. Med. Chem. 2008 , 16 , 6075 – 6085 .
- Gopalakrishnan , M. ; Sureshkumar , P. ; Kanagarajan , V. ; Thanusu , J. J. Sulfur Chem. 2007 , 28 , 383 – 392 .
- Gopalakrishnan , M. ; Thanusu , J. ; Kanagarajan , V. J. Sulfur Chem. 2008 , 29 , 179 – 185 .
- Kothari , S. ; Singhal , M. ; Vijayvergia , D. ; Vyas , R. ; Verma , B.L. J. Indian. Chem. Soc. 2000 , 77 , 329 – 331 .