Abstract
The corrosion inhibition and adsorption properties of Neem (Azadirachta indica – AZI) mature leaves extract as a green inhibitor of mild steel (MS) corrosion in nitric acid (HNO3) solutions have been studied using a gravimetric technique for experiments conducted at 30 and 60°C. The results disclose that the different concentrations of the AZI extract inhibit MS corrosion and that inhibition efficiency of the extract varies with concentration and temperature. For extract concentrations studied and ranging from 9.09 to 28.57 mg/L, the maximum inhibition efficiency was 80.5 and 80.07% both at 28.57 mg/L AZI at 30 and 60°C, respectively, in 2.0 N HNO3. The adsorption of the inhibitor on the MS surface was exothermic and consistent with the physical adsorption mechanism, best described by the Frumkin adsorption isotherm.
Introduction
Corrosion is the destruction of material resulting from an exposure and interaction with the environment. It is a major problem that must be confronted for safety, environmental, and economic reasons Citation1 in various chemical, mechanical, metallurgical, biochemical, and medical engineering applications, and more specifically in the design of a much more varied number of mechanical parts which equally vary in size, functionality, and useful lifespan. Several efforts have been made using corrosion preventive practices and the use of green corrosion inhibitors is one of them Citation2. To mention but a few, a general review of the chemistry and corrosion control properties of electroactive polymers is known as conductive polymers (CPs) used for corrosion protection in various environments and their potential benefits over common organic barrier coatings has been performed by Zarras et al. Citation3. Sol-gel derived hybrid coatings with the example of hybrid organo-ceramic corrosion protection coatings with encapsulated organic corrosion inhibitors have been analyzed by Khramov et al. Citation4. Zuo et al. Citation5 analyzed the influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions while the reactivity of polyester aliphatic amine surfactants as corrosion inhibitors for carbon steel in formation water (deep well water) have been studied by Alsabagh et al. Citation6. In line with the emergent concept of “Green Chemistry” and the related couple of principles of “Less hazardous synthesis” stating that wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment, and “Safer chemicals” whereby chemical products should be designed to preserve efficacy of function while reducing toxicity, the use of green inhibitors for the control of corrosion of metals Citation7 and alloys which are in contact with an aggressive environment is an accepted and growing practice Citation8 Citation9. Indeed, a large number of organic compounds are presently under study to investigate and optimize their corrosion inhibition potential. All these studies have revealed that organic compounds especially those with N, S, and O show significant corrosion inhibition efficiency. However, a certain proportion of these compounds is not only expensive but also shows some toxicity to living beings Citation10. It is needless to point out the importance of cheap and safe inhibitors of corrosion.
Plant extracts and the derived organic species (which are natural molecules) have therefore become important as an environmentally benign, readily available, renewable, and acceptable source for a wide range of inhibitors Citation11–16. They are the rich sources of ingredients which have very high inhibition efficiency Citation10 and are hence termed “Green Inhibitors” Citation14. Green corrosion inhibitors Citation17 are biodegradable and do not contain heavy metals or other toxic compounds. The successful use of naturally occurring substances to inhibit the corrosion of metals in both acidic and alkaline environments has been reported by some research groups Citation18–24 to mention but a few. Research efforts to find naturally organic substances or biodegradable organic materials to be used as effective corrosion inhibitors of a wide number of metals have been one of the key areas in our research group Citation25. In point of fact, the present article adds itself to the series of our four original research papers (one in press, one accepted for publication, and two currently under review) which relate to the exploration of four plants extracts (Azadirachta indica – AZI, Ocimum tenuiflorum, Aloe barbadensis, and Eucalyptus regnans) and their corrosion inhibition potential for zinc in acidic media.
Azadirachta indica (AZI) – the Neem plant
Our focus in this paper is on Neem (AZI), which is more specifically the extract from green mature Neem leaves from an over 100 years old Neem tree. Several studies have been carried out and have remained focused on the Neem plant parts for their various pharmacological activities (antiinflammatory, antipyretic, analgesic, immunostimulant, antifertility, anticarcinogenic, antimalarial, and hepatoprotective) Citation26–28 and medicinal properties Citation28 to mention but a few. The Neem extract has been only very occasionally involved in environmental engineering and environmental chemistry research with the analysis of the adsorption of Pb(II) from aqueous solution by AZI leaf powder by Bhattacharyya and Sharma Citation29, the adsorption and corrosion inhibitive properties of AZI in acid solutions Citation30 and the study of copper corrosion inhibition by AZI leaves extract in 0.5 M sulfuric acid by Valek and Martinez Citation31. AZI is well known in India and its neighboring countries for more than 2000 years as one of the most versatile medicinal plants having a wide spectrum of biological activity Citation28. Neem is an evergreen tree, cultivated in various parts of the Indian subcontinent. Neem has been extensively used in ayurveda Citation26, unani, and homoeopathic medicine and has become a cynosure of modern medicine. The Sanskrit name of the Neem tree is “Arishtha” meaning “reliever of sickness” and hence is considered as “Sarbaroganibarini” Citation28. The tree is still regarded as “village dispensary” in India. The importance of the Neem tree has been recognized by the US National Academy of Sciences, which published a report in 1992 entitled “Neem – a tree for solving global problems.”
Neem fruits, seeds, oil, leaves, bark, and roots have such uses as general antiseptics, antimicrobials, treatment of urinary disorders, diarrhea, fever and bronchitis, skin diseases, septic sores, infected burns, hypertensions, and inflammatory diseases. Neem oil and its isolates – nimbidin, nimbidiol, and nimbin – inhibit fungal growth on humans and animals Citation32. Neem leaf extracts and teas can treat malaria and the antimalarial action is attributable to gedunin, a limonoid. Given the wide spectrum of chemical species present in Neem leaves and their respective multifaceted chemical and biological properties, we therefore articulated that channeling Neem leaf extract for yet another use into green inhibition of corrosion studies may yield some interesting results. Neem extract has been little studied in environmental engineering with the equilibrium and kinetic biosorption studies of zinc from aqueous solution using AZI bark by King et al. Citation33, the removal of chromium (Cr(VI)) from aqueous solution using AZI leaf powder as absorbent by Venkateswarlu et al. Citation34, the successful scale-up of AZI suspension culture for azadirachtin production carried out in stirred tank bioreactor with two different impellers by Prakash and Srivastav Citation35, the adsorption and corrosion inhibitive properties of AZI in acid solutions Citation30 and the study of copper corrosion inhibition by AZI leaves extract in 0.5 M sulfuric acid by Valek and Martinez Citation31.
Research objectives
Mild steel (MS) is one of the most widely used metals in industry and is the most common form of steel (with 0.16–0.29% carbon) as its price is relatively low while it provides material properties that are acceptable for many applications. However, during industrial processes such as acid cleaning and pickling, MS corrodes easily implying that the use of an efficient inhibitor is very much necessary because the useful life of this valuable metal must be prolonged Citation36. Due to their industrial applications, several inhibitors have either been synthesized or chosen from organic compounds having heteroatoms in their molecular structures and some research on the use of natural occurring substances has also been intensified. Eddy and Ebenso Citation37 have recently studied the inhibition of the corrosion of MS by ethanol extract of Musa sapientum peels in H2SO4 has using gasometric and thermometric methods, whereas Eddy et al. Citation36 have additionally analyzed the adsorption and inhibitive efficiencies of amino-1-cyclopropyl-7-[(3R, 5S) 3, 5-dimethylpiperazin-1-YL]-6, 8-difluoro-4-oxo-uinoline-3-carboxylic acid on MS corrosion using gasometric and thermometric techniques and found it to be a good inhibitor for the corrosion of MS in H2SO4 solution. Other earlier studies on corrosion inhibition of MS include the study of alternating current and direct current of temperature effect on MS corrosion in acid media in the presence of benzimidazole derivatives by Popova et al. Citation38, the inhibition of MS corrosion in sulfuric acid using indigo dye and synergistic halide additives in aerated sulfuric acid solutions at 30–50°C by Oguzie et al. Citation39 and the inhibitory mechanism of MS corrosion in 2 M sulfuric acid solution by methylene blue dye using thermometric and gravimetric techniques by Oguzie et al. Citation40. All the more, in pursuit to find natural corrosion inhibitors, several studies have also been focused on the testing of certain plant extracts for the said purpose. Orubite and Oforka Citation41 have studied the inhibition of the corrosion of MS in hydrochloric acid solutions by the extracts of leaves of Nypa fruticans Wurmb, Oguzie Citation42 investigated the efficacy of Telfaria occidentalis extract as a corrosion inhibitor for MS in 2 M HCl and 1 M H2SO4 solutions, respectively, and assessed the effect of temperature and halide additives on the inhibition efficiency, whereas Sethuraman and Bothi Raja Citation43 evaluated the corrosion inhibition potential of Datura metel in acid medium on MS with a view to develop green corrosion inhibitors. Later, Li et al. Citation44 used berberine that was abstracted from coptis chinensis and its inhibition efficiency on corrosion of MS in 1 M H2SO4 was investigated through weight loss experiment, electrochemical techniques, and scanning electronic microscope (SEM) with energy disperse spectrometer (EDS). Oguzie Citation45 has conducted studies on the inhibitive effect of Occimum viridis extract on the acid corrosion of MS, and Chauhan and Gunasekaran Citation46 have investigated the inhibition effect of Zenthoxylum alatum plant extract on the corrosion of MS in 5 and 15% aqueous hydrochloric acid solution by weight loss and electrochemical impedance spectroscopy (EIS). Lately, Okafor et al. Citation47 have probed the inhibitive action of leaves (LV), seeds (SD), and a combination of leaves and seeds (LVSD) extracts of Phyllanthus amarus on MS corrosion in HCl and H2SO4 solutions using weight loss and gasometric techniques.
In the present study, we are trying to study corrosion of MS and the inhibition of the corrosion process by AZI extract. The few studies where AZI has been used for MS corrosion inhibition in acid solutions include studies by Mohanan et al. Citation48 wherein the biocidal and inhibitive effects of aqueous extract of AZI on MS in fresh water environment were investigated by pour plate technique and by weight loss measurements, potentiodynamic polarization, and alternating current impedance measurements and the study of Oguzie Citation30 whereby the protective effect and adsorption behavior of AZI extract in controlling MS corrosion in 1 M H2SO4 and 2 M HCl were studied. To the best of our knowledge, nothing has been specifically reported on the use of AZI extract for the inhibition of MS corrosion in nitric acid (HNO3) solution media. AZI leaves are often used in the medicinal and pharmaceutical industry. An additional beneficial use of Neem leaves to curb corrosion of MS would surely imply the successful utilization of this powerful and versatile natural resource in the metallurgical, materials science, and chemical engineering industries. The present study therefore probes the inhibitive and adsorption properties of AZI leaves extracts for MS corrosion using a gravimetric technique in an acidic media (HNO3 acid) with and without the extract at two temperatures (30 and 60°C). The thermodynamic parameters characterizing the adsorption process have also been calculated. In the least of a positive result would help reduce the economic cost of corrosion control as well as decrease the subsequent environmental threats from inhibitor usage because Neem leaves extract is non-toxic and biodegradable.
Results and discussions
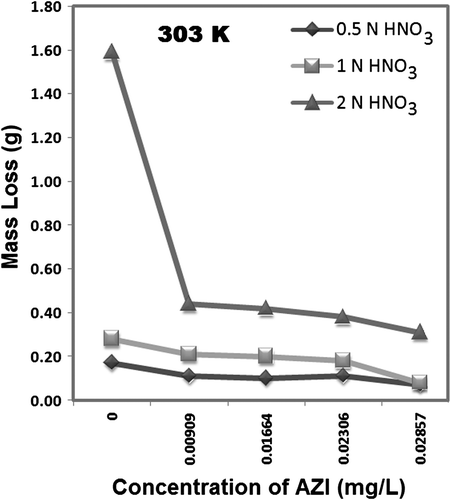
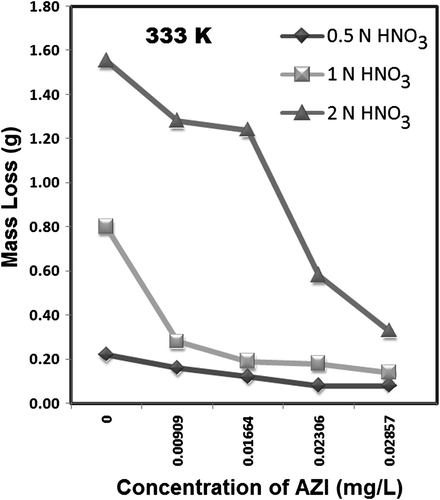
shows values of corrosion rate (CR) of MS in all the concentrations of HNO3 studied and it shows that CR increases with an increase in HNO3 concentration. The same trend of increasing CR is observed for either temperature studied in the experiments. shows the CR for the corrosion of MS at 0.5, 1.0, and 2.0 N HNO3 in the absence and presence of AZI extract at 303 and 333 K. It may be observed from the data in that an addition of an increased concentration of the inhibitor generally retards the CR of MS in the acid solutions. This is also seen and supported from the decreasing change in mass loss taking place at a particular acid concentration corresponding with an increase in inhibitor concentration ( and ).
Table 1. Corrosion rate for the corrosion of MS in HNO3 at 303 K.
Table 2. Corrosion rates for the corrosion of MS in 0.5, 1.0, and 2.0 N HNO3 solutions containing AZI extract.
Effect of Azadirachta indica (AZI) and acid concentration
From and , it is found that the rate of corrosion of MS is affected by concentration of HNO3, temperature, and concentration of AZI extract. When comparing and , it is deduced that the rate of MS corrosion increases as the concentration of HNO3 increases and also increases as the temperature is increased. An analysis and interpretation of trends in (or ) show that corrosion increases as the concentration of the acid increases, confirming that the rate of corrosion of MS in HNO3 increases with concentration. The mass loss taking place and recorded at the different concentrations of the AZI extract are lower than that of the blank solution (for the three acid concentrations) indicating that different concentrations of the AZI extract retard the corrosion of zinc. It is supposed to be due to adsorption of AZI extract on the surface of MS. This hypothesis is presently under verification and shall be reported in our next paper with details of the electrochemical studies reported and discussed therein.
Effect of temperature
and hence show the mass loss plots for the corrosion of MS in the presence of different concentrations of the AZI extract at 303 and 333 K, respectively. Comparing these trends, it is found that at a fixed concentration of the inhibitor and a fixed acid concentration, the mass loss taking place at 333 K is in most of the instances higher than that occurring at 303 K. This indicates that the inhibition efficiency of AZI extract decreases with increase in temperature. The decrease may be due to internal competition between forces of adsorption and desorption of the specific inhibitor molecule(s) participating in the corrosion inhibition reaction(s) at the active sites on the MS surface. These same competing forces of adsorption and desorption may also explain the occasional discrepancies in mass loss change observed in and . From , it can also be appreciated that the inhibition efficiency of AZI extract varies with its concentration. Optimum values of inhibition efficiency were obtained at an extract concentration of 28.57 mg/L, while the least values were obtained at an extract concentration of 9.09 mg/L. The differences between the trends for inhibition efficiency of AZI extract obtained at 303 and 333 K at the three acid concentrations over the range of AZI concentrations presently studied strongly suggest that the mechanisms of adsorption of the inhibitor on the MS surface is predominantly by physical adsorption. For a physical adsorption mechanism, inhibition efficiency of an inhibitor decreases with temperature, whereas for a chemical adsorption mechanism, values of inhibition efficiency increase with temperature Citation49 Citation50. Following the logic of the latter statement, it may be deduced from that for HNO3 concentrations of 0.5 and 1.0 N, for AZI concentrations up to 28.57 mg/L, chemical adsorption is involved in the corrosion inhibition reactions.
Table 3. Inhibition efficiencies of MS corrosion in 0.5, 1.0, and 2.0 N HNO3 solutions containing AZI extract.
Thermodynamics parameters
Values of activation energy for the corrosion reaction of MS in the presence and absence of different concentrations of the AZI extract have been calculated using the Arrhenius equation (Equation Equation1),
Table 4. Thermodynamic parameters for the adsorption of AZI extract on zinc surface (2.0 N H2SO4).
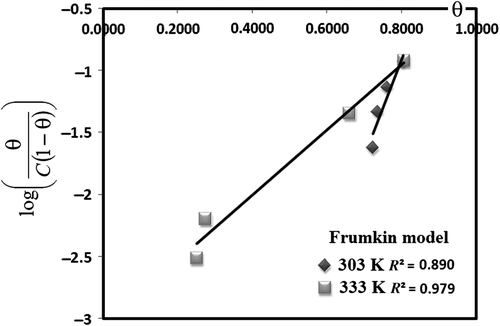
Adsorption isotherms
Adsorption isotherms are important in understanding the mechanism of inhibition of corrosion reactions. Various isotherm models, with two or three parameters, are available for modeling equilibrium data. Two-parameter isotherms are the most commonly used isotherms because of their simplicity and possibility of linearization. However, transformation of non-linear isotherm models to linear forms usually results in parameter estimation error. Three-parameter isotherms (Sips adsorption isotherm) have three adjustable parameters and cannot be estimated by linear regression. The adjustable parameters of all isotherms analyzed here were calculated using linear regression analysis. In order to describe the goodness-of-fit of the experimental data to the proposed models, the correlation coefficient (R 2) was calculated. The most frequently used adsorption isotherms are Frumkin, Temkin, Freundlich, Flory-Huggins, Bockris-Swinkel, El-Awardy, and Langmuir isotherms. All these isotherms can be represented as follows:
Experimental
Azadirachta indica (AZI) leaves extract stock solution
The stock solution of the AZI leaves extract was prepared by boiling 0.62 kg of air-dried Neem leaves in deionized water and left overnight. The contents of the extraction process were then mixed in a grinder, filtered, and the resulting solution was kept in a refrigerator at low temperatures of 2°C in order to prevent the contents from being altered or degraded due to the chemical, physical, and biological reactions it might otherwise undergo Citation52 due to open air exposure.
Mild steel (MS) coupon specimen preparation
Rectangular specimen sheets of MS were mechanically pressed cut to form different coupons (strips), each of dimension 5.0 cm long by 2.5 cm wide×0.045 cm thick. The MS used in the experiments had the following composition: C: 0.240; Mn: 0.470; Si: 0.28; Ni: 0.043; Cr: 0.061; Mo: 0.021; S: 0.020; P: 0.017; and the remainder was Fe. Each coupon was degreased by washing with ethanol, dried in acetone, and preserved in a dessicator. All reagents used for this study were Analar grade and double distilled water was used for their preparation. Specimens containing a small hole of 2 mm diameter near the upper edge were used for the determination of CR. The working surfaces of the MS coupons were carefully and lightly polished with grade P600 SiC polishing paper in order to remove any impervious oxide layer and eliminate the reactions that would have otherwise taken place with the acid and the oxide layer.
Inhibition efficiency (I) and degree of surface coverage (θ)
The mass loss method was employed for a room temperature at 303 and 333 K. The temperature for each run of the experiments was kept constant using a thermostat. In this procedure, the mass loss of the metal in uninhibited (with no AZI extract) and inhibited solutions was monitored and recorded. A 50 mL of test solutions were analyzed. From this data, the inhibition efficiency (%I) and degree of surface coverage (θ) were calculated Citation37 using Equations (Equation7) and (Equation8), respectively,
Conclusion
From the present study, it is concluded that AZI leaves extract can be used as an inhibitor for MS corrosion in HNO3 medium. While the green inhibitor molecule supposedly acts by being adsorbed on the MS surface, the overall inhibition is believed to be provided by a synergistic effect. It has also been found that the inhibitive action of AZI leaves extract is basically controlled by temperature and the concentration of the inhibitor.
The next step in the analysis of the corrosion inhibition of MS by AZI extract in nitric acid solutions shall constitute a thorough chemical and analytical investigation using NMR and/or IR spectroscopy, together with electrochemical studies so as to deduce which are the active components of the AZI leaves extract involved in the corrosion inhibition reaction, and also elucidate the corrosion inhibition mechanism. After this detailed study, our research group intends to isolate these active components and optimize on their application as green inhibitors which can find use in the inhibition of corrosion in industries where MS is used as a material of choice for the fabrication of machinery.
Acknowledgements
The authors are grateful to Dr. V.K. Agarwal (Chairman) of the Institute of Engineering and Technology (IET) Group of Institutions, Alwar, Rajasthan, India, for providing them the opportunity to establish a Computational and Green Chemistry Research Laboratory at IET whereat cropped up the idea to carry out the present study. We are also thankful to our other colleagues, laboratory technicians, and the anonymous reviewers whose criticisms have benefited in bringing this the manuscript to its present form.
References
- Thompson , N.G. , Yunovich , M. and Dunmire , D. 2007 . Cost of Corrosion and Corrosion Maintenance Strategies . Corr. Rev. , 25 : 247 – 262 .
- Anuradha , K. , Vimala , R. , Narayanasamy , B. , Arockia Selvi , J. and Rajendran , S. 2008 . Corrosion Inhibition of Carbon Steel in Low Chloride Media by an Aqueous Extract of Hibiscus rosa-sinensis Linn . Chem. Eng. Commun. , 195 : 352 – 366 .
- Zarras , P. , Anderson , N. , Webber , C. , Irvin , D.J. , Irvin , J.A. , Guenthner , A. and Stenger-Smith , J.D. 2003 . Progress in Using Conductive Polymers as Corrosion-Inhibiting Coatings . Radiat. Phys. Chem. , 68 : 387 – 394 .
- Khramov A.N. Voevodin N.N. Balbyshev V.N. Donley M.S Hybrid Organo-Ceramic Corrosion Protection Coatings with Encapsulated Organic Corrosion Inhibitors Thin Solid Films 2004 447 448 549–557
- Zuo , Y. , Zhao , P-H. and Zhao , J-M. 2003 . The Influences of Sealing Methods on Corrosion Behavior of Anodized Aluminum Alloys in NaCl Solutions . Surf. Coatings Technol. , 166 : 237 – 242 .
- Alsabagh , A.M. , Migahed , M.A. and Awad , H.S. 2006 . Reactivity of Polyester Aliphatic Amine Surfactants as Corrosion Inhibitors for Carbon Steel in Formation Water (Deep Well Water) . Corr. Sci. , 48 : 813 – 828 .
- Valdez , B. , Cheng , J. , Flores , F. , Schorr , M. and Veleva , L. 2003 . Application of Vapour Phase Corrosion Inhibitors for Silver Corrosion Control in the Electronics Industry . Corr. Rev. , 21 : 445 – 458 .
- Taylor , S.R. and Chambers , B.D. 2007 . The Discovery of Non-Chromate Corrosion Inhibitors for Aerospace Alloys Using High-Throughput Screening Methods . Corr. Rev. , 25 : 571 – 590 .
- Khaled , K.F. 2008 . New Synthesized Guanidine Derivative as a Green Corrosion Inhibitor for Mild Steel in Acidic Solutions . Int. J. Electrochem. Sci. , 3 : 462 – 475 .
- Bothi Raja , P. and Sethuraman , M.G. 2008 . Natural Products as Corrosion Inhibitor for Metals in Corrosive Media – A Review . Mater. Lett. , 62 : 113 – 116 .
- Rajendran , S. , Amalraj , A.J. , Joice , M.J. , Anthony , N. , Trivedi , D.C. and Sundaravadivelu , M. 2004 . Corrosion Inhibition by the Caffeine–Zn2+ System . Corr. Rev. , 22 : 233 – 248 .
- Mesbah , A. , Juers , C. , Lacouture , F. , Mathieu , S. , Rocca , E. , François , M. and Steinmetz , J. 2007 . Inhibitors for Magnesium Corrosion: Metal Organic Frameworks . Solid State Sci. , 9 : 322 – 328 .
- Okafor , P.C. , Osabor , V.I. and Ebenso , E.E. 2007 . Eco-Friendly Corrosion Inhibitors: Inhibitive Action of Ethanol Extracts of Garcinia kola for the Corrosion of Mild Steel in H2SO4 Solutions . Pigment Resin Technol. , 36 : 299 – 305 .
- Lebrini , M. , Traisnel , M. , Lagrenée , M. , Mernari , B. and Bentiss , F. 2008 . Inhibitive Properties, Adsorption and a Theoretical Study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as Corrosion Inhibitors for Mild Steel in Perchloric Acid . Corr. Sci. , 50 : 473 – 479 .
- Radojčić , I. , Berković , K. , Kovač , S. and Vorkapić-Furač , J. 2008 . Natural Honey and Black Radish Juice as Tin Corrosion Inhibitors . Corr. Sci. , 50 : 1498 – 1504 .
- Refaey , S.A.M. , Abd El Malak , A.M. , Taha , F. and Abdel-Fatah , H.T.M. 2008 . Corrosion and Inhibition of Cu–Zn Alloys in Acidic Medium by Using Isatin . Int. J. Electrochem. Sci. , 3 : 167 – 176 .
- Sharma S.K. Mudhoo A. Khamis E Green Corrosion Inhibitors: An Overview of Recent Research J. Corr. Sci. Eng http://www.icse.org/viewpaper.php?vol=1&pap=14 (accessed Oct 5, 2009)
- Abdel-Gaber , A.M. , Khamis , E. , Abo-Eldahab , H. and Adeel , S. 2008 . Materials Inhibition of Aluminium Corrosion in Alkaline Solutions Using Natural Compound . Chem. Phys. , 109 : 297 – 305 .
- Khamis , E. , Hefnawy , A. and El-Demerdash , A.M. 2007 . Acid Cleaning of Steel Using Arghel Echo Friendly Corrosion Inhibitor . Materialwissenschaft und Werkstofftechnik , 38 : 227 – 232 .
- Umoren , S.A. and Ebenso , E.E. 2008 . Studies of Anti-Corrosive Effect of Raphia Hookeri Exudates Gum – Halide Mixtures for Aluminium Corrosion in Acidic Medium . Pigment Resin Technol. , 37 : 173 – 182 .
- Okafor , P.C. and Ebenso , E.E. 2007 . Inhibitive Action of Carica Papaya Extracts on the Corrosion of Mild Steel in Acidic Media and their Adsorption Characteristics . Pigment Resin Technol. , 36 : 134 – 140 .
- El-Etre , A.Y. 2006 . Khillah Extract as Inhibitor for Acid Corrosion of SX 316 Steel . Appl. Surf. Sci. , 252 : 8521 – 8525 .
- Umoren , S.A. , Ogbobe , O. , Ebenso , E.E. and Ekpe , U.J. 2006 . Effect of Halide Ions on the Corrosion Inhibition of Mild Steel in Acidic Medium Using Polyvinyl Alcohol . Pigment Resin Technol. , 35 : 284 – 292 .
- Bendahou , M.A. , Benadellah , M.B.E. and Hammouti , B.B. 2006 . A Study of Rosemary Oil as a Green Corrosion Inhibitor for Steel in 2 M H3PO4 . Pigment Resin Technol. , 35 : 95 – 100 .
- Sharma , S.K. , Chaudhary , A. and Singh , R.V. 2008 . Gray Chemistry Verses Green Chemistry: Challenges and Opportunities . RASAYAN J. Chem. , 1 : 68 – 92 .
- Subapriya , R. and Nagini , S. 2005 . Medicinal Properties of Neem Leaves: A Review . Curr. Med. Chem.-Anti-Cancer Agents. , 5 : 149 – 156 .
- Dasgupta , T. , Banerjee , S. , Yadava , P.K. and Rao , A.R. 2004 . Chemopreventive Potential of Azadirachta indica (Neem) Leaf Extract in Murine Carcinogenesis Model Systems . J. Ethnopharmacol. , 92 : 23 – 36 .
- Biswas , K. , Chattopadhyay , I. , Banerjee , R.K. and Bandyopadhyay , U. 2002 . Biological Activities and Medicinal Properties of Neem (Azadirachta indica) . Curr. Sci. , 82 : 1336 – 1345 .
- Bhattacharyya , K.G. and Sharma , A. 2004 . Adsorption of Pb(II) from Aqueous Solution by Azadirachta indica (Neem) Leaf Powder . J. Hazard. Mater. , 113 : 97 – 109 .
- Oguzie , E.E. 2006 . Adsorption and Corrosion Inhibitive Properties of Azadirachta indica in Acid Solutions . Pigment Resin Technol. , 35 : 334 – 340 .
- Valek , L. and Martinez , S. 2007 . Copper Corrosion Inhibition by Azadirachta indica Leaves Extract in 0.5 M Sulphuric Acid . Mater. Lett. , 61 : 148 – 151 .
- Suresh , G.V. , Gopalakrishnan , G. and Masilamani , S. 2007 . Neem for Plant Pathogenic Fungal Control: The Outlook in the New Millennium. Neem: Today and in the New Millennium , 183 – 207 . Springer : HeidelbeRg .
- King , P. , Anuradha , K. , Beena Lahari , S. , Prasanna Kumar , Y. and Prasad , V.S.R.K. 2008 . Biosorption of Zinc From Aqueous Solution Using Azadirachta indica Bark: Equilibrium and Kinetic Studies . J. Hazard. Mater. , 152 : 324 – 329 .
- Venkateswarlu , P. , Venkata Ratnam , M. , Subba Rao , D. and Venkateswara Rao , M. 2007 . Removal of Chromium from an Aqueous Solution Using Azadirachta indica (Neem) Leaf Powder as an Adsorbent . Int. J. Phys. Sci. , 2 : 188 – 195 .
- Prakash , G. and Srivastav , A.K. 2007 . Azadirachtin Production in Stirred Tank Reactors by Azadirachta indica Suspension Culture . Process Biochem. , 42 : 93 – 97 .
- Eddy , N.O. , Ekwumemgbo , P. and Odoemelam , S.A. 2008 . Inhibition of the Corrosion of Mild Steel in H2SO4 by 5-amino-1-cyclopropyl-7-[(3R, 5S) 3, 5-dimethylpiperazin-1-YL]-6, 8-difluoro-4-oxo-uinoline-3-carboxylic Acid (ACPDQC) . Int. J. Phys. Sci. , 3 : 275 – 280 .
- Eddy , N.O. and Ebenso , E.E. 2008 . Adsorption and Inhibitive Properties of Ethanol Extracts of Musa sapientum Peels as a Green Corrosion Inhibitor for Mild Steel in H2SO4 . Afr. J. Pure Appl. Chem. , 2 : 46 – 54 .
- Popova , A. , Sokolova , E. , Raicheva , S. and Christov , M. 2003 . AC and DC Study of the Temperature Effect on Mild Steel Corrosion in Acid Media in the Presence of Benzimidazole Derivatives . Corr. Sci. , 45 : 33 – 58 .
- Oguzie , E.E. , Unaegbu , C. , Ogukwe , C.N. , Okolue , B.N. and Onuchukwu , A.I. 2004 . Inhibition of Mild Steel Corrosion in Sulphuric Acid Using Indigo Dye and Synergistic Halide Additives in Aerated Sulphuric Acid Solutions at 30–50°C . Mater. Chem. Phys. , 84 : 363 – 368 .
- Oguzie , E.E. , Onuoha , G.N. and Onuchukwu , A.I. 2005 . Inhibitory Mechanism of Mild Steel Corrosion in 2 M Sulphuric Acid Solution by Methylene Blue Dye . Mater. Chem. Phys. , 89 : 305 – 311 .
- Orubite , K.O. and Oforka , N.C. 2004 . Inhibition of the Corrosion of Mild Steel in Hydrochloric Acid Solutions by the Extracts of Leaves of Nypa fruticans Wurmb . Mater. Lett. , 58 : 1768 – 1772 .
- Oguzie , E.E. 2005 . Inhibition of Acid Corrosion of Mild Steel by Telfaria occidentalis . Pigment Resin Technol. , 34 : 321 – 326 .
- Sethuraman , M.G. and Bothi Raja , P. 2005 . Corrosion Inhibition of Mild Steel by Datura metel . Pigment Resin Technol. , 34 : 327 – 331 .
- Li , Y. , Zhao , P. , Liang , Q. and Hou , B. 2005 . Berberine as a Natural Source Inhibitor for Mild Steel in 1 M H2SO4 . Appl. Surf. Sci. , 252 : 1245 – 1253 .
- Oguzie , E.E. 2006 . Studies on the Inhibitive Effect of Occimum viridis Extract on the Acid Corrosion of Mild Steel . Mater. Chem. Phys. , 99 : 441 – 446 .
- Chauhan , L.R. and Gunasekaran , G. 2007 . Corrosion Inhibition of Mild Steel by Plant Extract in Dilute HCl Medium . Corr. Sci. , 49 : 1143 – 1161 .
- Okafor , P.C. , Ikpi , M.E. , Uwah , I.E. , Ebenso , E.E. , Ekpe , U.J. and Umoren , S.A. 2008 . Inhibitory Action of Phyllanthus amarus Extracts on the Corrosion of Mild Steel in Acidic Media . Corr. Sci. , 50 : 2310 – 2317 .
- Mohanan , S. , Manimegalai , M. , Rajeswari , P. , Maruthamuthu , S. and Palaniswamy , N. 2002 . Biocidal and Inhibitive Duality of Naturally Occurring Substance Azadiracta Indica on Mild Steel in Fresh Water . Bull. Electrochem. , 18 : 337 – 342 .
- Ebenso , E.E. 2003 . Effect of Halide Ions on the Corrosion Inhibition of Mild Steel in H2SO4 Using Methyl Red . Part 1, Bull. Electrochem. , 19 : 209 – 216 .
- Ebenso , E.E. 2004 . Effect of Methyl Red and Halide Ions on the Corrosion Inhibition of Aluminium in H2SO4 . Part 2, Bull. Electrochem. , 20 : 551 – 559 .
- Sheatty , D.S. , Shetty , P. and Nayak , H.V.S. 2006 . Inhibition of Mild Steel Corrosion in Acid Media by N-(2-thiophenyl) – N-phenyl thiourea . J. Chiliean Chem. Soc. , 51 : 849 – 853 .
- Sliwka-Kaszynska , M. , Kot-Wasik , A. and Namiesnik , J. 2003 . Preservation and Storage of Water Samples . Crit. Rev. Environ. Sci. Technol. , 33 : 31 – 44 .