Abstract
An efficient, simple, and green procedure for the synthesis of quinoxaline derivatives is described. The condensation of 1,2-diamines with 1,2-diketones using lithium bromide (LiBr) in H2O/EtOH as a green reaction media at room temperature affords the title compounds in high to excellent yields and in short reaction times.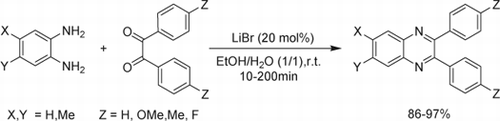
Introduction
Quinoxaline derivatives are an important class of nitrogen-containing heterocycles in medicinal chemistry as they have various biological activities, such as antimycobacterial Citation1 Citation2, antibacterial Citation3, antifungal Citation4, anihelmintic Citation4, antidepressant Citation5, and antitumor properties Citation6 Citation7. Moreover, these compounds have been applied for the preparation of various dyes Citation8. The condensation of 1,2-diamines with 1,2-diketones has been used as a useful protocol for the synthesis of quinoxalines. For this transformation, several catalysts and reagents have been reported, including o-iodoxybenzoic acid Citation9, ceric(IV) ammonium nitrate Citation10, zirconium tetrakis (dodecyl sulfate) Citation11, Yb(OTf)3 Citation12, (NH4)6Mo7O24.4H2O Citation13, sulfamic acid Citation14, H6P2W18O62.24H2O Citation15, oxalic acid Citation16, iodine in DMSO Citation17, polyaniline-sulfate salt Citation18, and KHSO4 Citation19. Other methods which have been applied for the synthesis of quinoxaline derivatives include heteroannulation of nitroketene N,S-aryliminoacetals with POCl3 Citation20, bi-catalyzed oxidative coupling of epoxides with ene-1,2-diamines Citation21, and cyclization of α-arylimino oximes of α-dicarbonyl compounds Citation22. However, many of the reported protocols are associated with one or more of the following disadvantages: need for anhydrous conditions; harsh reaction conditions; the use of expensive reagents; prolonged reaction times; moderate yields; and no agreement with the green chemistry protocols. Therefore, development of an efficient, cheap, simple, and environmentally friendly method for the preparation of quinoxaline derivatives is desirable.
Lewis acid-catalyzed reactions are currently of great interest because of their unique reactivity, selectivity, and need for mild conditions, but most of them are unusable in water Citation23 Citation24. Lithium bromide (LiBr) is a mild Lewis acid, which has been employed as catalyst in several organic transformations Citation25–34. The strong oxophilicity of Li+ activates oxygen-containing electrophiles to accept nucleophilic attack Citation35 Citation36. In most of the reported reactions, LiBr has been introduced as almost neutral Lewis acid catalyst.
The current environmental concerns encourage development of “greener” conditions, where possible, and the tight legislation on the maintenance of green conditions in synthetic processes that insists on preventing generation of waste, avoiding the use of non-green organic solvents, and minimizing the energy requirements Citation11 Citation13 Citation16 Citation37–41. The use of aqueous reaction media has received considerable attention in the context of green chemistry for several reasons: (1) it is cheap, safe, and environmentally benign; (2) performing reactions in aqueous medium eliminate the additional efforts to dry substrates and reagents before use, and thus reduce/eliminate the consumption of drying agents, energy, and time; and (3) the unique physical and chemical properties of water can be utilized to realize reactivity or selectivity that cannot be attained in organic solvents Citation11 Citation13 Citation16 Citation37–41.
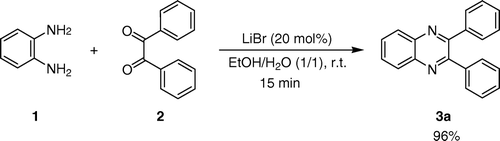
As part of our researches to develop efficient and environmentally benign synthetic methods in organic chemistry Citation11 Citation13 Citation16 Citation42–48, we report here an efficient, extremely mild, green, and simple method for the preparation of quinoxalines from aryl/alkyl 1,2-diamines and different 1,2-diketones using LiBr as an inexpensive, commercially available, and water-tolerant Lewis acid catalyst in H2O/EtOH mixture ().
Results and discussion
At first, effects of different proportions of LiBr to substrate, and also solvents were investigated on the condensation of benzene-1,2-diamine with benzil at room temperature (). The results are summarized in . As shown in , reasonable results were obtained when the reaction was carried out in the presence of 20 mol% of LiBr in EtOH/H2O (1/1, v/v).
Table 1. Effect of different proportions of LiBr to substrate, and also solvents on the condensation of benzene-1,2-diamine with benzil at room temperature.
To show the influence of oxophilicity of Li+ on activation of the carbonyl group of 1,2-diketones to accept nucleophilic attack of the amine group of 1,2-diamines, the reaction of benzene-1,2-diamine with benzil was examined in the presence of NaBr, KBr, and CsBr (20 mol%) in EtOH/H2O (1/1) at room temperature (); however, in these cases, the product was produced in low yields and in long reaction times. Thus, Li+ efficiently activates 1,2-diketones to progress the reaction Citation35 Citation36.
Table 2. The reaction of benzene-1,2-diamine with benzil in the presence of various alkaline metals bromides.
To recognize the generality and the scope of our method, different aromatic and aliphatic 1,2-diamines were reacted with structurally diverse 1,2-diketones. The results are displayed in . As indicates, when aromatic 1,2-diamines were used, all reactions proceeded efficiently and the desired quinoxalines were obtained in excellent yields and in short reaction times (, entries 1–13). However, aliphatic 1,2-diamines afforded the corresponding quinoxaline derivatives in slightly lower yields and longer reaction times (, entries 14–16). Furthermore, it was observed that the kind of 1,2-diketone had no significant effect on the reaction results.
Table 3. The green preparation of quinoxaline derivatives from 1,2-diamines and 1,2-diketones using LiBr in EtOH/H2O (1/1) at room temperature.
Experimental
All chemicals were purchased from Merck or Fluka Chemical Companies. The progress of the reactions was followed by TLC using silica gel SILG/UV 254 plates. The 1H NMR (250 or 500 MHz) and 13C NMR (62.9 or 125 MHz) were run on a Bruker Avance DPX-250, FT-NMR spectrometer (δ in ppm). Microanalyses were performed on a Perkin-Elmer 240-B microanalyzer. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
General procedure for the preparation of quinoxalines from 1,2-diamines and 1,2-diketones
To a mixture of 1,2-diketone (1 mmol), LiBr (0.017 g, 0.2 mmol), and EtOH/H2O [20 mL, 1/1 (v/v)] in a 50 mL round-bottomed flask was added 1,2-diamine (1 mmol), and the resulting mixture was stirred at room temperature for the appropriate time (). Afterward, H2O (20 mL) was added to the reaction mixture, and was allowed to stand at room temperature for 1 h. During this time, crystals of the pure product formed which were collected by filtration, and dried.
Selected spectral data of the products
2,3-Diphenylquinoxaline (3a). White solid; m.p. 130–131°C (Lit. Citation9 128–129°C); 1H NMR (CDCl3): δ 7.29–7.33 (m, 6H), 7.51 (m, 4H), 7.77 (m, 2H), 8.21 (m, 2H); 13C NMR (CDCl3): δ 128.1, 128.7, 129.1, 129.9, 131.0, 139.6, 141.7, 153.2; Anal. calcd. for C20H14N2: C, 85.08; H, 5.00; N, 9.92; Found: C, 85.29; H, 4.82; N, 10.13.
9-Methylacenaphtho[1,2-b]quinoxaline (3i). Pale yellow solid; m.p. 233–235°C; 1H NMR (500 MHz): δ 2.60 (s, 3H), 7.55 (d, J=8.2 Hz, 1H), 7.79 (t, 2H, J=7.5 Hz), 7.95 (s, 1H), 8.03–8.07 (m, 3H), 8.35 (t, 2H, J=6.3 Hz); 13C NMR (125 MHz): δ 22.2, 121.9, 122.1, 129.0, 129.2, 129.5, 129.6, 129.8, 130.4, 131.7, 132.4, 136.7, 140.0, 140.1, 141.7, 153.8, 154.5; Anal. calcd. for C19H12N2: C, 85.05; H, 4.51; N, 10.44; Found: C, 84.73; H, 4.69; N, 10.29.
9,10-Dimethylacenaphtho[1,2-b]quinoxaline (3k). Yellow solid; m.p. 303–305°C (Lit. Citation16 304–306°C); 1H NMR (CDCl3): δ 2.51 (s, 6H), 7.78 (m, 2H), 7.89 (s, 2H), 8.03 (m, 2H), 8.34 (m, 2H); 13C NMR (CDCl3): δ 20.3, 121.5, 127.8, 128.0, 128.6, 128.9, 129.1, 139.5, 140.00, 148.5, 153.3; Anal. calcd. for C20H14N2: C, 85.08; H, 5.00; N, 9.92; Found: C, 84.85; H, 5.19; N, 9.81.
Conclusion
In summary, we have introduced a highly efficient catalyst for the condensation of 1,2-diamines with 1,2-diketones. The promising points for the presented method include high yield, ease of handling, low cost of the catalyst, simple procedure and work-up, cleaner reaction profile, short reaction time, and compliance with the green chemistry protocols which make it a useful and attractive process for the rapid synthesis quinoxaline derivatives as biologically interesting compounds.
Acknowledgements
The authors thank Persian Gulf University and Payame Noor University (PNU) Research Councils for the financial support of this work.
References
- Waisser , K. ; Odlerova , Z. ; Beckert , R. ; Mayer , R. Pharmazie 1989 , 44 , 234 235 .
- Seitz , L.E. ; Suling , W.J. ; Reynolds , R.C. J. Med. Chem . 2002 , 45 , 5604 5606 .
- Badran , M.M. ; Moneer , A.A. ; Refaat , H.M. ; El-Malah , A.A. J. Chin. Chem. Soc . 2007 , 54 , 469 478 .
- Sakata , G. ; Makino , K. ; Karasawa , Y. Heterocycles 1988 , 27 , 2481 2515 , and references cited therein .
- Sarges , R. ; Howard , H.R. ; Browne , R.C. ; Label , L.A. ; Seymour , P.A. J. Med. Chem . 1990 , 33 , 2240 2254 .
- Hazeldine , S.T. ; Polin , L. ; Kushner , J. ; Paluch , J. ; White , K. ; Edelstein , M. ; Palomino , E. ; Corbett , T.H. ; Horwitz , J.P. J. Med. Chem . 2001 , 44 , 1758 1776 .
- Hazeldine , S.T. ; Polin , L. ; Kushner , J. ; White , K. ; Bouregeois , N.M. ; Crantz , B. ; Palomino , E. ; Corbett , T.H. ; Horwitz , J.P. J. Med. Chem . 2002 , 45 , 3130 3137 .
- Rangnekar , D.W. ; Sonawane , N.D. ; Sabnis , R.W. J. Heterocyclic Chem . 1998 , 35 , 1353 1356 .
- Heravi , M.M. ; Bakhtiari , K. ; Tehrani , M.H. ; Javadi , N.M. Oskooie , H.A. ARKIVOC 2006 , xvi , 16 22 .
- More , S.V. ; Sastry , M.N.V. ; Yao , C-F. Green Chem . 2006 , 8 , 91 95 .
- Hasaninejad , A. ; Zare , A. ; Zolfigol , M.A. ; Shekouhy , M. Synth. Commun . 2009 , 39 , 569 579 .
- Wang , L. ; Liu , J. ; Tian , H. ; Qian , C. Synth. Commun . 2004 , 34 , 1349 1357 .
- Hasaninejad , A. ; Zare , A. ; Mohammadizadeh , M.R. ; Karami , Z. J. Iran. Chem. Soc . 2009 , 6 , 153 158 .
- Darabi , H.R. ; Mohandessi , S. ; Aghapoor , K. ; Mohsenzadeh , F. Catal. Commun . 2007 , 8 , 389 392 .
- Heravi , M.M. ; Bakhtiari , K. ; Bamoharram , F.F. ; Tehrani , M.H. Monatsh. Chem . 2007 , 138 , 465 467 .
- Hasaninejad , A. ; Zare , A. ; Mohammadizadeh , M.R. ; Shekouhy , M. ARKIVOC 2008 , xiii , 28 35 .
- Bhosale , R.S. ; Sarda , S.R. ; Ardhapure , S.S. ; Jadhav , W.N. ; Bhusare , S.R. ; Pawar , R.P. Tetrahed. Lett . 2005 , 46 , 7183 7186 .
- Srinivas , C. ; Kumar , C.N.S.S.P. ; Rao , V.J. ; Palaniappan , S. J. Mol. Catal. A: Chem . 2007 , 265 , 227 230 .
- Oskooie , H.A. ; Heravi , M.M. ; Bakhtiari , K. ; Taheri , S. Monatsh. Chem . 2007 , 138 , 875 877 .
- Venkatesh , C. ; Singh , B. ; Mahata , P.K. ; Ila , H. ; Junjappa , H. Org. Lett . 2005 , 7 , 2169 2172 .
- Antoniotti , S. ; Duńach , E. Tetrahed. Lett . 2002 , 43 , 3971 3973 .
- Xekoukoulotakis , N.P. ; Hadjiantoniu-Maroulis , C.P. ; Maroulis , A.J. Tetrahed. Lett . 2000 , 41 , 10299 10302 .
- Santelli , M. ; Pons , J-M. Lewis Acids and Selectivity in Organic Synthesis . CRC Press : Boca Raton, FL , 1995 . pp 91 184 .
- Fringuelli , F. ; Pizzo , F. ; Vaccaro , L. J. Org. Chem . 2001 , 66 , 4719 4722 .
- Itoh , A. ; Hashimoto , S. ; Masaki , Y. Synlett 2005 , 18 , 2639 2640 .
- Kumar , S. ; Saini , A. ; Sandhu , J.S. ARKIVOC 2007 , xv , 18 23 .
- Ono , F. ; Negoro , R. ; Sato , T. Synlett 2001 , 10 , 1581 1583 .
- Seebach , D. ; Thaler , A. ; Blaser , D. ; Ko , S.Y. Helv. Chim. Acta 1991 , 74 , 1102 1118 .
- Prajapati , D. ; Lekhok , K.R. ; Sandhu , J.S. ; Ghosh , A.C. J. Chem. Soc., Perkin Trans . 1996 , 1 , 959 960
- Laskar , D.D. ; Prajapati , D. ; Sandhu , J.S. Synth. Commun . 2001 , 31 , 1427 1432 .
- Baruah , P.P. ; Gadhwal , S. ; Prajapati , D. ; Sandhu , J.S. Chem. Lett . 2002 , 1038 1039 .
- Saini , A. ; Kumar , S. ; Sandhu , J.S. Synlett 2006 , 12 , 1928 1932 .
- Firouzabadi , H. ; Karimi , B. ; Eslami , S. Tetrahed. Lett . 1999 , 40 , 4055 4058 .
- Firouzabadi , H. ; Iranpoor , N. ; Karimi , M. Synthesis 1999 , 1 , 58 60 .
- Basak , A. (née Nandi) Nayak , M.K. ; Chakraborti , A.K. Tetrahed. Lett . 1998 , 39 , 4883 4886 .
- Chakraborti , A.K. ; Basak , A. (née Nandi) Grover , V. J. Org. Chem . 1999 , 64 , 8014 8017 .
- Grieco , P.A. Organic Synthesis in Water ; London : Blackie Academic and Professional , 1998 .
- Minakata , S. ; Komatsu , M. Chem. Rev . 2009 , 109 , 711 724 .
- Tundo , P. ; Anastas , P. ; Black , D.S. ; Breen , J. ; Collins , T. ; Memoli , S. ; Miyamoto , J. ; Polyakoff , M. ; Tumas , W. Pure Appl. Chem . 2000 , 72 , 1207 1228 .
- Clark , J.H. Green Chem . 1999 , 1 , 1 8 .
- Li , C-J. Chem. Rev . 2005 , 105 , 3095 3165 .
- Zare , A. ; Parhami , A. ; Moosavi-Zare , A.R. ; Hasaninejad , A. ; Khalafi-Nezhad , A. ; Beyzavi , M.H. Can. J. Chem . 2009 , 87 , 416 421 .
- Zare , A. ; Hasaninejad , A. ; Beyzavi , M.H. ; Moosavi Zare , A.R. ; Khalafi-Nezhad , A. ; Asadi , F. ; Baramaki , L. ; Jomhori-Angali , S. ; Ghaleh-Golabi , R. Synth. Commun . 2009 , 39 , 139 157 .
- Zare , A. ; Moosavi-Zare , A.R. ; Hasaninejad , A. ; Parhami , A. ; Khalafi-Nezhad , A. ; Beyzavi , M.H. Synth. Commun . 2009 , 39 , 3156 3465 .
- Hasaninejad , A. ; Zare , A. ; Parhami , A. ; Moosavi-Zare , A.R. ; Bargebid , R. ; Beyzavi , M.H. ; Khalafi-Nezhad , A. ; Arghoon , A. ; Merajoddin , M. ; Moosavi , S.A. ; Dara , A. ; Shekouhy , M. Org. Prep. Proc. Int . 2009 , 41 , 291 299 .
- Zare , A. ; Hasaninejad , A. ; Shekouhy , M. ; Moosavi Zare , A.R. Org. Prep. Proc. Int . 2008 , 40 , 457 463 .
- Khalafi-Nezhad , A. ; Parhami , A. ; Zare , A. ; Moosavi Zare , A.R. ; Hasaninejad , A. ; Panahi , F. Synthesis 2008 , 4 , 617 621 .
- Zare , A. ; Hasaninejad , A. ; Beyzavi , M.H. ; Parhami , A. ; Moosavi Zare , A.R. ; Khalafi-Nezhad , A. ; Sharghi , H. Can. J. Chem . 2008 , 86 , 317 324 .