Abstract
The inhibition of corrosion of aluminum in hydrochloric acid solutions by the ethanolic extract of the leaves of Ananas sativum was studied using weight loss and hydrogen evolution methods. It was found that the plant extract retarded the acid induced corrosion of aluminum. Inhibition efficiency increased with increasing concentration of the extract and temperature. Adsorption studies revealed that Langmuir adsorption isotherm is the best adsorption model applicable to the adsorption of A. sativum on aluminum surface. Activation parameters such as activation energies (E a), activation enthalpy (ΔH o), and activation entropy (ΔS o) were evaluated from the effect of temperature on the corrosion and inhibition processes.
Introduction
The protection of metals against corrosion has attracted much attention globally as a result of huge losses of natural resources and finances that are sustained annually all over the world due to corrosion. The most important feature of aluminum is its corrosion resistance due to the presence of a thin, adherent, and protective surface oxide film. Because of this advantage, aluminum and its alloys are widely used in many industries such as reaction vessels, pipes, machinery, and chemical batteries Citation1–3.
However, when exposed to aggressive environments, such as the use of acid solutions for pickling, chemical and electrochemical etching of aluminum, industrial acid cleaning, cleaning of oil refinery equipment, oil well acidizing and acid descaling, these processes usually lead to substantial loss of the metal due to corrosion. Inhibitors are used to prevent metal dissolution and minimize acid consumption. Most of the efficient acid inhibitors are organic compounds that contain mainly nitrogen, sulfur or oxygen atoms in their structure Citation4–6. Despite the large number of organic inhibitors confirmed to be suitable for Al corrosion, there is always a need for developing new organic corrosion inhibitors. Besides, most of these inhibitors are toxic to the environment implying that their use may solve corrosion problem but enhance environmental problems.
The study of plant extracts as low-cost and eco-friendly corrosion inhibitors is of great interest from an environmental perspective and is attracting a significant level of attention. Green corrosion inhibitors have a promising future for the quality of the environment because they do not contain heavy metals or other toxic compounds. In addition, they are biodegradable and renewable source of materials.
Some reports have been highlighted on the successful application of plant extracts as corrosion inhibitors for aluminum in different media. The inhibitive action of the acid extracts of seeds, leaves, and bark from the Ficus virens plant toward the corrosion of aluminum in hydrochloric and sulfuric acids has been studied by Jain et al. Citation7. The use of aqueous solution of garlic in controlling the corrosion of Al immersed in NaOH solution has been reported Citation8. Application of the extract of different parts of Peepal (Fiscus religeosa) as inhibitors for the corrosion of aluminum in HCl has been studied Citation9. Ebenso et al. Citation10 reported that the ethanolic extract of Carica papaya and Azadirachta indica are good inhibitors for Al corrosion in acidic medium. The inhibitive action of the mucilage extracted from the modified stems of Opunita extract toward the acid corrosion of Al has been reported by El-Etre Citation11. The inhibitive action of extract of tobacco plant toward the corrosion of steel and aluminum has been investigated Citation12. Kliskic et al. Citation13 reported aqueous extract of Romarinus officinalis as a good inhibitor for the corrosion of Al–Mg alloy in chloride solution. Oguzie found that extract of Occimum basislicum Citation14 has the potential of inhibiting the corrosion of aluminum in acidic and alkaline media and Sansevieria trifaciata in alkaline medium Citation15. Leaves and seed extract of Gossypium hissutum L. Citation16 and Phyllanthus amarus Citation17 as effective corrosion inhibitors for aluminum in NaOH as well as Delonix regia Citation18 in acidic media has been reported by Abiola and coworkers. El-Hosary et al. studied the corrosion inhibition of aluminum and zinc in HCl by Hibiscus sabdariffa extract Citation19. In a related study, Noor Citation20 reported the corrosion inhibitive effect of the leaves extract of the same plant for aluminum in alkaline solutions. Inhibition of aluminum corrosion in NaOH by Ambrosia maritime (damsissa) has been reported by Addel-Gaber et al. Citation21. Obot and Obi-Egbedi Citation22 investigated the potential of Ipomoea involcrata in inhibiting corrosion of aluminum in NaOH. Other naturally occurring substances reported as effective corrosion inhibitor for aluminum also include aqueous extracts of some seed, leaves, fruits, and fruit peels in NaOH Citation23.
Series of reports have been highlighted in our laboratory on naturally occurring substances and plant extracts as green corrosion inhibitors. These include gum Arabic in inhibiting aluminum corrosion in alkaline medium Citation24, for mild steel and aluminum in acidic medium Citation25, Raphia hookeri exudates gum for mild steel and aluminum in acidic environment Citation26 Citation27, Vigna unguiculata for aluminum in acidic and alkaline media Citation28, exudates gums from Pachylobus edulis Citation29 and Dacrodyes edulis Citation30 for aluminum in HCl. The encouraging results obtained from previous studies and as part of our contribution to the growing interest on environmentally friendly corrosion inhibitors, the present work reports on the corrosion inhibitive properties of Ananas sativum for aluminum corrosion in acidic solutions using weight loss and hydrogen evolution methods at 303–333 K. A. sativum is a tropical fruit common in Nigeria and other African countries. The leaf of the plant is biodegradable and a renewable material.
Results and discussion
Corrosion inhibitive effect by Ananas sativum extract
The general mechanism for the corrosion of Al in acidic medium has been reported by Nugen and Foley as follows Citation31:
The corrosion of aluminum in 0.1 M HCl in the absence and presence of different concentrations of A. sativum in the temperature range 303–333 K was studied using weight loss technique. shows plots of weight loss against time for Al corrosion in the blank (0.1 M HCl) and in the presence of different concentrations of A. sativum extract at (a) 303 and (b) 333 K corresponding to the lowest and highest temperatures studied, respectively. From the plot, it is seen that at a given temperature the weight loss of Al increases as the period of immersion increased both in the absence and presence of the inhibitor indicating that the rate of corrosion of Al increases with time. Also the weight loss of Al is observed to increase with increase in temperature both in the blank solution and in the solution containing different concentrations of A. sativum. These observations imply that the corrosion of Al in HCl is influenced by immersion time, concentration of A. sativum and temperature.
Figure 1. Variation of weight loss against time for aluminum corrosion in 0.1 M HCl in the presence of different concentrations of extract at (a) 303 and (b) 333 K.
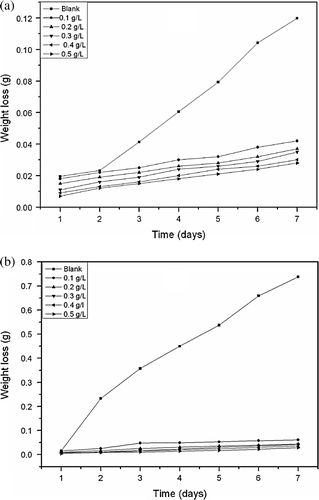
From the plot, weight loss for systems containing A. sativum was found to be lower compared to the blank indicating that different concentrations of A. sativum retard the corrosion of Al in 0.1 M HCl. The figure reveals that the inhibitor (plant extract) actually inhibited the HCl induced corrosion of aluminum to an appreciable extent. The figure also reveals that aluminum corrosion by HCl occurs not by simple homogenous process but by a heterogeneous one consisting of different or same rates. This assertion is made from the non-uniformity or non-linearity of the plots obtained Citation32.
The calculated values of corrosion rate (mpy) and inhibition efficiency (I%) for aluminum corrosion in 0.1 M HCl in the presence of different concentration of the plant extract from the weight loss measurements are shown in . Results presented in the table indicate that corrosion rate of aluminum in the acid medium was reduced in the presence of A. sativum extract compared to the blank solution. This is an indication that the extract inhibited acid induced corrosion of aluminum. It is also seen in the table that corrosion rate of Al in the presence of the extract decreases with increase in the extract concentration. Corrosion rate is also observed to increase with rise in temperature. Inhibition efficiency values were found to increase with increase in concentration of A. sativum and temperature. Maximum inhibition efficiency of 96.09% was obtained with extract concentration of 0.5 g/L at 333 K. Increase in inhibition efficiency with increase in temperature is suggestive of chemisorption of the A. sativum components onto the aluminum surface.
Table 1. Calculated values of corrosion rates (mpy) and inhibition efficiency (I%) for aluminum corrosion in 0.1 M HCl in the absence and presence of different concentrations of extract at different temperatures from weight loss measurements.
Inhibition of Al corrosion in 0.1 M HCl in the presence of the crude extract could be attributed to the adsorption of the phytochemical constituents of the extract on the aluminum surface which creates a barrier isolating the metal from the aggressive anions present in solution thus reducing the corrosion process. The results of the preliminary phytochemical screening reveal the crude extract contain alkaloids, tannins, cardiac glycosides, and saponnins. These substances contain oxygen and nitrogen atoms in their molecules which are regarded as centers of adsorption. The adsorption of these compounds on the aluminum surface makes a barrier for mass and charge transfers. Consequently, the metal is protected from the aggressive anions of the acid. The degree of protection increases with increase in extract concentration due to higher degree of surface coverage resulting from enhanced inhibitor adsorption. One of the major drawbacks of the use of plant extract as corrosion inhibitors is the inability to pinpoint the major active component that is responsible for the inhibiting action owing to the complex chemical composition of the crude extract. However, further investigation and the use of surface analytical techniques will enable the characterization of the active materials in the adsorbed layer and assist in identifying the most active ingredients which is currently being pursued in our laboratory.
Hydrogen evolution method
The inhibitive effect of A. sativum ethanolic extract on the corrosion of aluminum in HCl at a temperature of 303 K was also investigated using hydrogen evolution technique. This technique provides a rapid and sensitive means for gauging any interruption by an inhibitor with regard to gas evolution at the metal-corrodent interphase. Results obtained by this technique are corroborated by other well-established methods including weight loss and thermometry Citation11 Citation33, potentiostatic polarization Citation34, and impedance spectroscopy Citation35. is a typical plot showing the variation of volume of H2 gas evolved with time for 2 M HCl without and with different concentrations of the inhibitor (extract) at 303 K. The plots reveal slow rates of hydrogen evolution at the start of the reaction and after an incubation period, which correspond to the time interval needed by the corrodent to break down the pre-immersion oxide film on the aluminum surface, the volume of evolved H2 gas varies linearly with reaction time. It is also observed from the plots that the volume of hydrogen evolved was reduced on introduction of the extract onto the blank corrodent compared to the free acid and further decreases as the concentration of the extract increases.
Figure 2. Variation of volume of H2 evolved against time for aluminum, corrosion in 2 M HCl in the absence and presence of different concentrations of extract at 303 K.
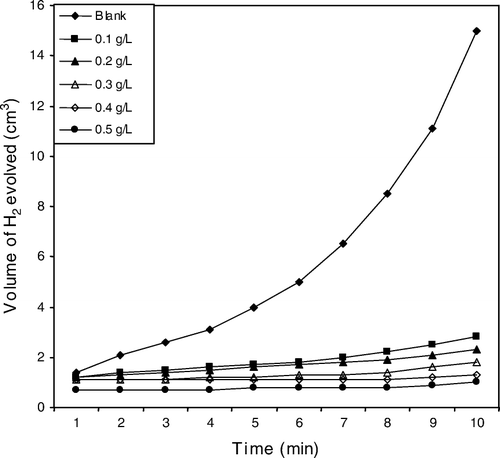
Corrosion rate and inhibition efficiency values for aluminum in 2 M HCl in the absence and presence of varying concentrations of the extract at 303 K from the hydrogen evolution measurements are given in . From the table, it is seen that the value of corrosion rate was high in the blank acid solution in the absence of the inhibitor. Addition of the plant extract to the acid solution led to a substantial reduction in the corrosion rate of aluminum and was found to decrease as the extract concentration increases. Inhibition efficiency and surface coverage increases with increase in concentration of the extract. Similar trend was observed for the weight loss techniques also employed in the present study.
Table 2. Calculated values of corrosion rate and inhibition efficiency for aluminum corrosion in 2 M HCl containing different concentrations of extract at 303 K from hydrogen evolution measurements.
Adsorption studies
One possible mechanism for corrosion inhibition using organic compounds is the adsorption of the inhibitor which blocks the metal surface and thus do not permit the corrosion to take place. The adsorption provides the information about the interaction among the adsorbed molecules themselves as well as their interaction with the metal surface Citation36. Two main types of interaction can describe the adsorption of organic compounds namely: physical adsorption and chemical adsorption. These are dependent on the electronic structure of the metal, the nature of the electrolyte, and the chemical structure of the inhibitor. Adsorption isotherms are very important to understand the mechanism of heterogeneous organo-electrochemical reactions Citation37 involving solid surfaces. In discussing adsorption isotherms, the degree of surface coverage (θ) is very useful. The degree of surface coverage values for different concentrations of A. sativum from the weight loss measurements obtained using θ=I%/100 assuming a direct relationship between surface coverage and inhibition efficiency were fitted into the most frequently used adsorption isotherms namely: Temkin, Frumkin, Langmuir, Freundlich, Hill de Boer, Parsons, Flory Huggins, Dhar-Flory Huggins, and Bockris Swinkles; and the correlation coefficients (R 2) were used to determine the best fitted isotherm. All of these isotherms are of the general form Citation38:
The usefulness of θ values in explaining the best isotherm to determining the adsorption process has shown that the experimental data obtained in this study fits well into Langmuir adsorption isotherm formulated as:
where θ is the degree of surface coverage, K
ads is the equilibrium constant of adsorption process, and is the free energy of adsorption values. Plot of C/θ against C as shown in gave straight lines which clearly show that aluminum corrosion inhibition in HCl by A. sativum extract at the temperatures studied obeys Langmuir adsorption isotherm. The correlation coefficient, slopes, and adsorption coefficients obtained from Langmuir isotherm plots are shown in . The values of correlation coefficient as shown in the table are quite good; an indication that the experimental data obtained in this study and hence adsorption of A. sativum extracts onto aluminum surface follows Langmuir isotherm. The values of K revealed that adsorption coefficient increases with increase in temperature. This is an indication that adsorption and hence inhibition efficiency increases with rise in temperature. Generally, K denotes the strength between adsorbate and adsorbent. Large values of K imply more efficient adsorption and hence better inhibition efficiency Citation39. Higher value of K at 333 K implies that more A. sativum extract was adsorbed onto aluminum surface leading to greater surface coverage and hence better protection efficiency than at 303 K. The Langmuir isotherm characterizes chemisorption of the adsorbed species and postulates monolayer adsorption of the adsorbate onto the adsorbent which is expected to have a slope of unity. This is clearly demonstrated in this study. The slope of unity obtained in this study is an indication that the adsorption of the extract components is approximated by Langmuir adsorption isotherm and that monolayer of the inhibitor species must have been attached to aluminum surface without lateral interaction between the adsorbed species Citation40.
Figure 3. Langmuir adsorption isotherm plot for aluminum corrosion in 0.1 M HCl for A. sativum extract at different temperatures.
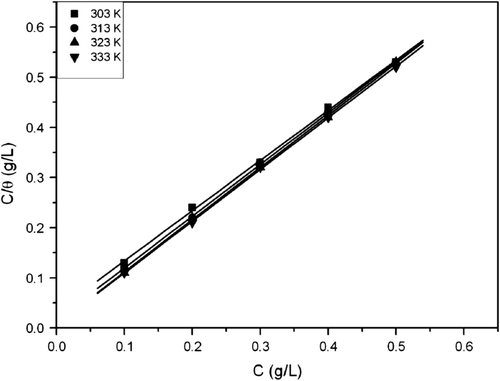
Table 3. Some parameters of linear regression from Langmuir isotherms plot for aluminum corrosion in 0.1 M HCl containing extract of A. sativum.
The free energy of adsorption values, , was obtained from Equation (Equation3). The values obtained are presented in . Results presented in the table indicate that the values of
are negative in all cases and ranged between −18.64 and −24.86 kJ/mol. The negative values indicate a spontaneous adsorption of the inhibitor molecules. The values of
are below −40 kJ/mol which indicate that the inhibitor function by physically adsorbing on the surface of the metal. Generally, values of
up to −20 kJ/mol are consistent with electrostatic interaction between charged molecules and a charged metal (which indicates physical adsorption) while those more negative than −40 kJ/mol involves charge sharing or transfer from the inhibitor molecules to the metal surface to form a co-ordinate type of bond (which indicates chemisorption) Citation32. Physical adsorption is a result of electrostatic attraction between charged metal surface and charged species in the bulk of the solution. Adsorption of negatively charged species is facilitated if the metal is positively charged. Positively charged species can also protect the positively charged metal surface acting with a negatively charged intermediate such as acid anions adsorbed on the metal surface.
Results of the present study have shown that A. sativum extract inhibits the acid induced corrosion of aluminum by virtue of adsorption of its components onto the metal surface. The inhibition process is a function of the metal, inhibitor concentration, and temperature as well as inhibitor adsorption abilities which is so much dependent on the number of adsorption sites. The mode of adsorption (physiosorption and chemisorption) observed could be attributed to the fact that A. sativum contains many different chemical compounds which some can adsorbed chemically and others adsorbed physically. This observation may be attributed to the fact that adsorbed organic molecules can influence the behavior of electrochemical reactions involved in corrosion processes in several ways. The action of organic inhibitors depends on the type of interactions between the substance and the metallic surface. The interactions can bring about a change either in electrochemical mechanism or in the surface available for the processes Citation41.
Effect of temperature
Temperature plays an important role on metal dissolution. The corrosion rate in acid solution, for example, increases exponentially with temperature increase because the hydrogen evolution overpotential increases Citation42. In order to access the effect of temperature on the corrosion and corrosion inhibition process, weight loss experiments were carried out in the temperature range 303–333 K in 0.1 M HCl in the absence and presence of different concentrations (0.1–0.5 g/L) of A. sativum extract. It was found that after 168 h immersion period, the corrosion rate in both uninhibited and inhibited acids increases with rise in temperature (). Inhibition efficiency was also found to increase with increase in temperature at all the concentrations of the extract studied. An Arrhenius-type dependence is observed between corrosion rate and temperature often expressed as:
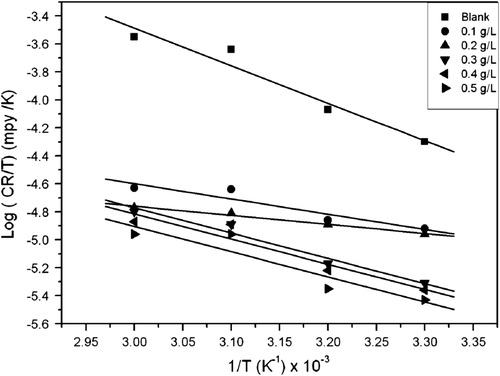
depicts an Arrhenius plot (logarithm of CR against the reciprocal of temperature (1/T)) for aluminum in 0.1 M HCl solution in the absence and presence of different extract concentrations. Satisfactory straight lines of high correlation coefficients were obtained. The values of activation energy were obtained from the slopes of the linear plots and are given in . It is clear that E a values in the presence of the different concentrations of the extract are lower than in their absence. The decrease in apparent activation energy in the presence of the extract denotes chemical adsorption while the reverse is usually attributed to physical adsorption Citation43. This conclusion is denoted by the increase in inhibition efficiency with increasing temperature (). Similar result has been reported by Okafor et al. Citation44 on the inhibition of acid corrosion of carbon steel using aqueous extract of P. amarus seeds and leaves. Moreover, the increase in activation energy is proportional to the inhibitor concentration, indicating that the energy barrier for the corrosion process is also increased Citation45.
Figure 4. Arrhenius plot for aluminum corrosion in 0.1 M HCl for blank and different concentrations of A. sativum.
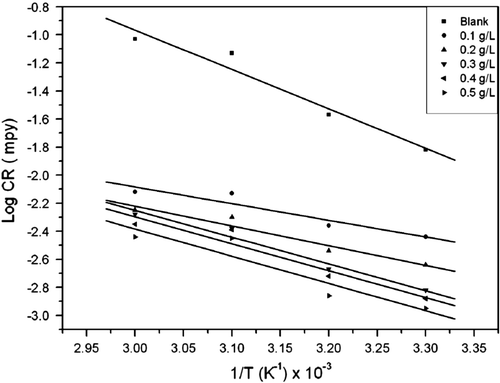
Table 4. Calculated values of activation parameters for aluminum corrosion in 0.1 M HCl in the absence and presence different concentrations of A. sativum extract.
An alternative formulation of Arrhenius equation is Citation46:
Experimental
Materials
Materials used for the study were Al sheets of type AA1060 and purity 98% obtained from System Metal Industries Calabar, Nigeria. The sheet was mechanically pressed cut into coupons of dimensions 5×4×0.08 cm. Each coupon was degreased by washing with absolute ethanol, dried in acetone, and preserved in a desiccator prior to use in corrosion testing. All reagents used for the study were Analar grade and were used as received without further purification. Double distilled water was used for the preparation of all reagents.
Preparation of extract of Ananas sativum
Samples of A. sativum were sun-dried and ground to fine powder. About 1.5 kg of the powdered sample was extracted using 80% ethanol for 72 h. The extract was concentrated initially using vacuum evaporator and finally by evaporation to dryness on a steam bath to obtain a solid extract devoid of ethanol. From the solid extract, different concentrations (0.1–0.5 g/L) were weighed and dissolved in 0.1 M HCl solution for weight loss measurements and in 2 M HCl for hydrogen evolution measurements.
Weight loss measurements
Weight loss measurement was carried out following the procedures reported elsewhere Citation25–30. The volume of test solution was kept at 100 mL. After 24 h of immersion, each coupon was retrieved from the solution, washed in solution of 70% HNO3, dried in acetone, and weighed. The samples were subsequently returned to their respective test solutions and the procedure repeated progressively for 168 h (7 days). The weight loss was taken as the difference between the weight at a given time and the original weight of the coupons.
From weight loss measurements, inhibition efficiency (I%), and corrosion rates (CR) were calculated using Equations (Equation11) and (Equation12), respectively:
where W 0 and W 1 are the initial and final weights of the coupon, respectively. W=(W 0–W 1) is the weight loss (mg), ρ is the density of the coupon (g/cm3), A is the area of the specimen (in2), and t is the exposure time (h).
Hydrogen evolution method
The apparatus and procedure used for hydrogen evolution method was similar to that described in literature Citation15 Citation24. The test solution was kept at 100 mL. The progress of the corrosion reaction was monitored by careful measurement of the volume of hydrogen gas evolved at fixed time intervals. From the volume of hydrogen gas evolved per minute, inhibition efficiency (I%) was calculated using Equation (Equation13):
where is the volume of hydrogen evolved at time t for inhibited solution and
is the volume of hydrogen evolved at time t for uninhibited solution.
Conclusion
On the basis of this study the following conclusions can be drawn:
-
The crude extract of A. sativum acts as inhibitor for aluminum corrosion in acidic medium.
-
Inhibition efficiency of the extract increases with increase in concentration of the inhibitor and also with increase in temperature.
-
The corrosion inhibition is probably due to the adsorption of the phytochemical constituents of the extract on the metal surface and blocking its active sites by phenomenon of chemical adsorption.
-
The extract was found to obey Langmuir adsorption isotherm from the fit of the experimental data at all the concentrations and temperatures studied.
-
The values of E a obtained in the presence of the extract were lower compared to the blank acid solution which further support the chemical adsorption proposed.
-
The values of
obtained are low and negative, which reveals the spontaneity of the adsorption process.
References
- Khaled , K.F. ; Al-Qahtani , M.M. Mater. Chem. Phys . 2009 , 113 , 150 158 .
- Amin , M.A. ; Mohsen , Q. ; Hazzaci , O.A. Mater. Chem. Phys . 2009 , 114 , 908 914 .
- Abdel-Rehim , S.S. ; Hassan , H.H. ; Amin , M.A. Appl. Surf. Sci . 2002 , 187 , 279 290 .
- Cao , G. ; Liang , C. J. Electrochem. Soc . 2007 , 154 , C144 C151 .
- Aljourani , J. ; Raeissi , K. ; Golozar , M.A. Corros. Sci . 2009 , 51 , 1836 1843 .
- Khaled , K.F. Mater. Chem. Phys . 2009 , 112 , 290 300 .
- Jain , T. ; Chowdhary , R. ; Mathur , S.P. Mater. Corros . 2006 , 57 , 422 426 .
- Priya , S.L.A. ; Chitra , A.A. ; Rajendran , S.A. ; Anuradha , K.B. Surf. Eng . 2005 , 21 , 229 231 .
- Jain , T. ; Chowdhary , R. ; Arora , P. ; Mathur , S.P. Bull. Electrochem . 2005 , 21 , 23 27 .
- Ebenso , E.E. ; Ekpe , U.J. ; Ibok , U.J. ; Umoren , S.A. ; Jackson , E. ; Oforka , N.C. ; Abiola , O.K. ; Martinez , S. Trans. SAEST 2004 , 39 , 117 123 .
- El-Etre , A.Y. Corros. Sci . 2003 , 45 , 2485 2495 .
- Davis , G.D. ; Von Fraunhofer , J.A. Mater. Perform . 2003 , 42 , 56 60 .
- Kliskic , M. ; Radosevic , J. ; Gudic , S. ; Katalinic , V. J. Appl. Electrochem . 2000 , 30 , 823 830 .
- Oguzie , E.E. ; Onouchuckwu , A.I. ; Okafor , P.C. ; Ebenso , E.E. Pigm. Resin Technol . 2006 , 35 , 63 70 .
- Oguzie , E.E. Corros. Sci . 2007 , 49 , 1527 1539 .
- Abiola , O.K. ; Otaigbe , J.O.E. ; Kio , O.J. Corros. Sci . 2009 , 51 , 1879 1881 .
- Abiola , O.K. ; Otaigbe , J.O.E. Corros. Sci . 2009 , 51 , 2790 2793 .
- Abiola , O.K. ; Oforka , N.C. ; Ebenso , E.E. ; Nwinuka , N.M. Anti-Corros. Methods Mater . 2007 , 54 , 219 224 .
- El-Hosary , A.A. ; Saleh , R.M. ; Shams El Din , A.M. Corros. Sci . 1972 , 12 , 879 904 .
- Noor , E.A. J. Appl. Electrochem. Sci . 2009 , 39 , 1465 1475 .
- Abdel-Gaber , A.M. ; Khamis , E. ; Abo-ElDahab , H. ; Abdeel , S. Mater. Chem. Phys . 2008 , 109 , 297 305 .
- Obot , I.B. ; Obi-Egbedi , N.O. Portug. Electrochim. Acta 2009 , 27 , 517 524 .
- Saleh , R.M. ; Ismail , A.A. ; El-Hosary , A.A. Corros. Sci . 1983 , 23 , 1239 1241 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Okafor , P.C. ; Ogbode , O. ; Oguzie , E.E. Anti-Corros. Methods Mater . 2006 , 53 , 277 282 .
- Umoren , S.A. Cellulose 2008 , 15 , 751 761 .
- Umoren , S.A. ; Obot , I.B. ; Obi-Egbedi , N.O. J. Mater. Sci . 2009 , 44 , 274 279 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Obi-Egbedi , N.O. Desalination 2009 , 247 , 561 572 .
- Umoren , S.A. ; Obot , I.B. ; Akpabio , L.E. ; Etuk , S.E. Pigm. Resin Technol . 2008 , 37 , 98 105 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Okafor , P.C. Portug. Electrochim. Acta 2008 , 26 , 267 282 .
- Umoren , S.A. ; Obot , I.B. ; Ebenso , E.E. ; Obi-Egbedi , N.O. Portug. Electrochim. Acta 2008 , 26 , 199 209 .
- Nguyen , T.H. ; Foley , R.T. J. Electrochem. Soc . 1982 , 129 , 27 32 .
- Bhajiwala , H.M. ; Vashi , R.T. Bull. Electrochem . 2001 , 17 , 441 448 .
- Mousa , M.N. ; Fouda , A.S. ; Taha , F.I. ; Elnenna , A. Bull. Korean Chem. Soc . 1988 , 9 , 191 195 .
- Abdallah , M. Portug. Electrochim. Acta 2004 , 22 , 161 175 .
- Aytac , A. ; Oxmen , U. ; Kabasakaloglu , M. Mater. Chem. Phys . 2005 , 89 , 176 181 .
- Damaskin , B.B. ; Petrii , O.A. ; Batrakov , V.V. Adsorption of Organic Compound on Electrodes ; New York : Plenum , 1971 .
- Bockris , J.O.M. ; Khan , S.U.M. ; Surface Electrochemistry: A Molecular Level Approach ; New York : Plenum , 1993 .
- Karakus , M. ; Sahin , M. ; Bilgic , S. Mater. Chem. Phys . 2005 , 92 , 565 571 .
- Refaey , S.A.M. ; Taha , F. ; Abd-E1-Malek , M. Appl. Surf. Sci . 2004 , 236 , 175 181 .
- Noor , E.A. Int. J. Electrochem. Sci . 2007 , 3 , 996 1017 .
- Aramaki , K. ; Hackermann , N. J. Electrochem. Soc . 1969 , 116 , 568 571 .
- de Souza , F.S. ; Spinelli , A. Corros. Sci . 2009 , 51 , 642 649 .
- Awad , M.I. J. Appl. Electrochem . 2006 , 36 , 1163 1168 .
- Okafor , P.C. ; Ikpi , M.E. ; Uwah , I.E. ; Ebenso , E.E. ; Ekpe , U.J. ; Umoren , S.A. Corros. Sci . 2008 , 50 , 2310 .
- Popova , A. ; Sokolova , E. ; Raicheva , S. ; Christov , M. Corros. Sci . 2003 , 45 , 33 58 .
- Fouda , A.S. ; Al-Sarawy , A.A. ; Ahmed , F.S. ; El-Abbasy , H.M. Corros. Sci . 2009 , 51 , 485 492 .