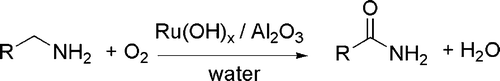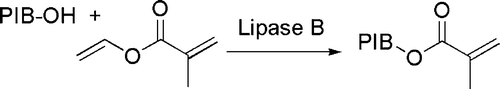Abstract
The literature of green chemistry has undergone a dramatic increase in the new millennium. Besides that, in ad hoc journals, papers of this type are published in journals of general, organic, and catalytic chemistry. The high proportion of communications within this area indicates that this is a hot topic. These reports mainly concern more environment-friendly synthetic methods, based on better catalytic systems, less harmful solvents and, more rarely, “alternative” physical techniques. Although the compliance with the green chemistry postulates is still partial, a trend in this direction is recognizable. For example, the number of preparative papers that introduce an environmental assessment is rapidly increasing.
Introduction
The key target of green or sustainable chemistry is making available to mankind useful compounds and materials, while causing no harm to the environment. This approach has acquired a central role in present day's chemistry, although the first embryo has long been present in the literature. A century ago, when chemical industry was just beginning its development on a large scale, a particularly clear-sighted scientist, G. Ciamician, observed that it was now possible to synthesize products identical to the natural ones. However, this was done in the laboratory by using harsh conditions and excess energy. The actual advancement, he meant, would be obtained when men would learn to run chemical reactions in the mild way nature does and would develop an environment-friendly chemistry Citation1.
Such concepts were much in advance of their time and embodied Citation2 most of the “12 Principles” of green chemistry as formulated by Anastas and Warner Citation3–5 at the end of the twentieth century. Nowadays, the increasing concern for a sustainable development and the perception of chemical industry as one of the most harmful human activity have created the conditions for the birth of green chemistry (based on the above principles) as an independent discipline.
The development of green chemistry
As mentioned above, sustainable chemistry in its mature form can be considered an established discipline since about 10 years. Thus, it could be worthwhile analyzing its development and understanding in which direction(s) it is moving, when entering the second decade of the twenty-first century. Reviewing the state of art of green chemistry has become impossible, since this topic is too extensive. What is attempted below, is offering a review of which topics can be classified within the field of green chemistry and correspond to the key concepts of this discipline. The overview compares what is expected from green chemistry on one hand, and in which way chemists answer this expectation, on the other one. As for the first issue, the principles by Anastas and Warner Citation3–5, which have been variously rephrased Citation6, express the main challenge in sustainable chemistry (and chemical engineering). These urge chemists to use alternative feedstocks, to avoid hazardous reagents or products and to minimize waste.
Green chemistry involves an interdisciplinary effort, guided by the principle “benign by design” Citation7–10. This requires to introduce the desirable aspects as early as possible when planning a process and to consider the whole lifecycle of the product, possibly by adopting a “cradle to cradle” approach. It is no more acceptable that “a new molecule is designed or a new process is discovered” Citation11 and then in separate steps the optimization of the synthesis and application are carried out. Rather, the chemical and the engineering aspects must be fused from the start Citation11–16 and the environmental aspects quantitatively assessed likewise at an early stage Citation17–20, giving priority to new processes that couple efficiency and protection of the environment. The targets are those indicated by the above mentioned 12 principles Citation3–6 or in related statements such as those due to Clark Citation21 and Sheldon Citation22. These require an enhanced incorporation of the reagent atoms in the product, the use of catalytic rather than stoichiometric reagents as well as devising new synthetic pathways, using less harmful solvents and new purification methods, introducing intensive processes that are less harmful.
On top of that, the process must be economically sound, otherwise the fact of being environment-friendly is useless. Indeed, in many cases industry resists – or has resisted – the introduction of this approach fearing a cost increase. However, the awareness is growing that there is no alternative choice because of the social pressure and regulation introduced. Moreover, several cases demonstrate that economically satisfactory solutions are possible by introducing the “green” approach Citation23–30.
Less easy is judging what is green, or how green is a new procedure proposed. The “revolutionary” idea is refocusing research and development on a lower environmental impact and better sustainability, rather than merely on a higher yield. Arriving at solutions that are satisfactory under all of the aspects is clearly a demanding task, which is not always fulfilled by a single paper or in a patent that claims to contribute to green chemistry. Indeed, in many cases the assertion is based on a single aspect. Sometimes it is claimed that a new catalyst improves the yield of a process under mild conditions, but no attention is paid to check whether such catalyst is harmful to man or environment, too expensive, labile or difficult to obtain. Likewise, maintaining mild conditions requires the use of expensive (economically or environmentally) reagents or solvents or of a large amount of energy, so that industrial implementation is not viable.
As a matter of fact, the label “green chemistry” in its full meaning applies only to a fraction of the published literature reviewed under this heading (see further below), because the overall consideration of the process is missing. This notwithstanding, it may be worthwhile analyzing the papers published with this keyword, because these correspond to what chemists feel green chemistry is. This may be compared with what the chemical industry expects from this kind of work. After all, the role that chemistry will have in the future and the perspective that will be opened depend on the kind of research chemists will do, which remains in part curiosity driven.
Green chemistry in the industry
As for the demand from the industry, several accounts are available, some stressing out the use of a renewable feedstock Citation31, other ones process innovation Citation15 Citation27 Citation32. A notable contribution is a survey among industrial experts that has been carried out in 2004 Citation33. These experts were interviewed on what could be done for enhancing the sustainability of chemical industry in Europe. To the question, which tool would they support, they listed first of all Computer Aided Molecular Design (75% support) and then in rapidly decreasing preference, Integrated Product and Process Design 55, Process Synthesis 50, Industrial Symbiosis and Ecology 50, Lifetime Process Optimization (40), Process Integration (35), and others. The reasoning was clear, one had to choose the right product to make first. Then the integration between the different aspects of the product's design and the process leading to it should be ensured, instead of focusing on a single aspect (that is, a holistic approach had to be used) Citation34.
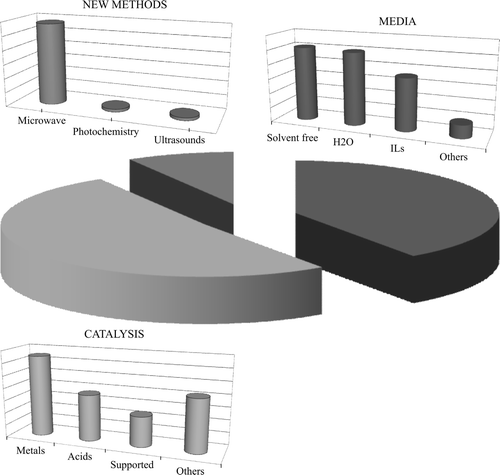
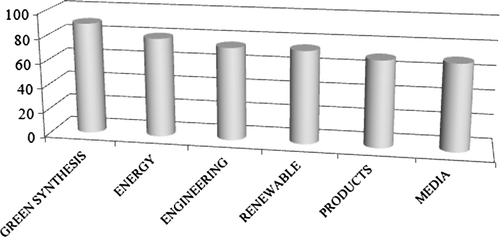
The best technology to use was the next question. Here the answers were more evenly distributed, with 10 items receiving between 65 and 90% support. It appears that these suggestions could be grouped in some large categories (see ). These were: a highly selective catalyzed synthesis (see category “green synthesis” in , 90% mean support); the use of green energy sources (80% mean support); the engineering aspects (both process intensification and economic small scale processes, 75% mean support); the use of a green feedstock (see category “renewable” in , 75% mean support); the reasoned (computer assisted) choice of the product, by taking into account all of the environmental aspects relative to the product itself and its synthesis (see category “products” in , 70% mean support); and finally, the choice of the medium and the purification technique (70% mean support).
Figure 1. Classification of the topics judged important for a green development by a panel of industry experts. Elaborated from data in Citation33.
As for the classes of reactions that require most urgently attention, a Round Table formed by experts from pharmaceutical industries Citation34 put forward five general processes that would have greatly beneficed from the introduction of green methods. These were: amide formation with good atom economy; activation of the hydroxyl group; reduction of amides avoiding hydrides; oxidation and epoxidation reactions in non-chlorinated solvents; and Mitsunobu reactions with a better atom economy. It does not appear for the moment that this statement has produced a significant redirection of the research toward such goals.
The literature on green chemistry
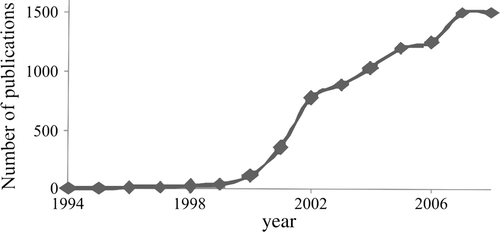
Since the 1990s, the increasing success of green chemistry has been indicated by the start of several series of well-attended international meetings, by the founding of interest groups or sections in most National Chemical Societies and by the introduction of journals aiming to cover the various aspects of this discipline. Among them, one may mention Green Chemistry (Royal Chemical Society, 1999), Green Chemistry Letters and Reviews (Taylor & Francis, 2007), and ChemSusChem (various European Chemical Societies and Wiley, 2008). Other journals are devoted to environmental safety and in turn include both long established titles, such as Environmental Science and Technology (American Chemical Society, 1967), Clean (Wiley, new name of Acta Hydrochimica et Hydrobiologica, 1973), and Environmental Impact Assessment Reviews (Elsevier, 1980), as well as new ones, such as Energy & Evironmental Sciences (by the Royal Chemical Society, 2008). Most importantly, the number of papers retrieved by Chemical Abstracts Footnote1 under the heading “green chemistry” (that is where these words are present in the abstract or as a keyword) has experienced a very rapid growth in the first few years of the new millennium and has now leveled well over 1000 publications per year (see ).
The large majority of the authors of such papers were from the academia. Taking into consideration those published in the decade 1999–2008, it has been examined in which journal this kind of research has been published. As shown in , by adding the first 18 bibliographic sources, one arrives at ca. 32% of the total number of published reports. Those published in Green Chemistry, a journal devoted to this topic that has been published over the entire decade 1999–2008, make by far the largest contribution (6.44%, 20% of the 18 most popular journals in the field). About a half of the papers in the other 17 sources have been published in journals of organic chemistry, ca. 14% in those of general chemistry and ca. 14% in those of catalysis. Notably, 1.21% of the total number of the references in this group are international patents and 10.46% Chinese patents. These are remarkable figures for this topic, sometimes considered of purely academic interest.
Table 1. Papers with the keyword “green chemistry” published in the decade 1999–2008. Journals most frequently chosen.
Thus, from the point of view of publications, green chemistry is essentially a sub-discipline of organic chemistry, with particular regard to catalytic reactions. However, there is also a clear identification of green chemistry as a discipline on its own, as indicated by the considerable fraction that is submitted to journals directly devoted to the topic (see for example, Green. Chem. and Int. J. Life Cycle Assess. in ).Footnote2 Another point worth notice is that almost a half of the considered reports (42%) is on journals reserved to rapid communications. This fact, along with the high rate of communications at major meetings, is an indication that this topic is highly regarded by the chemical community and advancement in this field is considered as a high priority.
The topics investigated were examined in more detail for the year 2008 in the above series of papers as well as in two further series including the papers published in that year in Green Chemistry and ChemSusChem (see (A)–(C); Green Chemistry Letters and Reviews is fully devoted to green synthesis).
Figure 3. Classification of the papers published in 2008: (a) in Green Chemistry; (b) in ChemSusChem; (c) classed as green chemistry, from any journal.
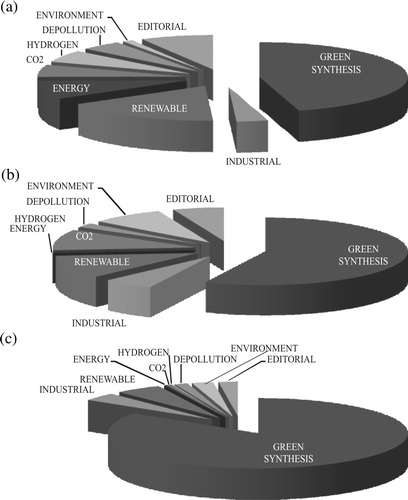
The topics are grouped in broad categories. It is apparent from the histograms that green synthesis is largely dominating, viz. about a half of the papers in the specialized journals and more than three-fourths in the general literature. In this category papers reporting “new” reactions or “new” conditions for a reaction have been grouped. This appears to be the main meaning that chemists (mostly from the academia) attach to the name “green chemistry,” whereas for the industry this represents only one of the questions, even though a quite important one (compare the share of “green synthesis” in and the support given to this topic in ). The experts from the industry attach a great importance to the optimization of the reaction course by taking care of the engineering aspects and of the products purification, topics less frequently addressed in the literature.
The use of renewable feedstocks receives a comparable attention both by the industrial panel and in specialized journals, less in the “general” literature. The same holds for the use of green sources of energy and related problems, such as solar light conversion and the generation and use of hydrogen, and for other environmental problems, including the CO2 cycle. These are more frequently addressed in Green Chemistry and ChemSusChem (and are deemed as quite important by the industry) than in papers with the keyword “green chemistry” published in journals of general interest.
Analysis of the investigated topics
A further refinement of the analysis reveals which innovative elements characterized the reactions assigned to the “green synthesis” category in the “general” 2008 literature (see ©). Three main fields can be recognized viz. “catalysis,” “reaction medium,” and “physical techniques” (in order of importance, see ). Some of the main directions of development within each of such fields are indicated below through some examples. The very limited choice is neither exhaustive nor even consistently representative. Further notice that quite often two labels are appropriate and in this case the paper has been considered in both groups.
Catalysis has been defined the “foundation pillar” of green chemistry. The better yields/increased selectivity/milder conditions that by definition characterize catalytic processes are of course equally qualifying for green chemistry Citation35, so that one may think that green chemistry is a section of catalytic chemistry. Homogeneous, heterogeneous, and supported (nano)catalysts Citation36 have all been applied for processes classified as “green.” In papers bearing this label usually the attention to the environmental characteristics is particularly high, with regard both to the catalyst operation (high turn-over number, reasonable stability, and limited cost) and to the reaction conditions (safety aspects).
Many of the reported catalyzed processes involve transition metals and their complexes. As an example, Ru complexes have been found to catalyze the hydrogenation of nitriles to primary amines with remarkable chemoselectivity. An effective catalyst is formed in situ from cheap starting materials, such as Ru(COD)(methylallyl2) and PPh3 Citation37. In another example, amides have been prepared from alcohols and amines by extrusion of dihydrogen in the presence of Ru(COD)Cl2, a N-heterocyclic carbene precursor and a phosphine Citation38. Another oxidation process leads from primary amines to amides in the presence of a supported ruthenium hydroxide catalyst Citation39 (see ).
Indeed, an important move toward green reactions is substituting abundant and cheap iron for scarce and not always environment-friendly noble metals in catalysis. This has been recently obtaining increasing interest and has been applied, e.g. for redox processes and for coupling reactions Citation40 Citation41. In the field of carbon–carbon bond forming reactions, iron catalyzed processes are becoming competitive, if compared to those using more expensive and toxic palladium- and nickel-based catalysts, with a significant economic and environmental advantage Citation42. Another quite active line of development involves the physical state of the active material, since catalysis by noble and transition metals is greatly improved when (supported) nanoparticles are used Citation36.
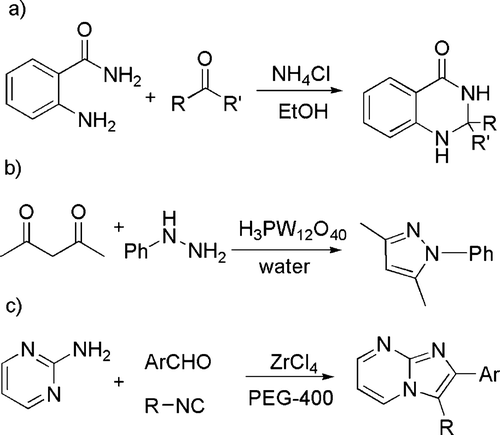
In another large group of reactions, the catalyst is a (Lewis) acid. Thus, several acid catalyzed condensation reactions that occur in high yields and short time qualify for the category of “click” chemistry (see ). An example is the condensation of 2-aminobenzamide with various alkyl, aryl, and alicyclic aldehydes or ketones to give 2,3-dihydroquinazolin-4(1H)-one derivatives (see (a)) in the presence of a catalytic amount of ammonium chloride in ethanol Citation43. Likewise, pyrazoles, diazepines, enaminones, and enamino esters have been smoothly obtained by the condensation of hydrazines (see (b)) or hydrazides, diamines or primary amines with 1,3-dicarbonyls. In the last case, 12-tungstophosphoric acid has been employed as a reusable catalyst in water Citation44. N-fused 2- and 3-aminoimidazoles have been prepared by means of a Ugi-type multi-component reaction mediated by zirconium(IV) chloride in polyethylene glycol-400 Citation45 (see (c)).
Nucleophilic substitution at acyl groups has been conveniently carried out in the presence of enzymes. A “green” application in the field of polymers is the transesterification of vinyl methacrylate by hydroxyl-terminated polyisobutylenes in the presence of Candida antarctica lipase B Citation46 (see ).
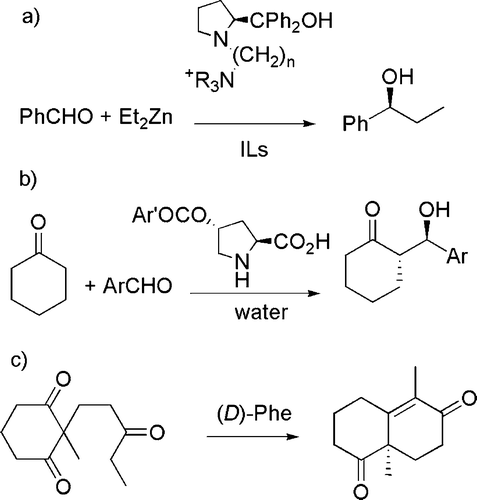
The use of chiral auxiliaries is another topic considered in this group, particularly when these are effectively recyclable. As an example, a ion-tagged prolinol has been found to induce the enantioselective alkylation of aldehydes by organometallic derivatives in ionic liquids and to be recyclable for more than 10 times Citation47 (see (a)). In a similar line, it was demonstrated that several 4-acyloxyproline derivatives are able to catalyze the direct asymmetric aldol condensation between cyclic ketones and benzaldehyde in water and can likewise be recycled Citation48 (see (b)). Analogously, an enantiomerically pure diketone (which can be used for the synthesis of diterpenes) has been prepared from cheap starting materials on a large scale (>125 g) Citation49 (see (c)).
Reactions carried out under solvent-free conditions or in an environment-friendly solvent form a large group. As an example, a tetranuclear zinc cluster, which is effective for the transesterification of a variety of methyl esters, in solution as well as under solvent-free conditions, has been prepared and tested up to the 100 g scale. In this case, the E factor (that is the mass ratio of waste to desired product) was low, 0.66 kg waste/kg product Citation50. Many processes affording highly stabilized products, such as condensation reactions leading to heteroaromatics, proceed more efficiently and have a wider scope when carried out in the absence of any solvent, particularly when induced by microwaves Citation51.
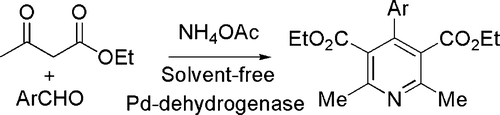
In a combined approach a condensation (Hanztsch) synthesis (with a strong acid as the catalyst) has been coupled with dehydrogenation (catalyzed by Pd dehydrogenase) for obtaining pyridines rather than dihydropyridines in a one-pot process (see ) Citation52.
Successful reactions under solvent-free conditions have often been obtained in the presence of supported catalysts, in most cases on (mesoporous) silica Citation53 Citation54.
Alternatively, shifting to water as the solvent is a positive move, as shown in many instances, including a method for the solid-phase synthesis of peptides that uses water-dispersible protected aminoacids Citation55. Ionic liquids are used fairly often Citation56–58, while only a limited attention has been devoted to purification methods, reasonably because this is a minor problem in small scale reactions.
As far as physical techniques are concerned, the use of “alternative” reaction conditions has been classified under this issue. Among the different approaches considered, microwaves are clearly the most largely used technique. Representative examples are the generation of ammonia from formamide (see (a), for the synthesis of N-heterocycles) Citation59, the nucleophilic substitution in aromatics Citation60, and the Wolff rearrangement of diazoketones (see (b)) Citation61.
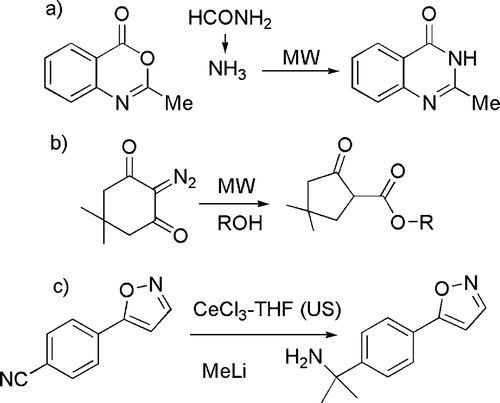
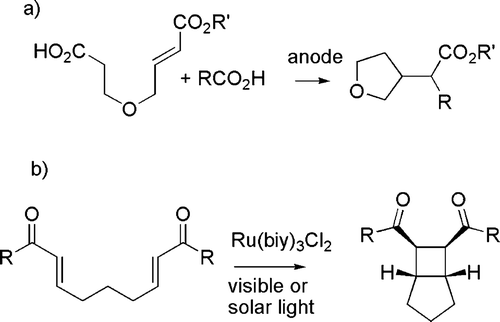
Ultrasounds have been less frequently used, but a number of reports are available ranging from the acceleration of a Knøvenhagel condensation Citation62 to the preparation of lanthanide complexes able to promote nucleophilic addition reactions (see c) Citation63. Different physical techniques such as photochemistry and electrochemistry have a minor role, although these are distinguished by the very mild conditions and, particularly in the former case, open reaction paths that have no thermal counterpart. Representative examples are the synthesis of five- and six-membered ring compounds by environmentally friendly radical cyclizations using Kolbe electrolysis (see (a)) Citation64 and the photochemical formation of cyclobutanes (see (b)) Citation65.
Other aspects are less extensively covered, as it appears in , but include important contributions on topics ranging from process intensification in chemical engineering Citation12 Citation66 to assess the role of wastewater treatment Citation3–5 Citation67 and of the reuse of biosolids Citation68. Most important is the assessment of the environmental quality both of chemical syntheses Citation69–71 and of the use of alternative sources of materials (e.g. solvents) Citation72 Citation73 and energy Citation74, as well as the role that regulations have on industrial chemistry Citation24.
Notice that a less frequent appearance of a topic in the present context does not mean that it is less investigated in absolute terms. This rather indicates that work in that area is not recognized as pertaining to green chemistry by the authors themselves, who have not inserted that label as keyword, or in the title or abstract.
Conclusion
Green chemistry is a multi-faceted discipline that has been created as a contribution of chemistry to sustainable development, avoiding damage to the environment. Obviously, the concern is greater in the industry, since social alarm and legislative limitation have a more direct impact in this case. Various panels from the industry have clearly outlined the directions that should be followed. Thus, important themes are not limited to the various aspects of synthesis and purification, but involve a more complete survey. The final aim is putting a new product on the market only after that this has been proven the (environmentally) correct decision. In order to open new perspectives, the use of renewable materials and energy must progress at the same time. The academic community appears to consider synthesis as the most important theme, more example than the environmental behavior of the synthesized material. For this reason, the postulates of green chemistry have permeated catalysis and, in part, organic chemistry. Reports of “new” syntheses are by far the main component of the literature identified as green chemistry. Nevertheless, many of the papers considered involve only partial results. These usually lack the early consideration of all the environmentally relevant aspects that should be peculiar of this discipline, as well as any attention to the engineering perspective of scaling up. This notwithstanding, one can certainly recognize a trend imparted by the popularity of green chemistry postulates. As a consequence, chemists pay a greater attention to the optimization of catalysis, to the elimination of toxic reagents and to the limitation or elimination of solvents (much less to the use of “alternative” physical methods). In addition, many preparative papers include an environmental assessment of the considered processes. If not immediately, in the long range this work will certainly contribute also to the industrial development.
Notes
1. Ca. 10,000 references retrieved by SciFinder Scholar® by using the keyword “green chemistry” for the decade 1999–2008, the same search by using ISI Web of Knowledge™, Google Scholar™, and Scirus (the last one limited to journals and patents) retrieves ca. 20,000, 15,000, and 4,000 entries, respectively.
2. ChemSusChem and Green Chemistry Letters and Reviews, which begun much later, have also a good share.
References
- (a) Ciamician , G. Bull. Soc. Chim. Fr. 4 1908 , 3 , i xxxvi (b) Ciamician G. Science 1912 36 385 394
- Albini , A. ; Fagnoni , M. ChemSusChem 2008 , 1 , 63 66 .
- Anastas , P.T. ; Warner , J.C. Green Chemistry: Theory and Practice ; Oxford : Oxford University Press , 1998 .
- Anastas , P.T. ; Zimmerman , J.B. Environ. Sci. Technol . 2003 , 37 , 94A 101A .
- Anastas , P.T. ; Beach , E.S. Green Chem. Lett. Rev . 2008 , 1 , 9 24 .
- Tang , S. ; Bourne , R. ; Smith , R. ; Polyakoff , M. Green Chem . 2008 , 10 , 268 269 .
- Warner , J.C. ; Cannon , A.S. ; Dye , K.M. Environ. Impact Assess. Rev . 2004 , 24 , 775 799 .
- McDonough , W. ; Braungart , M. ; Anastas , P.T. ; Zimmerman , J.B. Environ. Sci. Technol . 2003 , 37 , 434A 441A .
- Warner , J. Green Chem. Lett. Rev . 2007 , 1 , 1 2 .
- Anastas , P.T. Green Chem. Lett. Rev . 2007 , 1 , 3 4 .
- Anastas , P.T. Green Chem . 2008 , 10 , 607 .
- Dudukovic , M.P. Chem. Eng. Commun . 2009 , 196 , 252 266 .
- Doble , M. Chem. Eng. Prog . 2008 , 104 , 33 42 .
- Charpentier , J.C. Chimia 2002 , 56 , 119 125 .
- Hessel , V. ; Kralisch , D. ; Krtschil , U. Energy Environ. Sci . 2008 , 1 , 467 478 .
- Venzelaar , J. Chem. Eng. Technol . 2003 , 26 , 868 874 .
- Eissen , M. ; Mazur , R. ; Quebbemann , H.G. ; Pennemann , K.H. Helv. Chim. Acta 2004 , 87 , 524 535 .
- Smith , P. Clean Technology for the Manufacture of Speciality Chemicals ; Royal Society of Chemistry (Special Publication Cambridge) , 2001 ; Vol. 260 , p 24 .
- Augé , J. Green Chem . 2008 , 10 , 225 231 .
- Bare , J.C. ; Gloria , T.P. Environ. Sci. Technol . 2006 , 40 , 1104 1113 .
- Clark , J.H. Green Chem . 1999 , 1 , 1 8 .
- Sheldon , R.A. ; Arends , I. ; Hanefeld , U. Green Chemistry and Catalysis ; Weinheim : Wiley-VCH , 2007 .
- Koopmans , R.J. Soft Matt . 2006 , 2 , 537 543 .
- Wagemann , K. Green Chem . 2009 , 11 , 675 .
- Tucker , J.L. Org. Prep. Res. Dev . 2006 , 10 , 315 319 .
- Kulkarni , S.V. ; Chorghade , M.S. , Wiley : Hobboken, NJ , 2009 2 233 . Drug Discovery and Development .
- Kumar , R. ; Nagabhushana , K.S. ; Mal , N.K. Chem. Ind. Digest . 2009 , 22 , 135 138 .
- Tundo , P. ; Aricò , F. Chem. Int . 2007 , 29 , 5 .
- Mestres , R. Environ. Sci. Pollut. Res . 2005 , 12 , 128 132 .
- Lancaster , M. Educ. Chem . 2004 , 41 , 152 154 .
- Koopmans , R.J. Soft Matt . 2006 , 2 , 537 543 .
- Shonnard , D.R. Presented at 39th Middle Atlantic regional meeting of the American Chemical Society, Collegeville, PA, May 16–18, 2007; MARM-317 .
- Tsoka , C. ; Johns , W.R. ; Linke , P. ; Kokossis , A. Green Chem . 2004 , 6 , 401 406 .
- Constable , D.J.C. ; Dunn , P.J. ; Hayler , J.D. ; Humphrey , G.R. ; Leazer , J.L. Jr. ; Linderman , R.J. ; Lorenz , K. ; Manley , J. ; Pearlman , A. ; Wells , A. ; Zaks , A. ; Zhang , T.Y. Green Chem . 2007 , 9 , 411 420 .
- Anastas , P.T. ; Kirckhoff , M.M. ; Williamson , T.C. Appl. Catal. A General 2001 , 221 , 3 13 .
- Campelo , J.M. ; Diego , D. ; Luque , R. ; Marinas , J.M. ; Romero , A.A. ChemsSusChem . 2009 , 2 , 18 45 .
- Enthaler , S. ; Junge , K. ; Addis , D. ; Erre , G. ; Beller , M. ChemSusChem 2008 , 1 , 1006 1010 .
- Nordstrom , L.U. ; Vogt , H. ; Madsen , R. J. Am. Chem. Soc . 2008 , 130 , 17672 17673 .
- Kim , J.W. ; Yamaguchi , K. ; Mizuno , N. Angew. Chem. Int. Ed . 2008 , 47 , 9249 9251 .
- Correa , A. ; Garcia Mancheno , O. ; Bolm , C. Chem. Soc. Rev . 2008 , 37 , 1108 1117 .
- Enthaler , S. ; Junge , K. ; Beller , M. Angew. Chem. Int. Ed . 2008 , 47 , 3317 3321 .
- Czaplik , W.M. ; Mayer , M. ; Cvengros , J. ; von Wangelin , A.J. ChemSusChem 2009 , 2 , 396 417 .
- Shaabani , A. ; Maleki , A. ; Mofakham , H. Synth. Commun . 2008 , 38 , 3751 3754 .
- Chen , X. ; She , J. ; Shang , Z. ; Wu , J. ; Wu , H. Synthesis 2008 , 3478 3846 .
- Guchhait , S.K. ; Chetna , C. Synlett 2009 , 628 632 .
- Sen , M.Y. ; Puskas , J.E. ; Ummadisetty , S. ; Kennedy , J.P. Macromol. Rapid Commun . 2008 , 29 , 1598 1602
- Lombardo , M. ; Chiarucci , M. ; Trombini , C. Chem. Eur. J . 2008 , 14 , 11288 11291 .
- Giacalone , F. ; Gruttadauria , M. ; Lo Meo , P. ; Riela , S. ; Noto , R. Adv. Synth. Catal . 2008 , 350 , 2747 2760 .
- Lanfranchi , A. ; Baldovini , N. ; Hanquet , G. Synthesis 2008 , 3775 3778 .
- Iwasaki , T. ; Maegawa , Y. ; Hayashi , Y. ; Ohshima , T. ; Mashima , K. J. Org. Chem . 2008 , 73 , 5147 5150 .
- Thompson , M.J. ; Hurst , J.M. ; Chen , B. Synlett 2008 , 3183 3187 .
- De Paolis , O. ; Baffoe , J. ; Landge , S.M. ; Shainaz , M. ; Torok , B. Synthesis 2008 , 3423 3428 .
- Das , B. ; Kumar , A.S. ; Ravikanth , B. ; Damodar , K. ; Krishnaiah , M. J. Sulfur Chem . 2008 , 29 , 489 494 .
- Procopio , A. ; Das , G. ; Nardi , M. ; Oliverio , M. ; Pasqua , L. ChemSusChem 2008 , 1 , 916 919 .
- Hojo , K. ; Ichikawa , H. ; Fukumori , Y. ; Kawasaki , K. Int. J. Peptide Res. Ther . 2008 , 14 , 373 380 .
- Welton , T. Coord. Chem. Rev . 2004 , 248 , 2459 2477 .
- Plechkova , N.D. ; Seddon , K.R. Chem. Soc. Rev . 2008 , 37 , 123 150 .
- Malhotra , S.V. , Ionic Liquids in Organic Synthesis ; ACS : Wahington, DC , 2007 .
- Nouira , I. ; Kostakis , I.K. ; Dubouilh , C. ; Chosson , E. ; Iannelli , M. ; Besson , T. Tetrahed. Lett . 2008 , 49 , 7033 7063 .
- Seipel , K.R. ; Platt , Z.H. ; Nguyen , M. ; Holland , A.M. J. Org. Chem . 2008 , 73 , 4291 4294 .
- Presset , M. ; Coquerel , Y. ; Rodriguez , J. J. Org. Chem . 2009 , 74 , 415 418 .
- Zhang , X.R. ; Chao , W. ; Chuai , Y.T. ; Ma , Y. ; Hao , R. ; Zou , D.C. ; Wei , Y.G. ; Wang , Y. Org. Lett . 2006 , 8 , 2563 2566 .
- Reuman , M. ; Beish , S. ; Davis , J. ; Batchelor , M.J. ; Hutchings , M.C. ; Moffat , D.F.C. ; Connolly , P.J. ; Russell , R.K. J. Org. Chem . 2008 , 73 , 1121 1123 .
- Lebreux , F. ; Buzzo , F. ; Marko , I.E. Synlett 2008 , 2815 2820 .
- Ischay , M.A. ; Anzovino , M.E. ; Du , J. ; Yoon , T.P. J. Am. Chem. Soc . 2008 , 130 , 12886 12887 .
- Doble , M. Chem. Eng. Prog . 2008 , 104 , 33 83 .
- Høeibye , L. ; Clauson-Kaas , J. ; Wenzel , H. ; Larsen , H.F. ; Jacobsen , B.N. ; Dalgaard , O. Water Sci. Technol . 2008 , 58 , 963 968 .
- Peters , G.M. ; Rowley , H.V. Water 2008 , 35 , 80 83 .
- Zhang , X. ; Li , C. ; Fu , C. ; Chao , Z. ; Zhang , S. Ind. Eng. Chem. Res . 2008 , 47 , 1085 1094 .
- Guinee , J.B. ; Heijungs , R. Kirk-Othmer Encyclopedia of Chemical Technology , 5th ed 2005 14 805 .
- Eissen , M. ; Metzger , J.O. Chem. Eur. J . 2002 , 8 , 3580 3585 .
- Leitner , W. Green Chem . 2008 , 10 , 1023 .
- Horváth , I.T. Green Chem . 2008 , 10 , 1024 1028 .
- Balzani , V. ; Credi , A. ; Venturi , M. ChemSusChem 2008 , 1 , 26 58 .