Abstract
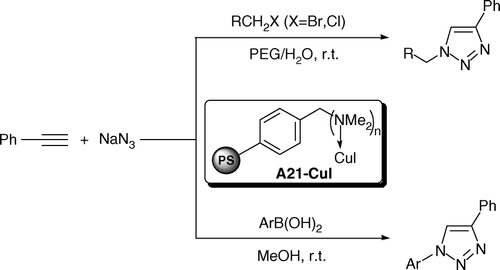
Polymer-supported copper-catalyzed one-pot synthesis of 1,4-disubstituted 1-alkyl- and 1-aryl-1,2,3-triazoles via 1,3-dipolar cycloaddition of alkyl halides or arylboronic acids, sodium azide, and phenylacetylene has been developed. Reactions went smoothly at room temperature using PEG/H2O or methanol as solvent to afford the corresponding triazoles in good to excellent yields. The catalyst could be recovered by simple filtration and reused several times with slightly decrease in its activity.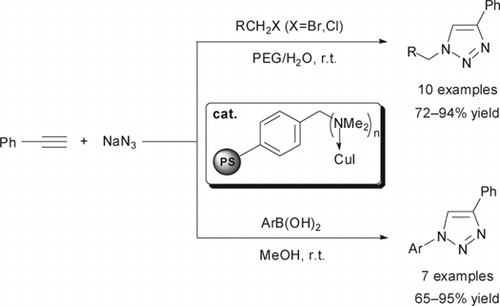
Introduction
The Huisgen 1,3-dipolar cycloaddition of azides and alkynes is one of the most powerful reactions to afford the 1,2,3-triazoles Citation1, which have shown interesting biological properties such as anti-allergic Citation2–4, anti-bacterial Citation5, and anti-HIV activity Citation6. An important advance in this field was the recent discovery that cycloadditions of terminal alkynes with organic azides catalyzed by CuI can be conducted at room temperature and are highly regioselective, leading to 4-substituted-1,2,3-triazoles. Consequently, this protocol has received considerable attention and found application in various research fields such as material chemistry Citation7–10, biological science Citation11–14, and medicinal chemistry Citation15–19. Despite these interesting applications, some limitations such as preparation of dangerous organic azides and metal contamination of the products are the problems need to be addressed.
Great efforts in green chemistry have been devoted in recent years to the application of eco-friendly heterogeneous catalysts Citation20 and multicomponent reactions (MCRs) Citation21. Immobilization of catalysts on polymer supports often offers advantages for carrying out organic transformations. Simple work-up, high selectivity, reusability of the catalysts, and low cost often make them more attractive than their homogeneous counterparts. MCRs have also received much attention lately, and have proven to be a very elegant and rapid way to access highly functional molecules from simple building blocks. We envisioned that the combination of heterogeneous catalysis system and the one-pot synthesis of 1,2,3-triazoles via in situ formation of organic azides will be advantageous. The azides could be generated in situ from the corresponding alkyl halides or arylboronic acids Citation22 avoiding the isolation step. The catalyst, in addition, could be easily recovered by simple filtration.
In this paper, we disclose a simple (dimethylamino) methyl-polystyrene-supported copper(I) iodide (A21-CuI) as an efficient and environmentally benign heterogeneous catalyst for the one-pot synthesis of 1,2,3-triazoles from alkyl halides or arylboronic acids, sodium azide, and phenylacetylene at ambient temperature ().
Results and discussion
The supported catalyst A21-CuI was prepared according to literature Citation23 and the copper content was found to be 1.31 mmol/g, determined by inductively coupled plasma (ICP) analysis. Initially, we focused on the one-pot synthesis of 1-alkyl substituted 1,2,3-triazoles. In an effort to develop an optimal catalytic system, different reaction parameters including solvent, base, as well as catalyst loading were studied via cycloaddition of benzyl chloride, sodium azide, and phenylacetylene at room temperature. The results are summarized in . Although the “click” reaction was reported in a wide range of solvent, such as DMSO/H2O, t BuOH/H2O, and CH2Cl2/H2O, only moderate yields were obtained in our cases (, entries 1–3). Encouraged by recent success in organic reactions with benign solvents, we turned our attention to PEG 400 and H2O. To our delight, 82% yield was obtained after 12 h when using PEG 400 as solvent. To further improve the solvent system, several combinations of PEG 400 and H2O were studied and finally PEG 400/H2O (1:1, v:v) was chosen as the best solvent. We assumed that this result was attributed to the phase-transfer catalytic nature of PEG 400. Water, in another aspect, could increase the solubility of sodium azide. The influences of different bases and catalyst loadings were also evaluated and good yield was obtained when employing Et3N as base and 10 mol% of catalyst (, entry 6).
Table 1. Screening of reaction parameters for one-pot synthesis of triazoles using benzyl chloride as substrate.a
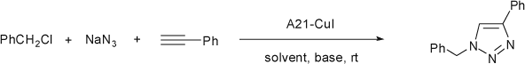
Under the optimized conditions, we carried out the cycloaddition reactions of various alkyl halides, sodium azide, and phenylacetylene (). It was found that both substituted benzyl halides and allyl halides could be efficiently converted to the desired products in good to excellent yields (72–94%). Among the benzyl halides, substrates bearing electron-withdrawing groups were more active. Other alkyl halides, such as C2H5Br and C4H9Br were less efficient and a prolonged time was required (, entries 9 and 10).
Table 2. Synthesis of 1,4-disubstituted 1-alkyl-1,2,3-triazoles.a
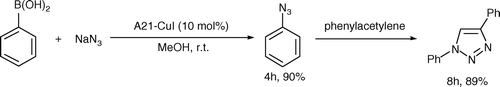
Having successfully developed an efficient system for preparation of 1-alkyl substituted triazoles, we considered whether our described procedure could be adapted toward one-pot synthesis of 1-aryl substituted triazoles. However, no reaction occurred at room temperature after 24 h when using iodobenzene as substrate (, entry 11). It was assumed that the reaction may be problematic with respect to the azidonation of iodobenzene, since the preparation of aryl azides relies upon a limited selection of transformations Citation24. Just recently, Tao et al. Citation22 reported a very interesting method in which copper(II) sulfate was used to promote the azidonation of arylboronic acids at room temperature. We considered that whether our supported CuI catalyst could be helpful in one-pot synthesis of 1-aryl substituted triazoles under mild conditions.
Initially, we tried the azidonation of phenylboronic acid according to the literature (). To our delight, the reaction proceeded fast in 4 h yielding the product in 90%. With this good result, we investigated the application of A21-CuI in one-pot 1,3-dipolar cycloaddition reaction. After consumption of the starting material (monitored by thin-layer chromatography (TLC)), phenylacetylene (1.1 equivalent) was added directly at room temperature to give the triazole in 89% yield. Other arylboronic acids were also employed in this one-pot two-step cycloaddition process. As shown in , each arylboronic acid could be applied in the reaction and a variety of functional groups on arylboronic acids were fully tolerated. It should be noted that our approach not only provided a simple way to prepare 1-aryl 1,2,3-triazoles in one-pot under mild conditions, the catalyst could also be easily recovered and reused for seven times (, entry 1).
Table 3. A21-CuI catalyzed the one-pot synthesis of 1-aryl 1,2,3-triazoles.a
Experimental
Chemicals were purchased from commercial suppliers and were used without further purification. All 1H and 13C NMR spectra were recorded with a Bruker Advance RX300 analyzer in CDCl3 with tetramethylsilane (TMS) as internal standard. The Cu content was determined with a Varian AA240 ICP analysis.
Typical procedure for the one-pot synthesis of 1-alkyl 1,2,3-triazoles
Alkyl halide (1 mmol), NaN3 (1.2 mmol), and phenylacetylene (1 mmol) were placed in an oven-dried round-bottomed flask. Subsequently, PEG 400/H2O (4 ml, 1:1), Et3N (1 mmol), and A21-CuI (77 mg, 10 mol%) were added. The reaction mixture was stirred vigorously at room temperature for a certain time (monitored by TLC). Then EtOAc (20 ml) was added and the catalyst was removed, washed with acetone and water, and dried under vacuum. The extracts were washed with water (2×10 ml), dried over Na2SO4, and evaporated under reduced pressure to give the crude product which was further purified by column chromatography on silica gel using hexane/ethyl acetate as eluent. Selected data: 1-benzyl-4-phenyl-1H-1,2,3-triazole: 1H NMR (300 MHz, CDCl3): ▵=5.46 (s, 2H), 7.25–7.39 (m, 8H), 7.67 (s, 1H), 7.77 (d, 2H, J=7.3 Hz); 13C NMR (75 MHz, CDCl3): ▵=53.8, 119.6, 125.4, 127.7, 127.9, 128.4, 128.6, 128.8, 130.4, 134.6, 147.9.
Typical procedure for the one-pot synthesis of 1-aryl 1,2,3-triazoles
Arylboronic acid (1 mmol) and NaN3 (1.2 mmol) were placed in an oven-dried round-bottomed flask. Subsequently, methanol (4 ml) and A21-CuI (77 mg, 10 mol%) were added. The reaction mixture was stirred vigorously at room temperature. Then phenylacetylene (1.1 mmol) was added into the flask and the resulting mixture was stirred vigorously for a certain time (monitored by TLC). Then CH2Cl2 (20 ml) was added and the catalyst was filtered off, washed with acetone and water, and dried under vacuum. The extracts were washed with water (2×10 ml), dried over Na2SO4, and evaporated under reduced pressure to give the crude product which was further purified by column chromatography on silica gel using hexane/ethyl acetate as eluent. Selected data: 1,4-diphenyl-1H-1,2,3-triazole: 1H NMR (300 MHz, CDCl3): δ=7.37 (t, J=7.2, 1H), 7.43–7.48 (m, 3H), 7.54 (t, J=7.5, 2H), 7.79 (d, J=7.5, 2H), 7.92 (d, J=7.2, 2H), 8.22 (s, 1H); 13C NMR (75 MHz, CDCl3): 120.7, 126.0, 128.5, 128.9, 129.0, 129.9, 130.4.
Conclusion
In summary, simple and efficient procedures for one-pot synthesis of 1,4-disubstituted-1,2,3-triazoles from corresponding alkyl halides and arylboronic acids have been described. Our protocols are advantageous over the reported procedures not only because of simple operation, high yielding, green reaction system, and mild conditions, but also because they avoided the isolation of the explosive organic azides. Moreover, the supported catalyst can be easily recovered by simple filtration and can be reused for several runs with almost consistent activities.
References
- Huisgen , R. In 1,3-Dipolar Cycloaddition Chemistry ; Padwa , A. ; Wiley : New York , 1984 .
- Buckle , D.R. ; Rockell , C.J.M. J. Chem. Soc. Perkin Trans . 1982 , 1 , 627 630 .
- Buckle , D.R. ; Outred , D.J. ; Rockell , C.J.M. ; Smith , H. ; Spicer , B.A. J. Med. Chem . 1983 , 26 , 251 254 .
- Buckle , D.R. ; Rockell , C.J.M. ; Smith , H. ; Spicer , B.A. J. Med. Chem . 1986 , 29 , 2262 2267 .
- Genin , M.J. ; Allwine , D.A. ; Anderson , D.J. ; Barbachyn , M.R. ; Emmert , D.E. ; Garmon , S.A. ; Graber , D.R. ; Grega , K.C. ; Hester , J.B. ; Hutchinson , D.K. ; Morris , J. ; Reischer , R.J. ; Ford , C.W. ; Zurenko , G.E. ; Hamel , J.C. ; Schaadt , R.D. ; Stapert , D. ; Yagi , B.H. J. Med. Chem . 2000 , 43 , 953 970 .
- Alvarez , R. ; Velazquez , S. ; San-Felix , A. ; Aquaro , S. ; De Clercq , E. ; Perno , C.F. ; Karlsson , A. ; Balzarini , J. Camarasa , M.J. J. Med. Chem . 1994 , 37 , 4185 4194 .
- Wu , P. ; Feldman , A.K. ; Nugent , A.K. ; Hawker , C.J. ; Scheel , A. ; Voit , B. ; Pyun , J. ; Frechet , J.M.J. ; Sharpless , K.B. ; Fokin , V.V. Angew. Chem. Int. Ed . 2004 , 43 , 3928 3932 .
- Aucagne , V. ; Hanni , K.D. ; Leigh , D.A. ; Lusby , P.J. ; Walker , D.B. J. Am. Chem. Soc . 2006 , 128 , 2186 2187 .
- Liu , Q.C. ; Zhao , P. ; Chen , Y.M. J. Polym. Sci. Part A: Polym. Chem . 2007 , 45 , 3330 3341 .
- Angelos , S. ; Yang , Y.W. ; Patel , K. ; Stoddart , J.F. ; Zink , J.I. Angew. Chem. Int. Ed . 2008 , 47 , 2222 2226 .
- Sivakumar , K. ; Xie , F. ; Cash , B.M. ; Long , S. ; Barnhill , H.N. ; Wang , Q. Org. Lett . 2004 , 6 , 4603 4606 .
- Agard , N.J. ; Prescher , J.A. ; Bertozzi , C.R. J. Am. Chem. Soc . 2004 , 126 , 15046 15047 .
- Costa , M.S. ; Boechat , N. ; Rangel , E.A. ; Da Silva , F.D. ; de Souza , A.M.T. ; Rodrigues , C.R. ; Castro , H.C. ; Junior , I.N. ; Lourenco , M.C.S. ; Wardell , S. ; Ferreira , V.F. Bioorg. Med. Chem . 2006 , 14 , 8644 8653 .
- Kumar , R. ; El-Sagheer , A. ; Tumpane , J. ; Lincoln , P. ; Wilhelmsson , L.M. ; Brown , T. J. Am. Chem. Soc . 2007 , 129 , 6859 6864 .
- Kolb , H.C. ; Sharpless , K.B. Drug Discov. Today 2003 , 8 , 1128 1137 .
- Manetsch , R. ; Krasiski , A. ; Radi , Z. ; Raushel , J. ; Taylor , P. ; Sharpless , K.B. ; Kolb , H.C. J. Am. Chem. Soc . 2004 , 126 , 12809 12818 .
- Whiting , M. ; Muldoon , J. ; Lin , Y.C. ; Silverman , S.M. ; Lindstrom , W. ; Olson , A.J. ; Kolb , H.C. ; Finn , M.G. ; Sharpless , K.B. ; Elder , J.H. ; Fokin , V.V. Angew. Chem. Int. Ed . 2006 , 45 , 1435 1439 .
- Sugawara , A. ; Sunazuka , T. ; Hirose , T. ; Nagai , K. ; Yamaguchi , Y. ; Hanaki , H. ; Sharpless , K.B. ; Omura , S. Bioorg. Med. Chem. Lett . 2007 , 17 , 6340 6344 .
- Tron , G.C. ; Pirali , T. ; Billington , R.A. ; Canonico , P.L. ; Sorba , G. ; Genazzani , A.A. Med. Res. Rev . 2008 , 28 , 278 308 .
- Ley , S.V. ; Baxendale , I.R. ; Bream , R.N. ; Jackson , P.S. ; Leach , A.G. ; Longbottom , D.A. ; Nesi , M. ; Scott , J.S. ; Storer , R.I. ; Taylor , S.J. J. Chem. Soc. Perkin Trans . 2000 , 1 , 3815 4195 ; and references cited therein .
- Zhu , J. ; Bienayme , H. Multicomponent Reactions , 1st ed. Weinheim : Wiley-VCH , 2005 .
- Tao , C.Z. ; Cui , X. ; Li , J. ; Liu , A.X. ; Liu , L. ; Guo , Q.X. Tetrahed. Lett . 2007 , 48 , 3525 3529 .
- Girard , C. ; Önen , E. ; Aufort , M. ; Beauvière , S. ; Samson , E. ; Herscovici , J. Org. Lett . 2006 , 8 , 1689 1692 .
- Scriven , E.F.V. ; Turnbull , K. Chem. Rev . 1988 , 88 , 297 368 .