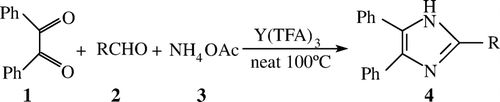
Abstract
Yttrium(III) trifluoroacetate is used as efficient catalyst for reaction of benzil, aldehydes, and ammonium acetate under mild and solvent-free conditions to afford the corresponding 2,4,5-triarylimidazoles in high yields and short reaction time. Furthermore, the catalyst can be recovered conveniently and reused several times in the reaction without a significant loss of catalytic activity.
Introduction
Triarylimidazole derivatives have many pharmaceutical activities and play important roles in biochemical processes Citation1. Some substituted triarylimidazoles are selective antagonists of the glucagon receptor Citation2 and inhibitors of IL-1 biosynthesis Citation3. There are several methods for the synthesis of substituted imidazoles Citation4. The reaction of aldehydes, benzil, and ammonium acetate with low yields after many hours refluxing in HOAc was the classical route to synthesize imidazoles Citation5. Later, the procedure of three components condensation of benzil, benzaldehyde derivatives, and ammonium acetate was frequently employed. In this procedure, [HeMIM]BF4 Citation6, Eu(OTf)3 Citation7, Keggin-type heteropolyacid Citation8, HClO4–SiO2 Citation9, iodine Citation10, zeolite HY and silica gel Citation11, silica sulfuric acid Citation12, NiCl2·6H2O Citation13, and Yb(OTf)3 Citation14 were used as catalysts to synthesize triarylimidazoles. Moreover, the synthesis of these triarylimidazoles has been usually carried out in the presence of microwave irradiation Citation15–18. But some of the synthesis methods suffer from one or more disadvantages to a large extent, such as harsh reaction conditions, poor yields, longer reaction times, and the use of hazardous and often expensive acid catalysts. Thus, the development of a simple and efficient method for the preparation of 2,4,5-triarylimidazoles is an active area of research with a scope for further improvements toward milder reaction conditions and higher product yields.
In recent times, the use of trifluoroacetates has received considerable attention as inexpensive, non-toxic, and readily available Lewis acid catalysts for various organic transformations. Trifluoroacetates such as Cu(TFA)2, Ag(TFA), Ni(TFA)2 Citation19, Fe(TFA)3 Citation20, and Bi(TFA)3 Citation21 were utilized as powerful catalysts to synthesize multifarious compounds. Our research group has successfully synthesized 3,4-dihydropyrimidin-2(1H)-ones and α,α′-bis(substituted benzylidene)cycloalkanones catalyzed by Cu(TFA)2 in high yields and short reaction times under solvent-free conditions Citation22 Citation23.
In this report, Yttrium(III) trifluoroacetate (Y(TFA)3) was employed as Lewis acid catalysts for the synthesis of 2,4,5-triarylimidazoles via solvent-free conditions ().
Results and discussion
Firstly, the reaction of benzaldehyde, benzil, and ammonium acetate was chosen as the model reaction to detect the catalytic activity of Y(TFA)3 and to investigate the optimized conditions. The reaction was carried out at different reaction temperatures (r.t., 70–100°C) and different reaction times (2–4 h). Furthermore, we changed the amount of catalyst from 1 to 5 mol%. Through thorough investigation, the best result in 97% yield was obtained by carrying out the reaction with 1:1:7 mole ratios of benzaldehyde, benzil, and ammonium acetate at 100°C and the dosage of 3 mol% catalysts for 3 h under solvent-free conditions.
Encouraged by these results, we have extended the methodology to a variety of aldehydes. Based on the optimum reaction conditions obtained above, the reactions of various aldehydes with benzil and ammonium acetate were investigated. The results are summarized in .
Table 1. Synthesis of 2,4,5-triarylimidazoles catalyzed by Y(TFA)3 under solvent-free conditions at 100°C for 3 h.
In the course of the study, the reactions proceeded completely to afford the corresponding 2,4,5-triarylimidazoles. We found that for aldehydes bearing either electron-releasing or electron withdrawing substituents in the meta or para positions, the reaction proceeded very efficiently in all cases. It seems that the effect of substituted groups on the aromatic aldehydes is not very strong. As expected in the case of propionaldehyde, as an aliphatic aldehydes, the desired product was obtained in lower yield (, 4j, 45%).
The recyclability of Y(TFA)3 was also investigated (, 4a). Of particular note was the fact that the Y(TFA)3 was very easy recovered quantitatively from the aqueous layers of the reaction mixture. It was reused in subsequent reactions and the yields of 2,4,5-triphenylimidazoles were 90%, 88%, 85%, and 80% in consecutive runs, respectively. Accord to the results above, Y(TFA)3 is efficient and reusable catalyst for the synthesis of 2,4,5-triarylimidazoles.
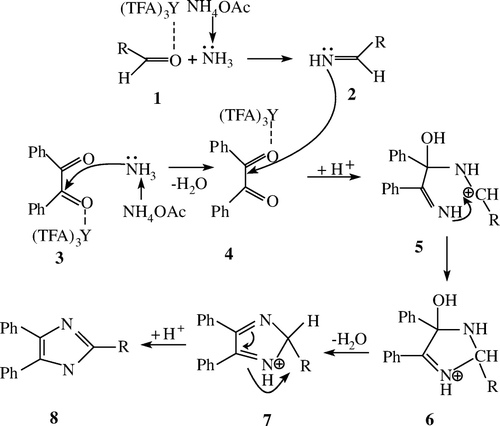
A probable mechanism for the synthesis of 2,4,5-triarylimidazoles may be presumed as shown below (). Ammonium acetate decomposes ammonia under the condition of heating which is the main source of nitrogen in the course of the reaction. Because of the strong withdrawing effect of trifluoroacetic group and the character of its metal cation, Y(TFA)3 is a strong Lewis acid catalyst. The carbonyl group can be activated to decrease the energy of transition state. It is highly probable that when the carbonyl oxygen is coordinated by Y(TFA)3, the carboxyl groups of benzyl 3 and aldehydes 1 can be activated. Then nucleophilic attack of the nitrogen of ammonia obtained from NH4OAc on the activated carbonyl group, produced the formation of aryl aldimine 4 and a-imino ketone 2, and it followed by the nucleophilic attack of the in situ generated imine 2 to carbonyl of aryl aldimine 4, obtaining the intermediate 5. Their subsequent intramolecular interaction results in cyclizations and eventually creates the formation of intermediate 6, which through the process of dehydration and dehydrogenation to afford the trisubstituted imidazoles 8.
Experimental
General
Melting points were measured on X-6 microscope melting points apparatus and uncorrected. IR spectra were measured on Spectrum GX spectrometer (KBr). 1H NMR spectra were recorded on Bruker AVANCE 600 spectrometer (600 MHz for 1H NMR), using CDCl3 as solvent and tetramethylsilane (TMS) as internal reference.
General procedure for preparation of Yttrium(III) trifluoroacetate (Y(TFA) 3)
Y(TFA)3 was prepared according to the reported procedure Citation24. Excessive Y2O3 was reacted with the trifluoroacetic acid. After completion of the reaction, the mixture was filtered, and then the filtrate was evaporated to dryness on water bath. The obtained crude product was recrystallized twice from water.
General procedure for preparation of 2,4,5-triarylimidazoles
Aldehyde (1 mmol), benzil (1 mmol), ammonium acetate (7 mmol), and Y(TFA)3 (0.03 mmol) were mixed. The mixture was stirred at 100°C for 3 h in 100 mL conical flask in water bath. When thin layer chromatography (TLC) indicated completion of the reaction, ethanol (10 mL) and de-ionized water (15 mL), was dumped into the conical flask and stirred for 10 min. The solid was filtered under suction, washed with ice-cold water, and dried to afford pure product. All products are known compounds, which were satisfactorily characterized by physical and spectra data.
2,4,5-Triphenylimidazole (4a)
Mp 278°C. IR (KBr): v=3413 (N–H), 3038 (C–H), 1596 (C=C), 1488 (C=N) cm–1. 1H NMR (600 MHz, CDCl3, TMS): δ=7.17–7.49 (m, 3C6H5), 12.69 (s, NH) ppm.
2-(4-Chlorophenyl)-4,5-diphenylimidazole (4b)
Mp 264–266°C. IR (KBr): v=3204 (N–H), 1613 (C=C), 1492 (C=N) cm–1. 1H NMR (600 MHz, CDCl3, TMS): δ=7.24–7.52 (m, 10H, Ph), 7.54 (d, 2H, J=10 Hz, C6H4Cl), 8.09 (d, 2H, J=10 Hz, C6H4), 12.77 (s, NH) ppm.
2-(2,4-Dichlorophenyl)-4,5-diphenylimidazole (4c)
Mp 174–176°C. IR (KBr): v=3439 (N–H), 3067 (C–H), 1594 (C=C), 1552 (C=N), 1101 (C–Cl) cm–1. 1H NMR (600 MHz, CDCl3, TMS): δ=12.7 (s, 1H), 7.78 (d, 1H), 7.83 (s, 1H), 7.23–7.58 (m, 12H) ppm.
2-(4-Hydroxyphenyl)-4,5-diphenylimidazole (4d)
Mp 262–264°C. IR (KBr): v=3630 (O–H), 3411 (N–H), 3058 (C–H), 1602 (C=C), 1485 (C=N) cm–1. 1H NMR (600 MHz, CDCl3, TMS): δ=7.51–7.86 (m, 10H), 6.99–7.04 (d, 2H), 7.87–7.90 (d, 2H), 12.61 (s, NH) ppm.
2-(4-Methoxyphenyl)-4,5-diphenylimidazole (4e)
Mp 230–232°C. IR (KBr): v=3409 (N–H), 3029 (C–H), 1613 (C=C), 1493 (C=N) cm–1. 1H NMR (600 MHz, CDCl3, TMS): δ=3.79 (s, OCH3), 7.03 (d, J=8.7 Hz, 2H), 7.33–7.51 (m, 10H, Ph), 8.00 (d, J=8.7Hz, 2H), 12.52 (s, NH) ppm.
Conclusion
In summary, we have developed a solvent-free procedure for the synthesis of 2,4,5-triarylimidazoles using Y(TFA)3 as a reusable catalyst. The mild conditions, short reaction time, inexpensive, readily availability of the catalyst, and simpler experimental procedure are the great advantages of the method. Thus, Y(TFA)3 could be an efficient, eco-friendly, and recyclable Lewis acid catalyst for the synthesis of 2,4,5-triarylimidazoles.
References
- Lombardino , J.G. ; Wiseman , E.H. J. Med. Chem . 1974 , 17 , 1182 1188 .
- Chang , L.L. ; Sidler , K.L. ; Cascieri , M.A. ; Laszlo , S. ; Koch , G. ; Li , B. ; MacCoss , M. ; Mantlo , N. ; O'Keefe , S. ; Pang , M. ; Rolando , A. ; Hagmann , W.K. Biorg. Med. Chem. Lett . 2001 , 11 , 2549 2553 .
- Gallagher , T.H. ; Fier-Thompson , S.M. ; Garigipati , R.S. ; Sorenson , M.E. ; Smietana , J.M. ; Lee , D. ; Bender , P.E. ; Lee , J.C. ; Laydon , J.T. ; Griswold , D.E. ; Chabot-Fletcher , M.C. ; Breton , J.J. ; Adams , J.L. Biorg. Med. Chem. Lett . 1995 , 5 , 1171 1176 .
- Tsuji , J. ; Sakai , K. ; Nemoto , H. ; Nagashima , H. J. Mol. Catal . 1983 , 18 , 169 176 .
- Sarshar , S. ; Siev , D. ; Mjalli , A.M.M. Tetrahed. Lett . 1996 , 37 , 835 838 .
- Xia , M. ; Lu , Y.D. J. Mol. Catal. A Chem . 2007 , 265 , 205 208 .
- Yu , C.M. ; Lei , M. ; Su , W.K. ; Xie , Y.Y. Synth. Commun . 2007 , 37 , 3301 3309 .
- Wolkenberg , S.E. ; Wisnoski , D.D. ; Leister , W.H. ; Wang , Y. ; Zhao , Z. ; Lindsley , C.W. Org. Lett . 2004 , 6 , 1453 1456 .
- Kantevari , S. ; Vuppalapati , S.V.N. ; Biradar , D.O. ; Nagarapu , L. J. Mol. Catal. A Chem . 2007 , 266 , 109 113 .
- Kidwaia , M. ; Mothsraa , P. ; Bansala , V. ; Somvanshib , R.K. ; Ethayathullab , A.S. ; Deyb , S. ; Singh , T.P. J. Mol. Catal. A Chem . 2007 , 265 , 177 182 .
- Balalaie , S. ; Arabanian , A. Monatsh. Chem . 2000 , 131 , 945 948 .
- Shaabani , A. ; Rahmati , A. J. Mol. Catal. A Chem . 2006 , 249 , 246 248 .
- Heravi , M.M. ; Bakhtiari , K. ; Taheri , H.A.S. J. Mol. Catal. A Chem . 2007 , 263 , 279 281 .
- Wang , L.M. ; Wang , Y.H. ; Tian , H. ; Yao , Y.F. ; Shao , J.H. ; Liu , B. J. Fluorine Chem . 2006 , 127 , 1570 1573 .
- Balalaie , S. ; Hashemi , M.M. ; Akhbari , M. Tetrahed. Lett . 2003 , 44 , 1709 1711 .
- Balalaie , S. ; Arabanian , A. Green Chem . 2000 , 2 , 274 276 .
- Usyatinsky , A.Y. ; Khemelnitsky , Y.L. Tetrahed. Lett . 2000 , 41 , 5031 5034 .
- Zhou , J.F. ; Song , Y.Z. ; Yang , Y.L. ; Zhu , Y.L. Synth. Commun . 2005 , 35 , 1369 1373 .
- Romano , A.M. ; Ricci , M. J. Mol. Catal. A Chem . 1997 , 120 , 71 74 .
- Adibi , H. ; Samimi , H.A. ; Beygzadeh , M. Catal. Comm . 2007 , 8 , 2119 2124
- Khosropour , A.R. ; Khodaei , M.M. ; Ghozati , K. Russ. J. Org. Chem . 2004 , 40 , 1332 1336 .
- Song , D.L. ; Wang , R.X. ; Chen , Y.L. ; Zhang , S.H. ; Liu , C.S. ; Luo , G. React. Kinet. Catal. Lett . 2008 , 95 , 385 390 .
- Song , D.L. ; Chen , Y.L. ; Wang , R.X. ; Liu , C.S. ; Jiang , H. ; Luo , G. Prep. Biochem. Biotechnol . 2009 , 39 , 201 207 .
- Yanagihara , N. ; Gotoh , T. ; Ogura , T. Polyhedron 1996 , 15 , 4349 4354 .
- Kidwai , M. ; Ruby , S. ; Rastogi , S. Bull. Korean Chem. Soc . 2005 , 26 , 2051 2053 .
- Kidwai , M. ; Mothsra , P. ; Bansal , V. ; Somvanshi , R.K. ; Ethayathulla , A.S. ; Dey , S. ; Singh , T.P. J. Mol. Catal. A Chem . 2007 , 265 , 177 182 .
- Shaabani , A. ; Rahmati , A. ; Aghaaliakbari , B. ; Safaei-Ghomi , J. Synth. Commun . 2006 , 36 , 65 70 .