Abstract
A simple, rapid, and highly efficient method has been attempted for the three-component condensation of benzil/benzoin, aldehydes, and ammonium acetate under microwave irradiation in the presence of a catalytic amount of bio-supported cellulose sulfuric acid under solvent-free conditions to afford the 2,4,5-triarylimidazole derivatives. The catalyst is easily prepared, inexpensive, separated simply by filtration, gives excellent yield of products with shorter reaction times, and is recyclable several times.
Introduction
The imidazole moiety is an important heterocyclic nucleus due to its widespread biological activity and use in synthetic chemistry. The imidazole ring system is one of the most important substructures found in a large number of natural products and pharmacologically active compounds such as the hypnotic agent etomidate Citation1 and the proton pump inhibitor omeprazole. In addition, they are used in photography as photosensitive compounds Citation2.
Because of their great importance, many synthetic strategies have been developed. In 1882, Radziszewski and Japp reported the first synthesis of the imidazole from 1,2-dicarbonyl compound, various aldehydes and ammonia, to obtain the 2,4,5-triphenyl imidazoles Citation3 Citation4. Also, Grimmett et al. proposed the synthesis of the imidazole using nitriles and esters Citation5. Another method is the four-component one-pot condensation of glyoxals, aldehydes, amines, and ammonium acetate in refluxing acetic acid which is the most desirable convenient method Citation6. Moreover, Zhang and Chen Citation7 described an efficient procedure to obtain unsymmetrical, C5 unsubstituted 2,4-diarylimidazoles. In this approach acetophenones are oxidized in situ to R-tosyloxyacetophenones, which then condense with arylamidines to obtain the desired compounds. Very recently, a literature survey reveals several methods for synthesis of 2,4,5-triarylimidazoles using ZrCl4 Citation8, zeolite HY/silica gel Citation9, sodium bisulfite (NaHSO3) Citation10, sulphanilic acid Citation11, iodine Citation12, and ionic liquids Citation13. However, many of these methodologies suffer from one or more disadvantages, such as low yields, high temperature requirement, prolonged reaction time, highly acidic conditions, requirement of excess of catalysts, and use of solvents. Therefore, there is a strong demand for a simple, highly efficient, and versatile method for the synthesis of 2,4,5-triarylimidazoles derivatives.
Organic synthesis in the absence of a solvent is a powerful tool for the generation of structurally diverse molecules. Solvent-free reactions often, have special selectivity, are easy to set-up and work-up, and are faster, which have aroused great interest Citation14. These aspects, coupled with the lower overall costs of running a reaction without solvent and no specially needed equipment, could become a decisive factor in industry.
In recent years, the uses of solid acids as heterogeneous catalysts have received tremendous attention in different areas of organic synthesis Citation15. Heterogeneous solid acids are advantageous over conventional homogeneous acid catalysts as they can be easily recovered from the reaction mixture by simple filtration and can be reused after activation or without activation thereby making the process economically more viable. Herein, we report a heterogeneous solid acid catalyst for the synthesis of 2,4,5-triarylimidazole derivatives, i.e. cellulose sulfuric acid (CSA).
Cellulose is a common material in plant cell walls and was first noted as such in 1838. It occurs naturally in almost pure form only in cotton fiber; in combination with lignin and any hemicellulose, it is found in all plant material. Cellulose is the most abundant natural material in the world and it has been widely studied during the past decades because it is a biodegradable material and a renewable resource. Cellulose and its derivatives have some unique properties such as inexpensive, extremely inert, biodegradable, and environmentally benign, which make them attractive alternatives for conventional organic or inorganic supports for catalytic applications Citation16. Recently, CSA has emerged as a promising biopolymeric solid support acid catalyst for various acid-catalyzed reactions Citation17–21.
The use of microwave for the synthesis of organic compounds under solvent-free conditions has been proven to be an efficient, safe, and environmentally benign technique, with the advantages of shorter reaction times, high yields, and easier manipulation Citation22–26.
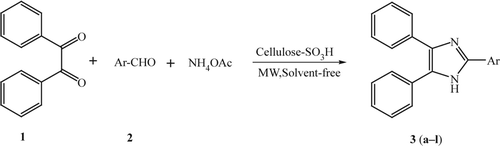
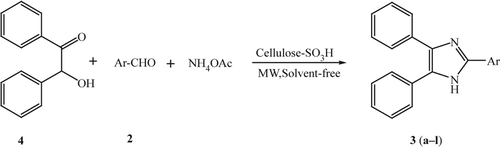
In continuation of our interest in microwave-assisted synthesis Citation27–29, herein we wish to report a simple and an efficient method for the one-pot three-component condensation of benzil/benzoin, aldehydes, and ammonium acetate for the synthesis of 2,4,5-triarylimidazole derivatives in the presence of CSA under solvent-free conditions using microwave irradiation ( and ).
Results and discussion
In order to find optimum reaction conditions, condensation of benzil, 4-chlorobenzaldehyde, and ammonium acetate in the presence catalytic amount of CSA was performed. The optimum molar ratio of benzil, p-chlorobenzaldehyde, and ammonium acetate was found to be 1:1:2.5 with CSA (0.1 g) under solvent-free conditions using microwave irradiation. Under these conditions, 2-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole (3c) was obtained in 98% yield after 1.5 min. We were encouraged by the results obtained with p-chlorobenzaldehyde. In a similar fashion, a variety of aromatic and heterocyclic aldehydes and benzil/benzoin subjected to this novel procedure yielded the corresponding 2,4,5-triarylimidazole in high to excellent yields. The results are summarized in . Moreover, reaction work-up is simple and the catalyst can be easily separated from the product and reused several times, without affecting the yield of the desired product and reaction times, thus making it environmentally benign. The procedure gives the products high to excellent yields and avoids problems associated with solvent use such as cost, handling, and specifically safety related to fire hazard due to occurrence of sparks in microwave oven.
Table 1. Microwave-assisted CSA catalyzed one-pot synthesis of 2,4,5-triarylimidazole derivatives under solvent-free conditions.
As shown in , the reaction of benzaldehyde, benzil, and ammonium acetate was also carried out in the presence of various Lewis acids (HgCl2 and SnCl2·2H2O), protic acids (H2SO4 and HCl), clays (EPZG and EPZ 10), and solid acid (CSA). The reaction rate and best yield were obtained with CSA. It is noteworthy that in the absence of a catalyst under the reaction conditions, no product formation was observed after 10 min. This result indicates that the catalyst exhibits a high catalytic activity in this transformation.
Table 2. Microwave-assisted effect of different catalyst for the synthesis of 3a under solvent-free conditions.
In , we have compared our results with results obtained by some other reported procedures for the synthesis of 2-(4-nitrophenyl)-4,5-diphenyl-1H-imidazole (3g). The data presented in this table show the promising feature of this method in terms of reaction rate and the yield of product compared with those reported in the literature.
Table 3. Comparisons of some other reported procedures with the present method for the synthesis of 2-(4-nitrophenyl)-4,5-diphenyl-1H-imidazole (, entry 3g).
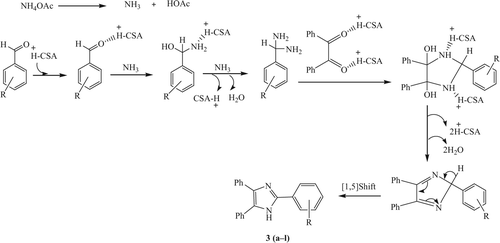
Further investigation involved the reusability of the catalyst due to the importance of reusability for large-scale operations from an industrial point of view. Therefore, the recovery and reusability of CSA were examined. The catalyst can be separated and reused after being extracted with CH2Cl2 (15 ml). The reusability of the catalyst was investigated in the reaction of benzaldehyde, benzil, and ammonium acetate (, entry 3a) in the presence of 0.1 g CSA. The results illustrated in showed that the catalyst could be used five times without any loss of activity. The proposed mechanism of this reaction is as shown in .
Table 4. Recovery and reuse of CSA in the reaction of benzil, benzaldehyde, and ammonium acetate (, entry 3a).
Experimental
The materials were obtained from commercial suppliers and were used without further purification. The uncorrected melting points of compounds were taken in an open capillary in a paraffin bath. The progresses of the reactions were monitored by thin layer chromatography (TLC) using ethyl acetate:n-hexane (1:9) as a solvent system. IR spectra were recorded on Perkin–Elmer FT spectrophotometer in KBr disc. 1H NMR spectra were recorded on an 80 MHz FT-NMR spectrometer in CDCl3 as a solvent and chemical shift values are recorded in units δ (ppm) relative to tetramethylsilane (Me4Si) as an internal standard. All experiments under microwave irradiation were carried out in a Model 800T microwave oven (BPL, Appliances and Utilities Ltd., India) having a maximum power output of 800 W and a 2450 MHz frequency.
The CSA was prepared according to literature Citation21.
General procedure for the synthesis of 2,4,5-triary-1H-imidazoles 3(a–l)
Benzil/benzoin (1 mmol), ammonium acetate (2.5 mmol), and CSA (0.1 g) were taken in a beaker (50 ml) and to this aldehyde (1 mmol) was added. The reaction mixture was mixed properly with the help of a glass rod and placed in a microwave oven at the power of 180 W and irradiated for a period of 10 sec at a time. After each irradiation, the reaction mixture was removed from the microwave oven for shaking. The total period of microwave irradiation was 1–3 min (). After TLC (petroleum ether:ethyl acetate = 9:1 as eluent) indicated the starting material of benzil/benzoin and aldehyde had disappeared, the reaction mixture was extracted with CH2Cl2 (15 ml) and to separate the catalyst. Then the filtrate was evaporated under reduced pressure to give crude product. The crude product was purified by recrystallization from ethanol and gave the corresponding 2,4,5-triarylimidazole (3a–l) in high to excellent yields.
Spectral data of principal compounds
2,4,5-Triphenyl-1H-imidazole (3a)
IR (KBr): 3450 (N–H), 3050 (C–H), 1600 (C = C), 1580 (C = N) cm−1. 1H NMR (CDCl3, 80 MHz; δ, ppm): 7.15–8.00 (m, 15H, Ph), 9.20 (br s, NH). EIMS (m/z,%): 297 (M + 1).
2-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazole (3c)
IR (KBr): 3450 (N–H), 1600 (C = C), 1580 (C = N) cm−1. 1H NMR (CDCl3, 80 MHz; δ, ppm): 7.10–7.60 (m, 10H, Ph), 7.35 (d, 2H, J = 10 Hz, Ar), 7.85 (d, 2H, J = 10 Hz, Ar). EIMS (m/z,%): 331 (M + 1).
2-(4-Methylphenyl)-4,5-diphenyl-1H-imidazole (3d)
IR (KBr): 3450 (N–H), 1600 (C = C), 1585 (C = N) cm−1. 1H NMR (CDCl3, 80 MHz; δ, ppm): 2.30 (s, CH3), 7.10–7.60 (m, 10H, Ph), 7.70 (d, 2H, J = 10 Hz, Ar), 7.30 (d, 2H, J = 10 Hz, Ar). EIMS (m/z,%): 311 (M + 1).
2-(4-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (3e)
IR (KBr): 3450 (N–H), 1610 (C = C), 1575 (C = N), 1385 (C–O) cm−1. 1H NMR (CDCl3, 80 MHz; δ, ppm): 3.90 (s, OCH3), 7.05 (d, 2H, J = 8.8 Hz, Ar), 7.30–7.80 (m, 10H, Ph), 7.90 (d, 2H, J = 8.8 Hz, Ar). EIMS (m/z,%): 327 (M + 1).
2-(4-Nitrophenyl)-4,5-diphenyl-1H-imidazole (3g)
IR (KBr): 3400 (N–H), 1580 (C = N), 1515 (NO2), 1335 (NO2) cm−1. 1H NMR (CDCl3, 80 MHz; δ, ppm): 7.15–7.70 (m, 10H, Ph), 7.90–8.25 (AB, 4H, J = 0.9 Hz, Ar). EIMS (m/z,%): 342 (M + 1).
2-(4-Dimethylaminophenyl)-4,5-diphenyl-1H-imidazole (3h)
IR (KBr): 3050 (C–H), 2850 (C–H), 1615 (C = C), 1600 (C = N), 1360 (C–N) cm−1. 1H NMR (CDCl3, 80 MHz; δ, ppm): 2.90 (s, 2CH3), 6.60 (d, 2H, J = 8.9 Hz, Ar), 7.10–7.60 (m, 10H, Ph), 7.70 (d, 2H, J = 8.9 Hz, Ar). EIMS (m/z,%): 340 (M + 1).
2-(2-Furyl)-4,5-diphenyl-1H-imidazole (3k)
IR (KBr): 639, 719, 874, 1169, 1210, 1660, 2470, 2993, 3316 cm−1. 1H NMR (CDCl3/DMSO, 200 MHz; δ, ppm): 7.21 (m, 1H, NH), 7.46–7.58 (m, 4H, Ar), 7.60–7.70 (m, 3H, Ar), 7.96–8.02 (m, 6H, Ar). EIMS (m/z,%): 287 (M + 1).
Conclusion
In conclusion, CSA as a stable, efficient, and environmentally benign catalyst was prepared and employed for the one-pot three-component synthesis of 2,4,5-triarylimidazole derivatives via the condensation of benzil/benzoin, aldehyde, and ammonium acetate under solvent-free conditions using microwave irradiation. In view of green chemistry, the catalyst is recyclable for up to four uses without much loss of activity, rendering the process more economic. Moreover, the present methodology gives excellent yields with shorter reaction time and simple work-up procedure.
Acknowledgements
We are grateful to the Head of the Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, Maharashtra 431 004, India, for providing the laboratory facilities.
References
- Wauquier , A. , Van Den Broeck , W.A.E. , Verheyen , J.L. and Janssen , P.A.J. 1978 . Eur. J. Pharmacol. , 47 : 367 – 377 .
- Satoru , I. 1989 . Chem. Abstr. , 111 : 214482
- Radziszewski , B. 1882 . Chem. Ber. , 15 : 1493 – 1496 .
- Japp , F.R. and Robinson , H.H. 1882 . Chem. Ber. , 15 : 1268 – 1270 .
- Grimmett M.R. Katritzky A.R. Rees C.W. Scriven E.F.V. Comprehensive Heterocyclic Chemistry Pergamon: NewYork 1996 77 220
- Krieg , B. and Mancke , G.Z. 1967 . Naturforschg , 226 : 132 – 253 .
- Zhang , P.F. and Chen , Z.C. 2001 . Synthesis , 14 : 2075 – 2077 .
- Sharma , G.V.M. , Jyothi , Y. and Lakshmi , P.S. 2006 . Syn. Commun. , 36 : 2991 – 3000 .
- Balalaie , S. , Arabanian , A. and Hashtroudi , M.S. 2000 . Mont. Fur. Chem. , 131 : 945 – 948 .
- Sangshetti , J.N. , Kokare , N.D. , Kothakar , S.A. and Shinde , D.B. 2008 . Mont. Fur. Chem. , 139 : 125 – 127 .
- Mohammed , A.F. , Kokare , N.D. , Sangshetti , J.N. and Shinde , D.B. 2007 . J. Korean. Chem. Soci. , 51 : 418 – 422 .
- Kidwai , M. , Mothsra , P. , Bansal , V. and Goyal , R. 2006 . Mont. Fur. Chem. , 137 : 1189 – 1194 .
- Siddiqui , S.A. , Narkhede , U.C. , Palimkar , S.S. , Daniel , T. , Lahoti , R.J. and Srinivasan , K.V. 2005 . Tetrahedron , 61 : 3539 – 3546 .
- Tannaka , K. and Toda , F. 2000 . Chem. Rev. , 100 : 1025 – 1074 .
- Clark , J.H. 2002 . Acc. Chem. Res. , 35 : 791 – 797 .
- Klemm , D. , Heublein , B. , Fink , H.P. and Bohn , A. 2005 . Angew Chem. Int. Ed. , 44 : 3358 – 3393 .
- Ahmad , S. and Ali , M. 2007 . Appl. Catal. A. , 331 : 149 – 151 .
- Reddy , P.N. , Reddy , Y.T. , Reddy , M.N. , Rajitha , B. and Crooks Peter , A. 2009 . Syn. Commun. , 39 : 1257 – 1263 .
- Ahmad , S. , Abbas , R. and Zahra , B. 2008 . Catal. Comm. , 9 : 13 – 16 .
- Shaabani , A. , Rahmati , A. and Badri , Z. 2008 . Catal. Comm. , 9 : 13 – 16 .
- Sadaphal , S.A. , Sonar , S.S. , Ware , M.N. and Shingare , M.S. 2008 . Green Chem. Lett. Rev. , 1 : 191 – 196 .
- Caddick , S. 1995 . Tetrahed. Lett. , 51 : 10403 – 10432 .
- Mekheimer , R.A. and Sadek , K.U. J. 2009 . Hetero. Chem. , 46 : 149 – 151 .
- Radia , M. , Bernardoa , V. , Bechia , B. , Castagnoloa , D. , Paganoa , M. and Bottaa , M. 2009 . Tetrahed. Lett. , 50 : 6572 – 6575 .
- Jayagobia , M. and Raghunathan , R. 2009 . Tetrahed. Lett. , 50 : 6886 – 6890 .
- Seo , J. , Michaelian , N. , Owens , S.C. , Dashner , S.T. , Wong , A.J. , Barron , A.E. and Carrasco , M.R. 2009 . Org. Lett. , 11 : 5210 – 5213 .
- Karale , B.K. , Chavan , V.P. , Mane , A.S. , Hangarge , R.V. , Gill , C.H. and Shingare , M.S. 2002 . Syn. Comm. , 32 : 497 – 503 .
- Madje , B.R. , Shindalkar , S.S. , Ware , M.N. and Shingare , M.S. 2005 . Org. Chem: An Ind. J. , 2 : 95 – 98 .
- Shindalkar , S.S. , Madje , B.R. and Shingare , M.S. 2007 . Mendeleev Commun. , 17 : 43 – 44 .